Lithium-Ion Batteries: Thermal Behaviour Investigation of Unbalanced Modules
Abstract
:1. Introduction
2. Module Description
3. Mathematical Modelling of the Module
3.1. Governing Equations
3.1.1. Electrochemical Equations of the Cells
3.1.2. Electrochemical-Thermal Equations of the Cells
3.1.3. Thermo-Hydrodynamic Equations of the Module
3.2. Boundary Conditions
3.3. Numerical Solution and Validation
4. Results and Discussion
4.1. Module Design Considerations
4.1.1. Influence of the Cooling
4.1.2. Influence of the Air Flux
4.1.3. Influence of the Spacing
4.1.4. Validation of the Computational Fluid Dynamics Model
4.2. Unbalanced Battery Module
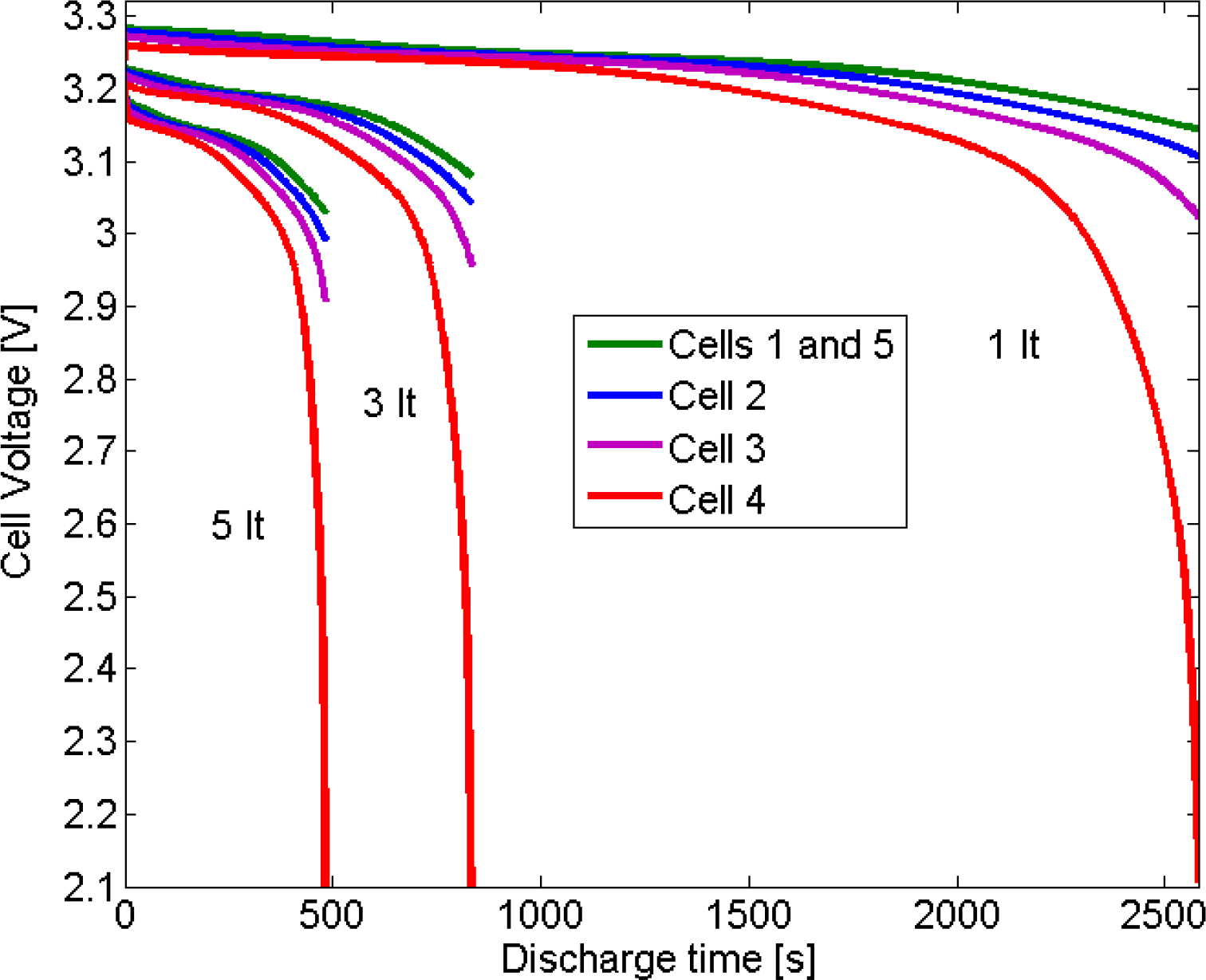
4.2.1. Voltage of the Cells
4.2.2. Temperature Distribution
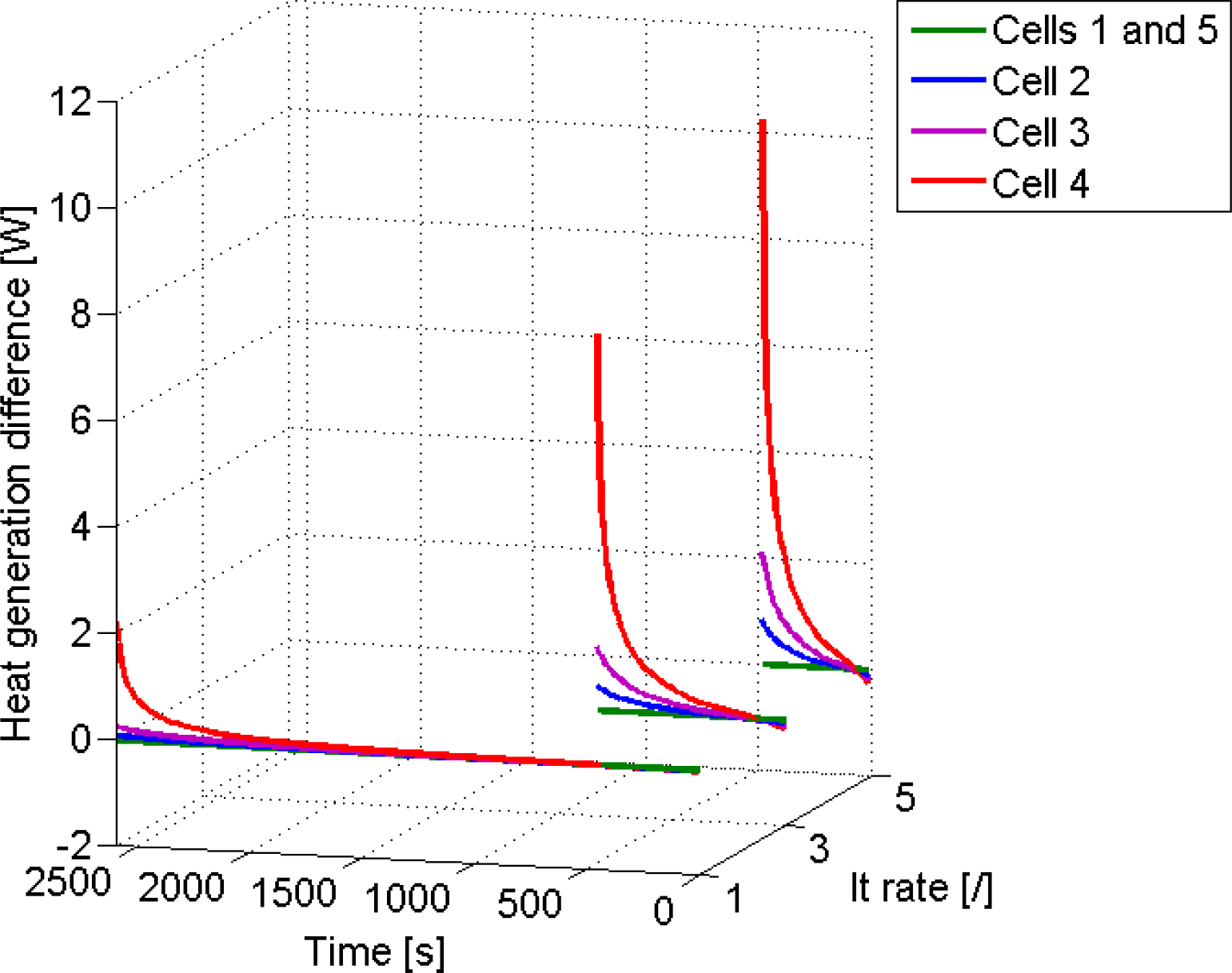
4.2.3. Heat Generation
5. Conclusions
List of Symbols (Variables and Parameters)
| Symbols | |
|---|---|
| as | Active surface area (1/m) |
| c | lithium ions concentration (mol/m3) |
| cp | Heat capacity (J/kg·K) |
| d | Diameter at the start of the spiral or internal diameter of the cell (m) |
| D | Diffusion coefficient (m2/s) |
| D | Diameter taken at the end of the spiral or cell diameter (m) |
| Ea | Activation energy (J/mol) |
| F | Faraday constant, 96,485 C/mol |
| h | Convection heat transfer coefficient W/m2·K |
| i0 | Exchange current density of an electrode reaction (A/m2) |
| jLi | Transfer current due to the intercalation or deintercalation of lithium ions (A/m3) |
| k | Electrochemical reaction rate constant (m/s) |
| k | Thermal conductivity (W/m·K) |
| L | Total length of the spiral constituting the internal structure of the cell (m) |
| L | Length of the cells in the vertical direction (i.e./height) (m) |
| N | Total number of layers in the jelly roll structure of the cell (/) |
| N | Total number of cells in the battery module (NT·NL) (/) |
| NL | Number of cells in the longitudinal direction of the battery module (/) |
| NT | Number of cells in the transverse direction of the battery module (/) |
| Q | Heat generation (W/m3) |
| r | Radius of the spherical particle (m) |
| R | Universal gas constant, 8314 J/mol·K |
| ST | Transverse distance between two consecutive cells on the same row (m) |
| t | Time (s) |
| t+ | Transference number of lithium ions (/) |
| T | Temperature (°C) |
| U | Open circuit voltage (V) |
| v | Fluid velocity at the entrance of the module (m/s) |
| V | Volume of layer (m3) |
| w | Width of the different layers constituting the jelly roll (m) |
| Greek letters | |
|---|---|
| α | Charge transfer coefficient (/) |
| ϵ/Volume | fraction (/) |
| η | Overpotential (V) |
| κ | Ionic conductivity (S/m) |
| ϕ | Potential (V) |
| ρ | Density (kg/m3) |
| σ | Electrical conductivity (S/m) |
| Subscripts | |
|---|---|
| a | Anode |
| c | Cathode |
| e or l | Electrolyte phase |
| eff | Effective |
| f | Fluid |
| i | Inlet |
| max | Maximum |
| measured | Measured by experiment |
| model | Modeled by simulation |
| o | Outlet |
| s | Solid phase |
| w | Wall |
| Abbreviations | |
|---|---|
| DoD | Depth of discharge |
| EV | Electric vehicle |
| HEV | Hybrid electric vehicle |
| LFP | Lithium iron phosphate |
| MUMPS | Multifrontal Massively Parallel Solver |
| SoC | State of charge |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van den Bossche, P.; Omar, N.; Al Sakka, M.; Samba, A.; Gualous, H.; van Mierlo, J. The Challenge of PHEV Battery Design and the Opportunities of Electrothermal Modeling. In Lithium-Ion Batteries: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 11. [Google Scholar]
- Van den Bossche, P.; Vergels, F.; van Mierlo, J.; Matheys, J.; van Autenboer, W. SUBAT: An assessment of sustainable battery technology. J. Power Sources 2006, 162, 913–919. [Google Scholar]
- Omar, N.; Daowd, M.; van den Bossche, P.; Hegazy, O.; Smekens, J.; Coosemans, T.; van Mierlo, J. Rechargeable Energy Storage Systems for Plug-in Hybrid Electric Vehicles-Assessment of Electrical Characteristics. Energies 2012, 5, 2952–2988. [Google Scholar]
- Mulder, G.; Omar, N.; Pauwels, S.; Meeus, M.; Leemans, F.; Verbrugge, B.; de Nijs, W.; van den Bossche, P.; Six, D.; van Mierlo, J. Comparison of commercial battery cells in relation to material properties. Electrochim. Acta 2013, 87, 473–488. [Google Scholar]
- Van Mierlo, J.; van den Bossche, P.; Maggetto, G. Models of energy sources for EV and HEV: Fuel cells, batteries, ultracapacitors, flywheels and engine-generators. J. Power Sources 2004, 128, 76–89. [Google Scholar]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar]
- Pesaran, A.A. Battery thermal models for hybrid vehicle simulations. J. Power Sources 2002, 110, 377–382. [Google Scholar]
- Sabbah, R.; Kizilel, R.; Selman, J.R. Active (air-cooled) vs. passive (phase change material) thermal management of high power lithium-ion packs: Limitation of temperature rise and uniformity of temperature distribution. J. Power Sources 2008, 182, 630–638. [Google Scholar]
- Fathabadi, H. High thermal performance lithium-ion battery module including hybrid active-passive thermal management system for using in hybrid/electric vehicles. Energy 2014, 70, 529–538. [Google Scholar]
- Warner, J. Lithium-Ion Battery Packs for EVs. In Lithium-Ion Batteries: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 7. [Google Scholar]
- Goli, P.; Legedza, S.; Dhar, A.; Salgado, R.; Renteria, J.; Balandin, A. Graphene-enhanced hybrid phase change materials for thermal management of Li-ion batteries. J. Power Sources 2014, 248, 37–43. [Google Scholar]
- Renteria, J.; Nika, D.; Balandin, A. Graphene Thermal Properties: Applications in Thermal Management and Energy Storage. J. Appl. Sci. 2014, 4, 525–547. [Google Scholar]
- Koo, B.; Goli, P.; Sumant, A.; dos Santos Claro, P.; Rajh, T.; Johnson, C.; Balandin, A.; Shevchenko, E. Toward Lithium Ion Batteries with Enhanced Thermal Conductivity. ACS Nano 2014, 8, 7202–7207. [Google Scholar]
- Evanoff, K.; Khan, J.; Balandin, A.; Magasinski, A.; Ready, W.; Fuller, T.; Yushin, G. Towards Ultrathick Battery Electrodes: Aligned Carbon Nanotube-Enabled Architecture. Adv. Mater. 2012, 24, 533–537. [Google Scholar]
- Ma, Y.; Teng, H.; Thelliez, M. Electro-Thermal Modeling of a Lithium-ion Battery System. SAE Int. J. Engines 2010, 3, 306–317. [Google Scholar]
- Wang, T.; Tseng, K.; Zhao, J.; Wei, Z. Thermal investigation of lithium-ion battery module with different cell arrangement structures and forced air-cooling strategies. Appl. Energy 2014, 134, 229–238. [Google Scholar]
- He, F.; Li, X.; Ma, L. Combined experimental and numerical study of thermal management of battery module consisting of multiple Li-ion cells. Int. J. Heat Mass Transf. 2014, 72, 622–629. [Google Scholar]
- Li, X.; He, F.; Ma, L. Thermal Management of cylindrical batteries investigated using wind tunnel testing and computational fluid dynamics simulation. J. Power Sources 2013, 238, 395–402. [Google Scholar]
- Xing, Y.; Ma, E.; Tsui, K.; Pecht, M. Battery Management Systems in Electric and Hybrid Vehicles. Energies 2011, 4, 1840–1857. [Google Scholar]
- Capron, O.; Samba, A.; Omar, N.; Gualous, H.; van den Bossche, P.; van Mierlo, J. Battery Thermal Modelling—Assessment of heat distribution and optimization of battery design concept, Proceedings of the 2013 International Conference on Renewable Energy Research and Applications (ICRERA), Madrid, Spain, 20–23 October 2013; pp. 304–309.
- Doyle, M.; Fuller, T.; Newman, J. Modeling of Galvanostatic Charge and Discharge of the Lithium/Polymer/Insertion Cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar]
- Fuller, T.; Doyle, M.; Newman, J. Simulation and Optimization of the Dual Lithium Ion Insertion Cell. J. Electrochem. Soc. 1994, 141, 1–10. [Google Scholar]
- Saw, L.; Ye, Y.; Tay, A.A.O. Electrochemical-thermal analysis of 18650 Lithium Iron Phosphate cell. Energy Convers. Manag. 2013, 75, 162–174. [Google Scholar]
- Prada, E.; Di Domenico, D.; Creff, Y.; Bernard, J.; Sauvant-Moynot, V.; Huet, F. Simplified Electrochemical and Thermal model of LiFePO4, Graphite Li-Ion Batteries for fast charge applications. J. Electrochem. Soc. 2012, 159, A1508–A1519. [Google Scholar]
- Jiang, F.; Peng, P.; Sun, Y. Thermal analyses of LiFePO4/graphite battery discharge processes. J. Power Sources 2013, 243, 181–194. [Google Scholar]
- Inui, Y.; Kobayashi, Y.; Watanabe, Y.; Watase, Y.; Kitamura, Y. Simulation of temperature distribution in cylindrical and prismatic lithium ion secondary batteries. Energy Convers. Manag. 2007, 48, 2103–2109. [Google Scholar]
- Somasundaram, K.; Birgersson, E.; Mujumdar, A. Thermal-electrochemical model for passive thermal management of a spiral-wound lithium-ion battery. Power Sources 2012, 203, 84–96. [Google Scholar]
- Ye, Y.; Shi, Y.; Tay, A.A.O. Electro-thermal cycle life model for lithium iron phosphate battery. J. Power Sources 2012, 217, 509–518. [Google Scholar]
- Somasundaram, K. Mathematical Modeling of Transport Phenomena in Electrochemical Energy Storage Systems. Ph.D. Thesis, Department of Mechanical Engineering, National University of Singapore, Singapore, 2012. [Google Scholar]
- PEC. Available online: http://www.peccorp.com/SBT0550-tabs-glance.html accessed on 2 February 2015.
- Samba, A.; Louahlia-Gualous, H.; le Masson, S.; Nörterhäuser, D. Two-phase thermosyphon loop for cooling outdoor telecommunication equipments. Appl. Therm. Eng. 2013, 50, 1351–1360. [Google Scholar]
- Zukauskas, A. Heat transfer from tubes in crossflow. Adv. Heat Transf. 1972, 8, 93–160. [Google Scholar]
- Martinez, C.; Sorlien, D.; Goodrich, R.; Chandler, L.; Magnuson, D. Using Cell Balancing to Maximize the Capacity of Multi-cell Li-Ion Battery Modules. Available online: http://www.intersil.com/content/dam/Intersil/documents/an16/an167.pdf accessed on 25 June 2015.













| Physics | Governing Equations | Mathematical Expressions |
|---|---|---|
| Chemical kinetics | Butler–Volmer equation | |
| Electrode overpotential | η=(ϕs−ϕe) – U | |
| Exchange current density | ||
| Charge conservation | Solid phase | |
| Electrolyte phase | ||
| Mass Transfer | Species conservation in solid phase | |
| Species conservation in electrolyte | ||
| Parameters | Anode | Separator | Cathode |
|---|---|---|---|
| Thickness [24], (m) | 3:4 ×10−5 | 2:5 × 10−5 | 8 × 10−5 |
| Active material volume fraction [24], (/) | 0:58 | 0:55 | 0:374 |
| Filler volume fraction [24], (/) | 0:0326 | – | 0:0535 |
| Volume fraction electrolyte, (/) | 0:3874 | 0:45 | 0:5725 |
| Particle radius [24], (m) | 5 × 10−6 | – | 5 × 10−8 |
| Electrolyte phase Li+ diffusion [24], (m2/s) | 2 × 10−10 | ||
| Initial electrolyte concentration [23], (mol/m3) | 2000 | ||
| Maximum solid phase concentration [24], (mol/m3) | 30, 555 | – | 22, 806 |
| Regions | cp (J/kg·K) | ρ (kg/m3) | kT (W/m·K) |
|---|---|---|---|
| Cell internal region | 1034:2 | 3345:5 | 0:33434 (kT;r) 57:515 (kTang) |
| negative electrode [23] | 1437:4 | 1347:33 | 1:04 |
| positive electrode [23] | 750 | 3600 | 0:20 |
| separator electrode [23] | 1978:16 | 1008:98 | 0:344 |
| negative current collector [23] | 385 | 8933 | 398 |
| positive current collector [23] | 875 | 2770 | 170 |
| Conservation Equations | Mathematical Expressions |
|---|---|
| Conservation of mass | |
| Conservation of momentum | |
| Conservation of energy |
| Cases | ReD (/) | Present Study, NuD (/) | Zukauskas [32], NuD (/) | Deviation (%) |
|---|---|---|---|---|
| Figure 7a,b | 538 | 8.91 | 10.01 | 11 |
| Figure 7c,d | 1229 | 21.32 | 19.81 | 7 |
| Cell number | Initial Depth of Discharge |
|---|---|
| 2 | 5% |
| 3 | 10% |
| 4 | 20% |
© 2015 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capron, O.; Samba, A.; Omar, N.; Coosemans, T.; Bossche, P.V.d.; Van Mierlo, J. Lithium-Ion Batteries: Thermal Behaviour Investigation of Unbalanced Modules. Sustainability 2015, 7, 8374-8398. https://doi.org/10.3390/su7078374
Capron O, Samba A, Omar N, Coosemans T, Bossche PVd, Van Mierlo J. Lithium-Ion Batteries: Thermal Behaviour Investigation of Unbalanced Modules. Sustainability. 2015; 7(7):8374-8398. https://doi.org/10.3390/su7078374
Chicago/Turabian StyleCapron, Odile, Ahmadou Samba, Noshin Omar, Thierry Coosemans, Peter Van den Bossche, and Joeri Van Mierlo. 2015. "Lithium-Ion Batteries: Thermal Behaviour Investigation of Unbalanced Modules" Sustainability 7, no. 7: 8374-8398. https://doi.org/10.3390/su7078374







