Fast Removal of Polybrominated Diphenyl Ethers from Aqueous Solutions by Using Low-Cost Adsorbents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Characterization of Low-Cost Adsorbents
2.3. Pretreatment of Low-Cost Adsorbents
2.4. Adsorption Experiments
2.5. Algal Biotoxicity Assays
2.6. Adsorption Rate Constants
3. Results and Discussion
3.1. Characterization of Low-Cost Adsorbents
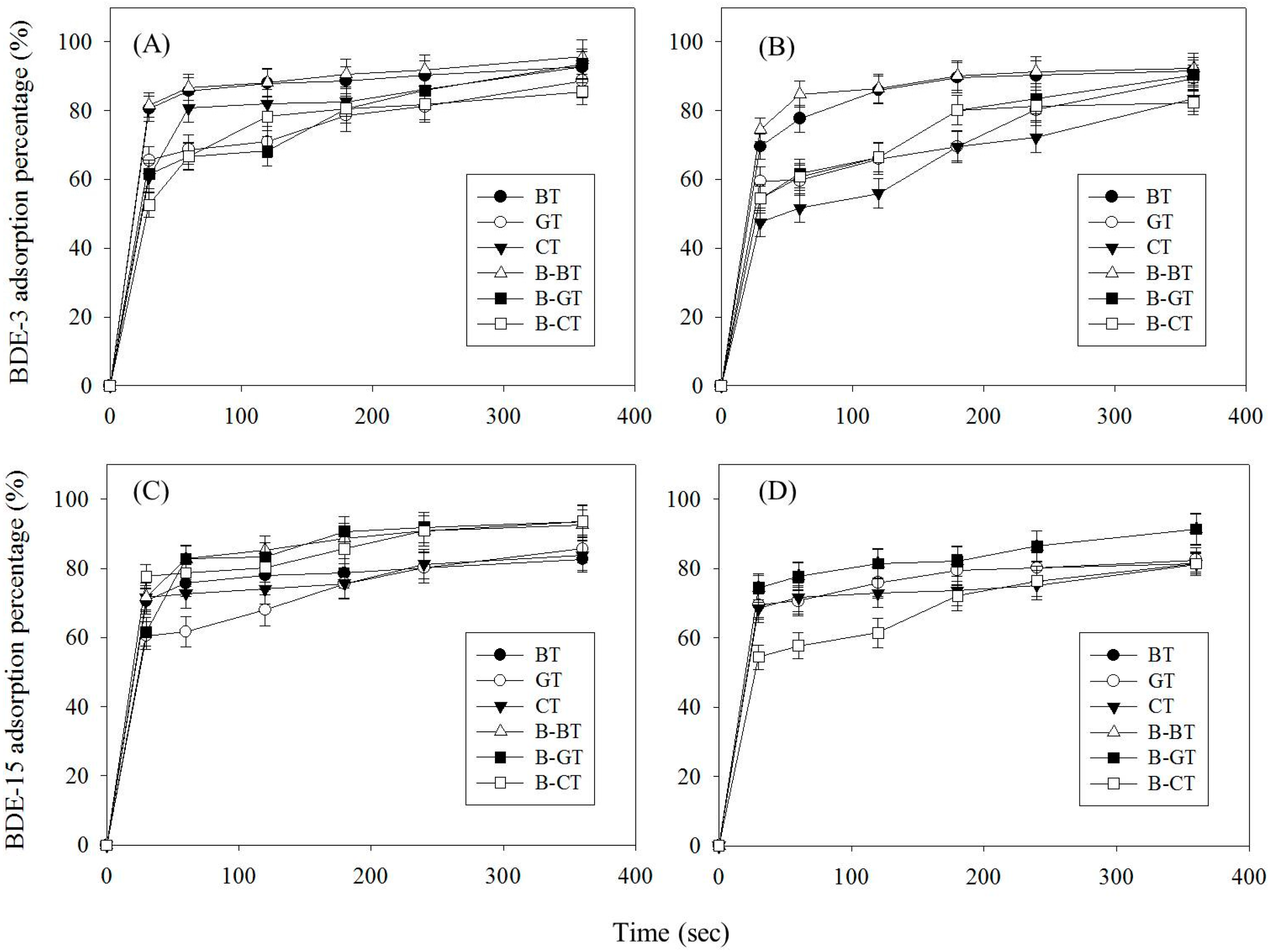
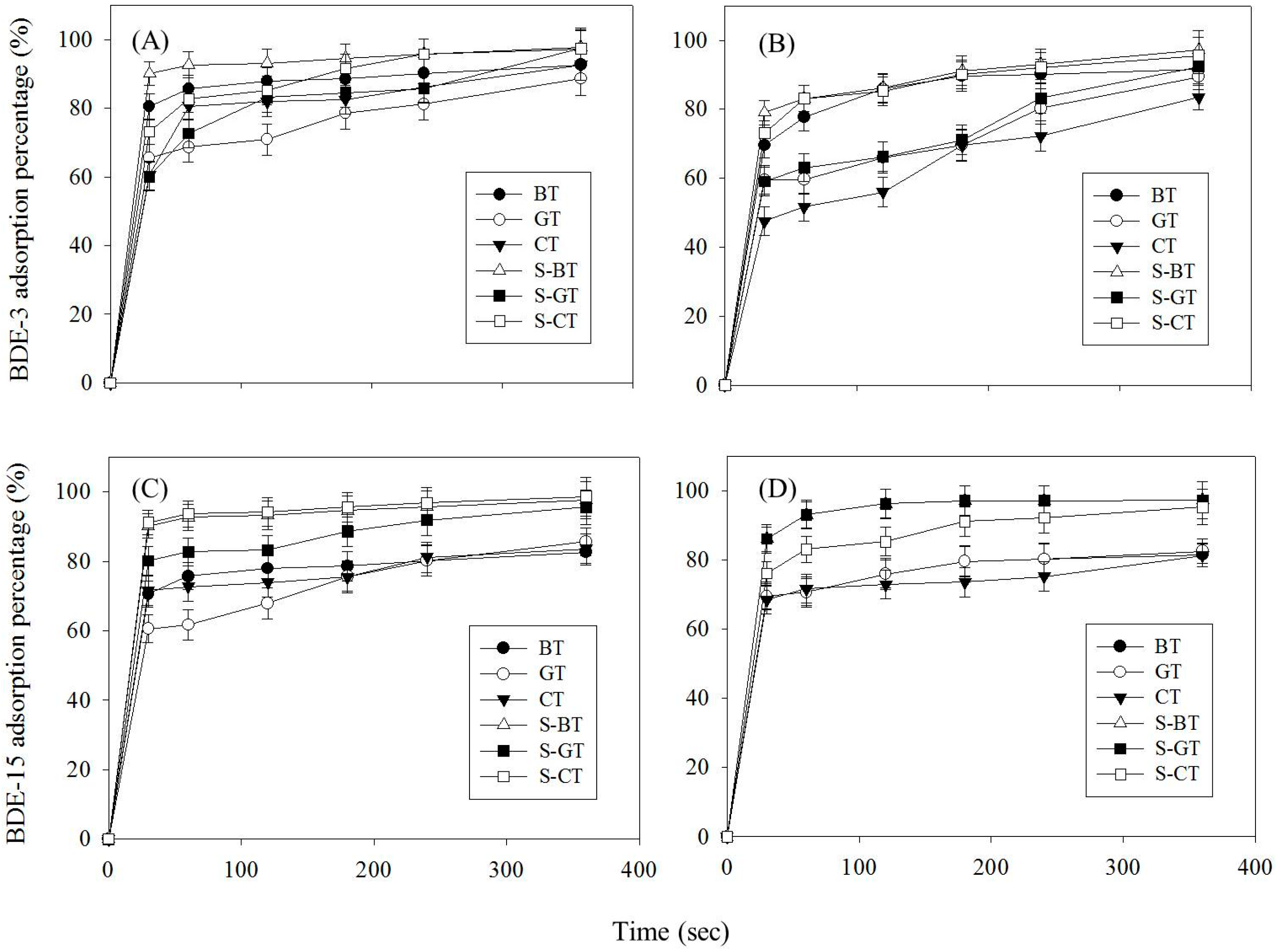
3.2. Analysis of Adsorption Capacity
3.3. Algal Biotoxicity of BDE-3 and BDE-15
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hooper, K.; McDonald, T.A. The PBDEs: An emerging environmental challenge and another reason for breast-milk monitoring programs. Environ. Health Perspect. 2000, 108, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Darnerud, P.O.; Eriksen, G.S.; Johannesson, T.; Larsen, P.B.; Viluksela, M. Polybrominated diphenyl ethers: Occurrence, dietary exposure, and toxicology. Environ. Health Perspect. 2001, 109, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.; Langford, K.H.; Scrimshaw, M.D.; Lester, J.N. Polybrominated diphenyl ether (PBDE) flame retardants. Sci. Total Environ. 2001, 275, 1–17. [Google Scholar]
- Hua, I.; Kang, N.; Chad, T.J.; Fabrega-Duque, J. Heterogeneous photochemical reactions of decabromodiphenyl ether. Environ. Toxicol. Chem. 2003, 22, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Soderstrom, G.; Sellstrom, U.; De Wit, A.C.; Tysklind, M. Photolytic debromination of decabromodiphenyl ether (BDE 209). Environ. Sci. Technol. 2004, 38, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Filley, T.R.; Jafvert, C.T.; Nies, L.; Hua, I.; Bezares-Cruz, J. Photodegradation of decabromodiphenyl ether adsorbed onto clay minerals, metal oxides, and sediment. Environ. Sci. Technol. 2006, 40, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Ratola, N.; Alves, A.; Santos, L.; Lacorte, S. Pine needles as passive bio-samplers to determine polybrominated diphenyl ethers. Chemosphere 2011, 85, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Van den Eede, N.; Dirtu, A.C.; Ali, N.; Neels, H.; Covaci, A. Multi-residue method for the determination of brominated and organophosphate flame retardants in indoor dust. Talanta 2012, 89, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, J.; Feng, L.; Yu, G.; Lin, H.; Wang, L.N. Photodegradation of 2,2′,4,4′-tetrabromodiphenyl ether in nonionic surfactant solutions. Chemosphere 2008, 73, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Rayne, S.; Ikonomou, M.G.; Whale, M.D. Anaerobic microbial and photochemical degradation of 4,4’-dibromodiphenyl ether. Water Res. 2003, 37, 551–560. [Google Scholar] [CrossRef]
- Peng, Y.H.; Chen, M.K.; Shih, Y.H. Adsorption and sequential degradation of polybrominated diphenyl ethers with zerovalent iron. J. Hazard. Mater. 2013, 260, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gu, C.; Ye, M.; Bian, Y.; Cheng, Y.; Wang, F.; Yang, X.; Song, Y.; Jiang, X. Debromination of polybrominated diphenyl ethers by attapulgite-supported Fe/Ni bimetallic nanoparticles: Influencing factors, kinetics and mechanism. J. Hazard. Mater. 2015, 298, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.S.F.; Alves, A.; Madeira, L.M. Chemical and photochemical degradation of polybrominated diphenyl ethers in liquid systems—A review. Water Res. 2016, 88, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Taiwo, E.A.; Adesina, A. Electrochemical regeneration of a native activated carbon. Chem. Biochem. Eng. Q. 2005, 19, 269–273. [Google Scholar]
- Zhou, M.H.; Lei, L.C. Electrochemical regeneration of activated carbon loaded with p-nitrophenol in a fluidized electrochemical reactor. Electrochim. Acta 2005, 51, 4489–4496. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, D.; Namasivayam, C. Experimental and kinetic studies on methylene blue adsorption by coir pith carbon. Bioresour. Technol. 2007, 98, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Bukallah, S.B.; Rauf, M.A.; Alali, S.S. Removal of methylene blue from aqueous solution by adsorption on sand. Dyes Pigments 2007, 74, 85–87. [Google Scholar] [CrossRef]
- Aroguz, A.Z.; Gulen, J.; Evers, R.H. Adsorption of methylene blue from aqueous solution on pyrolyzed petrified sediment. Bioresour. Technol. 2008, 99, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H. Spent tea leaves: A new non-conventional and low-cost adsorbent for removal of basic dye from aqueous solutions. J. Hazard. Mater. 2009, 161, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.H.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.H.; Wu, Y.C. Potential low-cost biosorbent for copper removal: Pineapple leaf powder. J. Environ. Eng. 2012, 138, 286–292. [Google Scholar] [CrossRef]
- Monanty, A.K.; Tripathy, P.C.; Misra, M.; Parija, S.; Sahoo, S. Natural fiber pineapple chemical modification of pineapple leaf fiber: Graft copolymerization of acrylonitrile onto defatted pineapple leaf fibers. J. Appl. Polym. Sci. 2000, 77, 3035–3043. [Google Scholar] [CrossRef]
- Lopattananon, N.; Panawarangkul, K.; Sahakaro, K.; Ellis, B. Performance of pineapple leaf fiber-natural rubber composites: The effect of fiber surface treatments. J. Appl. Polym. Sci. 2006, 102, 1974–1984. [Google Scholar] [CrossRef]
- Bouaziz, F.; Koubaa, M.; Kallel, F.; Chaari, F.; Driss, D.; Ghorbel, R.E.; Chaabouni, S.E. Efficiency of almond gum as a low-cost adsorbent for methylene blue dye removal from aqueous solutions. Ind. Crop. Prod. 2015, 74, 903–911. [Google Scholar] [CrossRef]
- Hameed, B.H. Removal of cationic dye from aqueous solution using jackfruit peel as non-conventional low-cost adsorbent. J. Hazard. Mater. 2009, 162, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.H.; Lin, Y.T.; Hong, D.Y.; Sharma, Y.C.; Chen, S.C.; Tripathi, K. Effective removal of copper ions from aqueous solution using base treated black tea waste. Ecol. Eng. 2014, 67, 127–133. [Google Scholar] [CrossRef]
- De Wit, C.A. An overview of brominated flame retardants in the environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- Hale, R.C.; Alaee, M.; Manchester-Neesvig, J.B.; Stapleton, H.M.; Ikonomou, M.G. Polybrominated diphenyl ether flame retardants in the North American environment. Environ. Int. 2003, 29, 771–779. [Google Scholar] [CrossRef]
- Hites, R.A. Polybrominated diphenyl ethers in the environment and in people: A meta-analysis of concentrations. Environ. Sci. Technol. 2004, 38, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.H.; Tai, Y.T. Reaction of decabrominated diphenyl ether by zerovalent iron nanoparticles. Chemosphere 2010, 78, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, A. Zur Physiologie der Speicherung kondensierter anorganischer Phosphate in Chlorella. Beitr. Phys. Morphol. Algen 1962, 1, 157–166. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 735–742. [Google Scholar] [CrossRef]
- Ho, Y.S.; Porter, J.F.; Mckay, G. Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: Copper, nickel and lead single component systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]
- Cay, S.; Uyanik, A.; Ozajik, A. Single and binary component adsorption of copper(II) and cadmium(II) from aqueous solutions using tea-industry waste. Sep. Purif. Technol. 2004, 38, 273–280. [Google Scholar] [CrossRef]
- Weng, C.H.; Lin, Y.T.; Tzeng, T.W. Removal of methylene blue from aqueous solution by adsorption onto pineapple leaf powder. J. Hazard. Mater. 2009, 170, 417–424. [Google Scholar] [CrossRef] [PubMed]
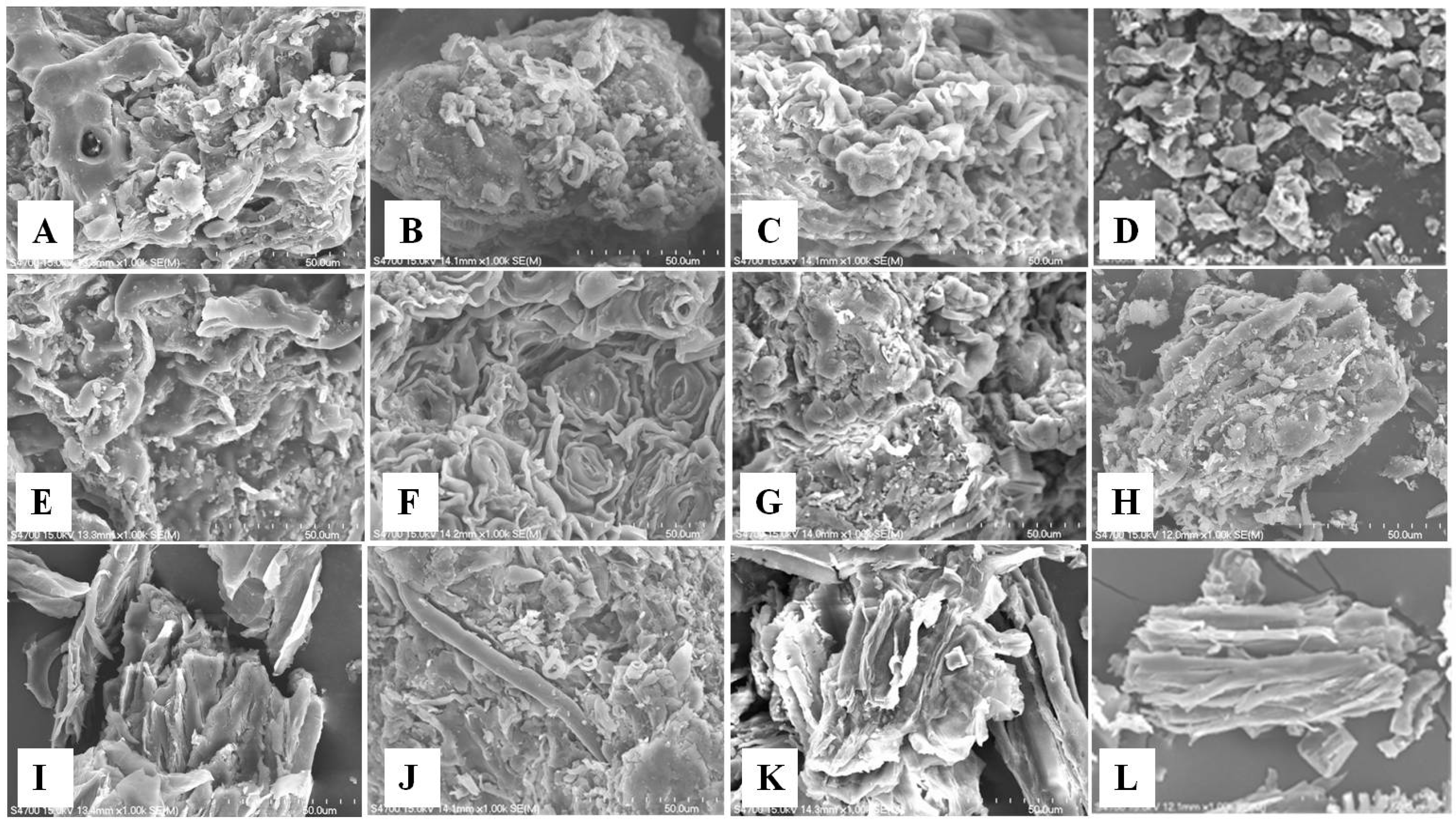

| Adsorbents | pH | pHzpc | FTIR | Particle Size |
|---|---|---|---|---|
| BT | 5.07 | 2.6 | O-H, C-H, C-O, C=O, C=C, C-N | <0.149 mm |
| A-BT | 4.86 | 2.6 | O-H, C-H, C-O, C=O, C=C, C-N | <0.149 mm |
| B-BT | 5.67 | 2.7 | O-H, C-H, C-O, C=O, C=C, C-N | <0.149 mm |
| S-BT | 5.07 | 2.8 | O-H, C-H, C-O, C=O, C=C, C-N | <0.149 mm |
| GT | 4.45 | 2.8 | O-H, C-H, C=O, N-H, C-O | <0.149 mm |
| A-GT | 4.10 | 2.1 | O-H, C-H, C=O, N-H, C-O | <0.149 mm |
| B-GT | 5.12 | 2.5 | O-H, C-H, C=O, N-H, C-O | <0.149 mm |
| S-GT | 4.58 | 2.6 | O-H, C-H, C=O, N-H, C-O | <0.149 mm |
| CT | 6.53 | 2.5 | COOH, O-H, C=O, C-H, N-H, C=C | <0.149 mm |
| A-CT | 6.07 | 2.0 | COOH, O-H, C=O, C-H, N-H, C=C | <0.149 mm |
| B-CT | 8.12 | 2.7 | COOH, O-H, C=O, C-H, N-H, C=C | <0.149 mm |
| S-CT | 6.40 | 2.5 | COOH, O-H, C=O, C-H, N-H, C=C | <0.149 mm |
| BDE-3 | BDE-15 | |||
|---|---|---|---|---|
| 0.01 g·L−1 | 0.002 g·L−1 | 0.01 g·L−1 | 0.002 g·L−1 | |
| BT | 92.7 ± 4.43 | 91.4 ± 4.02 | 82.6 ± 3.71 | 81.5 ± 3.36 |
| GT | 88.7 ± 4.57 | 89.4 ± 3.82 | 85.3 ± 4.03 | 82.4 ± 3.55 |
| CT | 92.7 ± 4.86 | 83.5 ± 3.55 | 83.1 ± 4.17 | 81.2 ± 3.27 |
| A-BT | 97.8 ± 4.95 | 95.2 ± 4.97 | 91.8 ± 3.79 | 85.3 ± 3.51 |
| A-GT | 91.9 ± 4.41 | 91.3 ± 4.12 | 85.6 ± 4.15 | 84.2 ± 3.46 |
| A-CT | 90.9 ± 4.03 | 85.3 ± 3.64 | 91.8 ± 4.14 | 75.3 ± 2.92 |
| B-BT | 95.5 ± 5.04 | 92.4 ± 4.26 | 92.5 ± 4.39 | 91.4 ± 4.51 |
| B-GT | 93.6 ± 4.38 | 90.4 ± 4.28 | 93.3 ± 4.45 | 91.6 ± 4.46 |
| B-CT | 85.6 ± 3.83 | 82.3 ± 3.52 | 93.5 ± 4.78 | 81.4 ± 2.95 |
| S-BT | 97.8 ± 5.53 | 97.3 ± 5.57 | 97.7 ± 5.34 | 97.2 ± 5.45 |
| S-GT | 97.4 ± 5.31 | 92.2 ± 4.82 | 95.6 ± 4.98 | 97.1 ± 5.35 |
| S-CT | 97.3 ± 5.45 | 95.5 ± 5.34 | 98.5 ± 5.56 | 95.3 ± 5.16 |
| S-BT | S-GT | S-CT | ||||
|---|---|---|---|---|---|---|
| BDE-3 | BDE-15 | BDE-3 | BDE-15 | BDE-3 | BDE-15 | |
| Initial concentration (mg·L−1) | 4.79 | 4.68 | 5.12 | 4.91 | 4.63 | 4.44 |
| Weight of adsorbents (g·L−1) | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 |
| qe (mg·L−1) | 4.66 | 4.55 | 4.72 | 4.78 | 4.43 | 4.21 |
| k2 (g·(mg·L−1)−1·s−1) | 2.57 × 10−2 | 6.61 × 10−2 | 8.16 × 10−3 | 6.04 × 10−2 | 2.52 × 10−2 | 2.84 × 10−2 |
| r2 | 0.993 | 0.998 | 0.941 | 0.998 | 0.997 | 0.996 |
| Blank (without Any PBDEs) (μg·kg−1) | BDE-3 | BDE-15 | |||
|---|---|---|---|---|---|
| Before Adsorption (μg·kg−1) | After Adsorption (μg·kg−1) | Before Adsorption (μg·kg−1) | After Adsorption (μg·kg−1) | ||
| BT | 1461 ± 24 | 1021 ± 14 | 1367 ± 16 | 696 ± 12 | 1343 ± 18 |
| GT | 1511 ± 28 | 786 ± 8 | 1401 ± 13 | 568 ± 8 | 1278 ± 11 |
| CT | 1507 ± 29 | 968 ± 12 | 1396 ± 14 | 556 ± 6 | 1253 ± 11 |
| A-BT | 1503 ± 29 | 985 ± 12 | 1387 ± 13 | 658 ± 8 | 1264 ± 15 |
| A-GT | 1488 ± 26 | 845 ± 12 | 1427 ± 15 | 548 ± 8 | 1254 ± 11 |
| A-CT | 1472 ± 23 | 845 ± 11 | 1354 ± 13 | 651 ± 7 | 1278 ± 13 |
| B-BT | 1531 ± 32 | 867 ± 11 | 1408 ± 14 | 632 ± 6 | 1298 ± 14 |
| B-GT | 1521 ± 31 | 931 ± 14 | 1432 ± 15 | 632 ± 6 | 1308 ± 12 |
| B-CT | 1481 ± 24 | 951 ± 11 | 1406 ± 14 | 598 ± 6 | 1346 ± 14 |
| S-BT | 1459 ± 25 | 976 ± 12 | 1421 ± 18 | 678 ± 11 | 1401 ± 21 |
| S-GT | 1473 ± 22 | 886 ± 11 | 1471 ± 21 | 641 ± 9 | 1394 ± 18 |
| S-CT | 1463 ± 24 | 1011 ± 13 | 1457 ± 16 | 617 ± 9 | 1421 ± 18 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, R.; Jien, S.-H.; Weng, C.-H.; Lee, T.-W.; Liao, C.-S. Fast Removal of Polybrominated Diphenyl Ethers from Aqueous Solutions by Using Low-Cost Adsorbents. Sustainability 2017, 9, 102. https://doi.org/10.3390/su9010102
Chang R, Jien S-H, Weng C-H, Lee T-W, Liao C-S. Fast Removal of Polybrominated Diphenyl Ethers from Aqueous Solutions by Using Low-Cost Adsorbents. Sustainability. 2017; 9(1):102. https://doi.org/10.3390/su9010102
Chicago/Turabian StyleChang, Renin, Shih-Hao Jien, Chih-Huang Weng, Tsung-Wei Lee, and Chien-Sen Liao. 2017. "Fast Removal of Polybrominated Diphenyl Ethers from Aqueous Solutions by Using Low-Cost Adsorbents" Sustainability 9, no. 1: 102. https://doi.org/10.3390/su9010102






