Binary Component Sorption of Cadmium, and Copper Ions onto Yangtze River Sediments with Different Particle Sizes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sediment Samples and Characterization
2.2. Sample Extraction and Analysis
2.3. Sorption and Desorption Experiments
2.4. Sorption Models
2.4.1. Sorption Isotherm
2.4.2. Sorption Kinetics
2.5. Heavy Metal Distribution Coefficient (Kd)
3. Results and Discussion
3.1. Concentration and Distribution of Cu and Cd in Sediments
3.2. Kinetic Sorption of Cu and Cd on Sediments
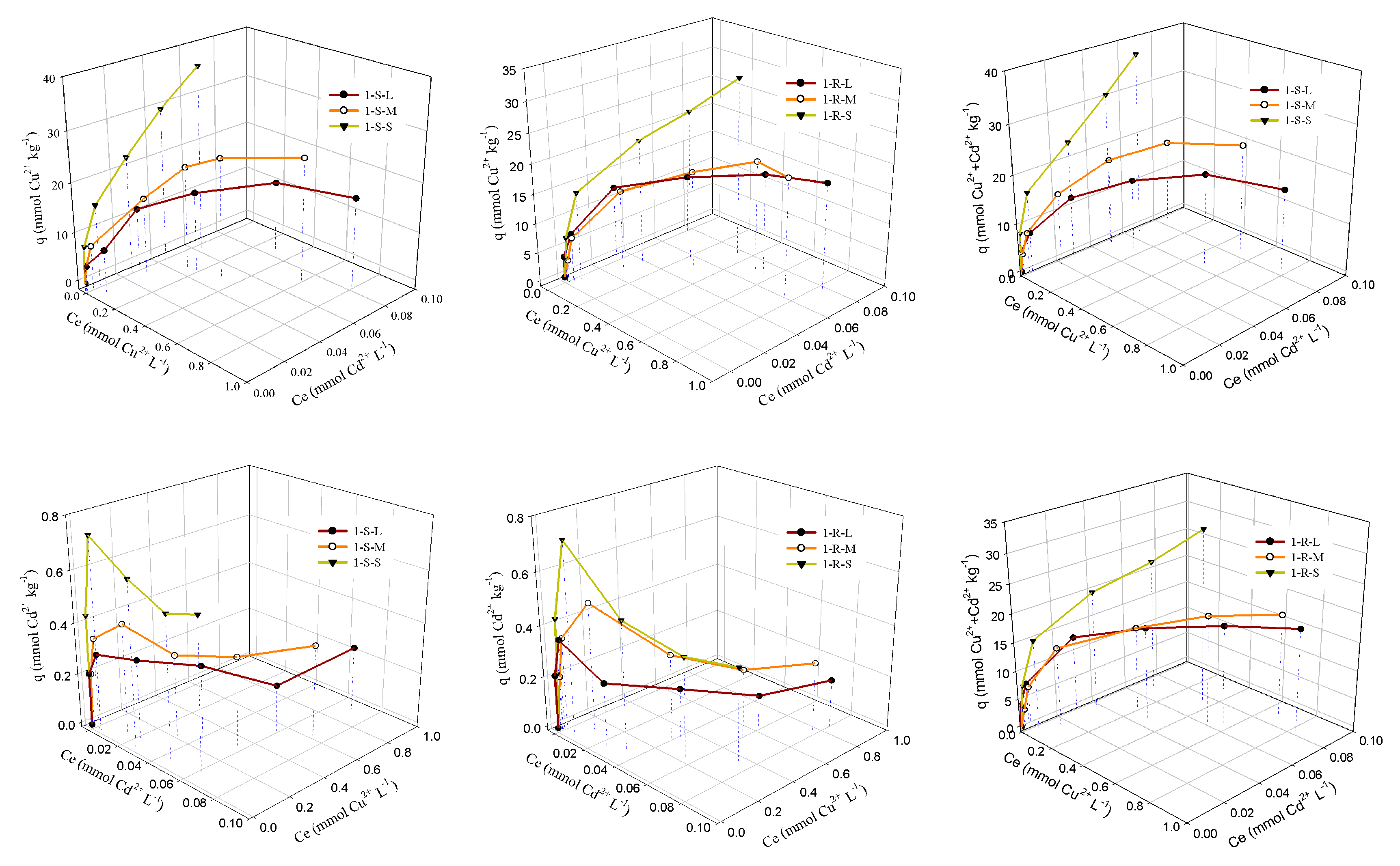
3.3. Sorption Isotherms of Cu and Cd on Sediments
3.4. Distribution Coefficient of Cu and Cd on Sediments
3.5. Desorption of Cu and Cd
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ndiba, P.; Axe, L.; Boonfueng, T. Heavy Metal Immobilization through Phosphate and Thermal Treatment of Dredged Sediments. Environ. Sci. Technol. 2008, 42, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Nobi, E.P.; Dilipan, E.; Thangaradjou, T.; Sivakumar, K.; Kannan, L. Geochemical and geo-statistical assessment of heavy metal concentration in the sediments of different coastal ecosystems of Andaman Islands, India. Estuar. Coast. Shelf Sci. 2010, 87, 253–264. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.-G.; Zeng, G.-M.; Jiang, M.; Yang, Z.-Z.; Cui, F.; Zhu, M.-Y.; Shen, L.-Q.; Hu, L. Effects of sediment geochemical properties on heavy metal bioavailability. Environ. Int. 2014, 73, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.K.; Singhal, D.C.; Sharma, M.K. Adsorption of zinc on bed sediment of River Hindon: Adsorption models and kinetics. J. Hazard. Mater. 2004, 114, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Du, Q.; Tang, H. Surface Complexation Model for the Heavy Metal Adsorption on Natural Sediment. Environ. Sci. Technol. 1998, 32, 870–875. [Google Scholar] [CrossRef]
- Apte, A.D.; Tare, V.; Bose, P. Extent of oxidation of Cr(III) to Cr(VI) under various conditions pertaining to natural environment. J. Hazard. Mater. 2006, 128, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Liang, L.; Dickey, M.J.; Yin, X.; Dai, S. Reductive Precipitation of Uranium(VI) by Zero-Valent Iron. Environ. Sci. Technol. 1998, 32, 3366–3373. [Google Scholar] [CrossRef]
- Ajima, M.N.O.; Nnodi, P.C.; Ogo, O.A.; Adaka, G.S.; Osuigwe, D.I.; Njoku, D.C. Bioaccumulation of heavy metals in Mbaa River and the impact on aquatic ecosystem. Environ. Monit. Assess. 2015, 187, 768. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Fujita, M.; Furumai, H.; Kasuga, I.; Kurisu, F. Sorption behavior of heavy metal species by soakaway sediment receiving urban road runoff from residential and heavily trafficked areas. J. Hazard. Mater. 2009, 164, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.L.; Apte, S.C.; Batley, G.E. Effect of Short-Term Resuspension Events on the Oxidation of Cadmium, Lead, and Zinc Sulfide Phases in Anoxic Estuarine Sediments. Environ. Sci. Technol. 2000, 34, 4533–4537. [Google Scholar] [CrossRef]
- Cabral, M.; Toure, A.; Garçon, G.; Diop, C.; Bouhsina, S.; Dewaele, D.; Cazier, F.; Courcot, D.; Tall-Dia, A.; Shirali, P.; et al. Effects of environmental cadmium and lead exposure on adults neighboring a discharge: Evidences of adverse health effects. Environ. Pollut. 2015, 206, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Ghrefat, H.; Yusuf, N. Assessing Mn, Fe, Cu, Zn, and Cd pollution in bottom sediments of Wadi Al-Arab Dam, Jordan. Chemosphere 2006, 65, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.K.; Sharma, M.K. Adsorption of Cadmium on Bed Sediments of River Hindon: Adsorption Models and Kinetics. Water Air Soil Pollut. 2002, 137, 1–19. [Google Scholar] [CrossRef]
- Bettinetti, R.; Giarei, C.; Provini, A. Chemical Analysis and Sediment Toxicity Bioassays to Assess the Contamination of the River Lambro (Northern Italy). Arch. Environ. Contam. Toxicol. 2003, 45, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.J. Transport phases of transition metals in the Amazon and Yukon Rivers. Geol. Soc. Am. Bull. 1977, 88, 829–843. [Google Scholar] [CrossRef]
- Hussein, H.; Farag, S.; Kandil, K.; Moawad, H. Tolerance and uptake of heavy metals by Pseudomonads. Process Biochem. 2005, 40, 955–961. [Google Scholar] [CrossRef]
- Wang, S.; Terdkiatburana, T.; Tadé, M.O. Adsorption of Cu(II), Pb(II) and humic acid on natural zeolite tuff in single and binary systems. Sep. Purif. Technol. 2008, 62, 64–70. [Google Scholar] [CrossRef]
- Turki, A. Metal Speciation (Cd, Cu, Pb and Zn) in Sediments from Al Shabab Lagoon, Jeddah, Saudi Arabia. JKAU Mar. Sci. 2007, 18, 191–210. [Google Scholar] [CrossRef]
- Loska, K.; Wiechula, D. Application of principal component analysis for the estimation of source of heavy metal contamination in surface sediments from the Rybnik Reservoir. Chemosphere 2003, 51, 723–733. [Google Scholar] [CrossRef]
- Morillo, J.; Usero, J.; Gracia, I. Heavy metal distribution in marine sediments from the southwest coast of Spain. Chemosphere 2004, 55, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Ren, L.; Wang, X.; Liu, W. Pollution characteristics and potential ecological risk assessment of metals in the sediments of Xiaoqing River, Jinan. Environ. Sci. Pollut. Res. 2017, 24, 15001–15011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Feng, H.; Chang, J.; Qu, J.; Xie, H.; Yu, L. Heavy metal contamination in surface sediments of Yangtze River intertidal zone: An assessment from different indexes. Environ. Pollut. 2009, 157, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Ansari Dezfoli, A.R.; Mehrabian, M.A.; Hashemipour, H. Comparative study of Zn(II) and Cd(II) ions adsorption on charged carbon nano tubes: Molecular dynamics approach. Adsorption 2013, 19, 1253–1261. [Google Scholar] [CrossRef]
- Ding, C.; Cheng, W.; Wang, X.; Wu, Z.-Y.; Sun, Y.; Chen, C.; Wang, X.; Yu, S.-H. Competitive sorption of Pb(II), Cu(II) and Ni(II) on carbonaceous nanofibers: A spectroscopic and modeling approach. J. Hazard. Mater. 2016, 313, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Escudero, C.; Poch, J.; Villaescusa, I. Modelling of breakthrough curves of single and binary mixtures of Cu(II), Cd(II), Ni(II) and Pb(II) sorption onto grape stalks waste. Chem. Eng. J. 2013, 217, 129–138. [Google Scholar] [CrossRef]
- Kongsuwan, A.; Patnukao, P.; Pavasant, P. Binary component sorption of Cu(II) and Pb(II) with activated carbon from Eucalyptus camaldulensisDehn bark. J. Ind. Eng. Chem. 2009, 15, 465–470. [Google Scholar] [CrossRef]
- Zang, F.; Wang, S.; Nan, Z.; Ma, J.; Li, Y.; Zhang, Q.; Chen, Y. Immobilization of Cu, Zn, Cd and Pb in mine drainage stream sediment using Chinese loess. Chemosphere 2017, 181, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kwak, M.Y.; Shin, W.S. Competitive sorption of lead and cadmium onto sediments. Chem. Eng. J. 2009, 152, 376–388. [Google Scholar] [CrossRef]
- Allison, L.E. Wet combustion apparatus and procedure for organic and inorganic carbon in soil. Soil Sci. Soc. Am. Proc. 1960, 24, 36–40. [Google Scholar] [CrossRef]
- Nemati, K.; Bakar, N.K.A.; Abas, M.R.; Sobhanzadeh, E. Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J. Hazard. Mater. 2011, 192, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Chi, Q.; Yan, M. Handbook of Elemental Abundance for Applied Geochemistry; Geological Publishing House: Beijing, China, 2007; pp. 140–142. [Google Scholar]
- Vosoogh, A.; Saeedi, M.; Lak, R. Metal fractionation and pollution risk assessment of different sediment sizes in three major southwestern rivers of Caspian Sea. Environ. Earth Sci. 2017, 76, 292. [Google Scholar] [CrossRef]
- Ramos, L.; González, M.J.; Hernández, L.M. Sequential Extraction of Copper, Lead, Cadmium, and Zinc in Sediments from Ebro River (Spain): Relationship with Levels Detected in Earthworms. Bull. Environ. Contam. Toxicol. 1999, 62, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Ok, Y.S.; Kim, S.-H.; Cho, J.-S.; Heo, J.-S.; Delaune, R.D.; Seo, D.-C. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Lair, G.J.; Gerzabek, M.H.; Haberhauer, G. Sorption of heavy metals on organic and inorganic soil constituents. Environ. Chem. Lett. 2007, 5, 23–27. [Google Scholar] [CrossRef]
- Cerqueira, B.; Covelo, F.E.; Andrade, L.; Vega, A.F. The influence of soil properties on the individual and competitive sorption and desorption of Cu and Cd. Geoderma 2011, 162, 20–26. [Google Scholar] [CrossRef]

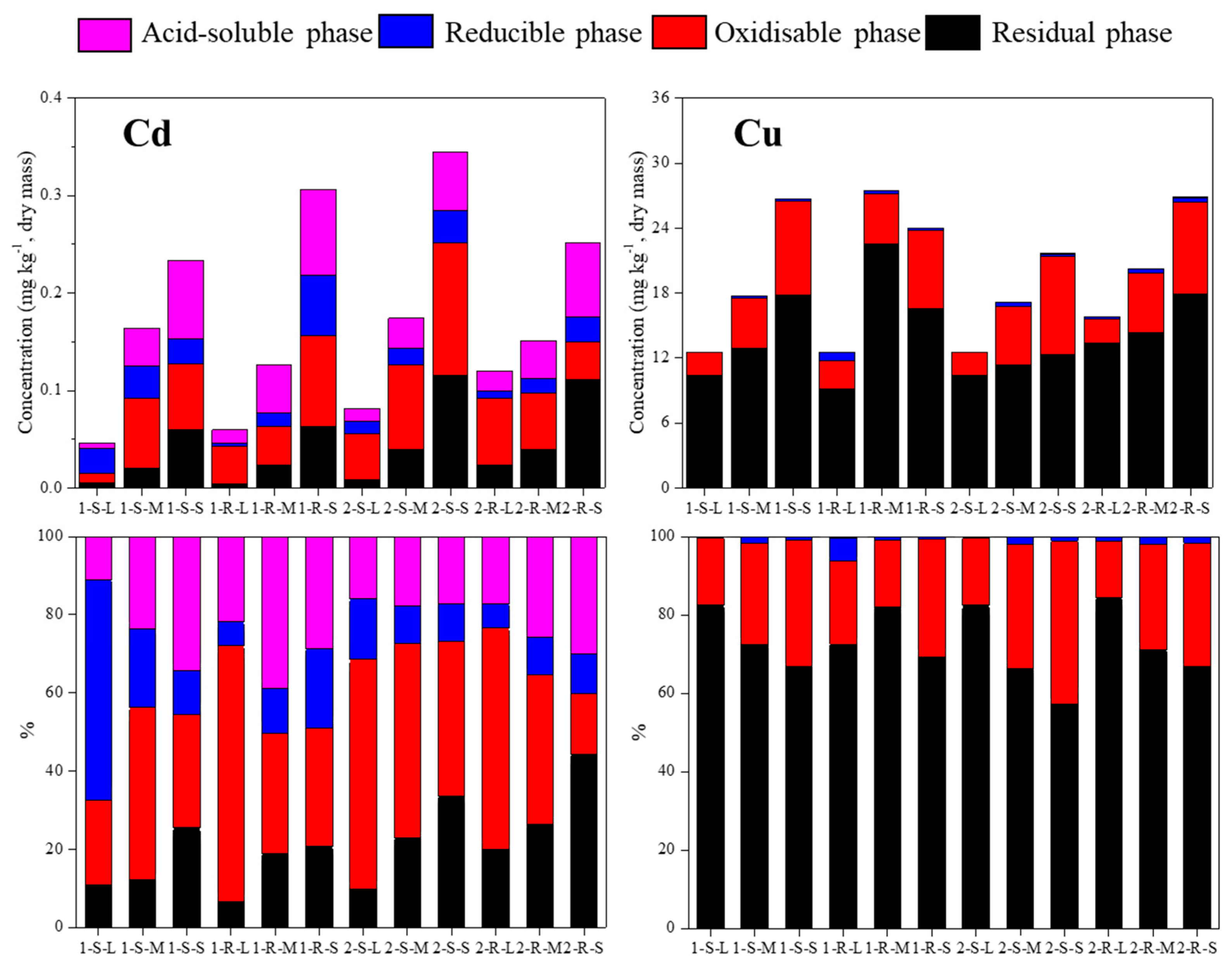



| Sample | site | pH | OM (%) | Cu Content (mg kg−1) | Cd Content (mg kg−1) | Particle Size (d0.5) (μm) | Specific Surface Area (m3 g−1) | Sample Number | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 106°32′59″E, 29°32′46″N | Submerged sediment | 7.3 | 3.44 | 19.0 | 0.148 | 319 | 0.0872 | 1-S-L |
| 156 | 0.133 | 1-S-M | |||||||
| 72.9 | 0.436 | 1-S-S | |||||||
| Riparian sediment | 7.4 | 3.41 | 21.3 | 0.164 | 322 | 0.0627 | 1-R-L | ||
| 165 | 0.124 | 1-R-M | |||||||
| 67.9 | 0.406 | 1-R-S | |||||||
| 2 | 106°32′46″E, 29°33′51″N | Submerged sediment | 7.2 | 3.88 | 17.1 | 0.200 | 317 | 0.0941 | 2-S-L |
| 151 | 0.123 | 2-S-M | |||||||
| 71.2 | 0.379 | 2-S-S | |||||||
| Riparian sediment | 7.6 | 3.55 | 21.0 | 0.174 | 316 | 0.0742 | 2-R-L | ||
| 163 | 0.130 | 2-R-M | |||||||
| 69.3 | 0.353 | 2-R-S |
| Sediment | Double Constant | Pseudo-First-Order | Pseudo-Second-Order | Elovich | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | R2 | Qe | K1 | R2 | Qe | K2 | h | R2 | K | α | R2 | ||
| Cu | 1-S-L | 7.30 | 2.58 × 10−2 | 0.981 | 7.70 | 9.76 | 0.999 | 7.83 | 3.23 | 198 | 0.998 | 2.67 × 108 | 2.88 | 0.971 |
| 1-S-M | 7.48 | 1.69 × 10−2 | 0.990 | 7.75 | 12.0 | 0.999 | 7.83 | 5.04 | 308 | 0.999 | 3.58 × 108 | 2.89 | 0.974 | |
| 1-S-S | 7.68 | 5.25 × 10−3 | 0.999 | 7.77 | 18.9 | 0.999 | 7.79 | 17.5 | 1.07 × 103 | 0.999 | 4.21 × 108 | 2.87 | 0.952 | |
| 1-R-L | 7.17 | 3.25 × 10−2 | 0.973 | 7.68 | 8.46 | 0.998 | 7.84 | 2.47 | 152 | 0.998 | 1.83 × 108 | 2.85 | 0.969 | |
| 1-R-M | 7.50 | 1.64 × 10−2 | 0.989 | 7.77 | 11.9 | 0.999 | 7.85 | 5.04 | 310 | 0.998 | 3.57 × 108 | 2.89 | 0.966 | |
| 1-R-S | 7.72 | 7.99 × 10−4 | 0.999 | 7.73 | 31.3 | 0.999 | 77.4 | 1.38 | 8.25 × 103 | 0.999 | 4.24 × 108 | 2.88 | 0.942 | |
| Cd | 1-S-L | 0.371 | 5.35 × 10−2 | 0.992 | 0.409 | 8.38 | 0.964 | 0.422 | 35.2 | 6.27 | 0.989 | 5.84 × 105 | 46.0 | 0.997 |
| 1-S-M | 0.388 | 4.35 × 10−2 | 0.988 | 0.421 | 8.66 | 0.984 | 0.432 | 40.1 | 7.48 | 0.997 | 5.63 × 106 | 50.5 | 0.988 | |
| 1-S-S | 0.415 | 2.52 × 10−2 | 0.984 | 0.437 | 10.1 | 0.999 | 0.444 | 59.9 | 11.8 | 0.999 | 1.61 × 107 | 50.9 | 0.973 | |
| 1-R-L | 0.348 | 6.08 × 10−2 | 0.998 | 0.386 | 8.81 | 0.944 | 0.400 | 37.1 | 5.93 | 0.973 | 9.30 × 104 | 43.6 | 0.953 | |
| 1-R-M | 0.385 | 4.33 × 10−2 | 0.987 | 0.418 | 8.61 | 0.984 | 0.429 | 40.0 | 7.37 | 0.998 | 5.61 × 105 | 50.8 | 0.988 | |
| 1-R-S | 0.431 | 8.18 × 10−3 | 0.998 | 0.438 | 16.5 | 0.999 | 0.441 | 197 | 38.3 | 0.999 | 2.34 × 106 | 60.0 | 0.981 | |
| Sediment | Cu2+ | Cd2+ | Cu2+ + Cd2+ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| K (L mmol−1) | QMAX (mmol kg−1) | R2 | K (L mmol−1) | QMAX (mmol kg−1) | R2 | K (L mmol−1) | QMAX (mmol kg−1) | R2 | |
| 1-S-L | 15.3 | 19.9 | 0.931 | 1.16 × 104 | 0.274 | 0.884 | 49.4 | 18.1 | 0.946 |
| 1-S-M | 26.3 | 25.2 | 0.988 | 2.59 × 103 | 0.376 | 0.947 | 21.8 | 25.6 | 0.990 |
| 1-S-S | 57.3 | 48.2 | 0.990 | 5.26 × 103 | 0.638 | 0.922 | 47.2 | 40.4 | 0.971 |
| 1-R-L | 70.6 | 17.1 | 0.986 | 4.15 × 105 | 0.226 | 0.628 | 59.6 | 17.2 | 0.975 |
| 1-R-M | 15.2 | 20.7 | 0.980 | 3.57 × 103 | 0.345 | 0.686 | 14.7 | 20.8 | 0.983 |
| 1-R-S | 14.6 | 34.7 | 0.9856 | 7.80 × 103 | 0.490 | 0.661 | 14.6 | 35.5 | 0.985 |
| Sediment | Cu2+ | Cd2+ | Cu2+ + Cd2+ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| q | BCu | BCd | R2 | q | BCd | BCu | R2 | q | BCd | BCu | R2 | |
| 1-S-L | 19.9 | 25.3 | 0.106 | 0.986 | 0.315 | 6.91 × 103 | 173 | 0.986 | 20.3 | 109 | 23.0 | 0.960 |
| 1-S-M | 26.4 | 28.1 | 1.40 | 0.990 | 0.432 | 3.52 × 103 | 117 | 0.967 | 25.6 | 31.9 | 19.1 | 0.990 |
| 1-S-S | 62.3 | 44.5 | 10.6 | 0.990 | 0.641 | 2.54 × 103 | 89.2 | 0.922 | 47.8 | 0.00240 | 61.7 | 0.988 |
| 1-R-L | 17.9 | 78.7 | 28.8 | 0.989 | 0.240 | 6.26 × 103 | 39.5 | 0.646 | 17.2 | 0.00310 | 76.5 | 0.985 |
| 1-R-M | 20.7 | 15.2 | 0.0902 | 0.979 | 0.302 | 5.32 × 103 | 40.5 | 0.753 | 20.3 | 94.3 | 7.64 | 0.994 |
| 1-R-S | 36.1 | 14.2 | 2.30 | 0.986 | 0.490 | 3.41 × 103 | 23.0 | 0.661 | 33.8 | 11.4 | 14.8 | 0.986 |
| Cu | Cd | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 40 | 60 | 80 | 100 | 1 | 2 | 4 | 6 | 8 | 10 | |
| 1-S-L | 610 | 416 | 164 | 62.6 | 36.7 | 21.3 | 724 | 55.4 | 13.2 | 6.69 | 3.09 | 3.76 |
| 1-S-M | 413 | 318 | 263 | 133 | 71.4 | 39.4 | 400 | 98.8 | 24.3 | 8.87 | 5.96 | 4.74 |
| 1-S-S | 1.93 × 103 | 2.04 × 103 | 2.51 × 103 | 1.32 × 103 | 976 | 703 | 1.42 × 103 | 1.54 × 103 | 137 | 22.9 | 11.5 | 9.08 |
| 1-R-L | 283 | 362 | 227 | 52.2 | 29.8 | 21.0 | 514 | 116 | 9.35 | 4.47 | 2.34 | 2.22 |
| 1-R-M | 147 | 2542 | 152 | 54.9 | 35.8 | 25.4 | 371 | 120 | 35.0 | 7.98 | 4.24 | 3.34 |
| 1-R-S | 919 | 620 | 373 | 211 | 111 | 86.6 | 1.11 × 103 | 1.28 × 103 | 118 | 14.5 | 6.74 | 4.76 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Zhao, G.; Sun, J. Binary Component Sorption of Cadmium, and Copper Ions onto Yangtze River Sediments with Different Particle Sizes. Sustainability 2017, 9, 2089. https://doi.org/10.3390/su9112089
Fan J, Zhao G, Sun J. Binary Component Sorption of Cadmium, and Copper Ions onto Yangtze River Sediments with Different Particle Sizes. Sustainability. 2017; 9(11):2089. https://doi.org/10.3390/su9112089
Chicago/Turabian StyleFan, Jianxin, Guoliang Zhao, and Jiaoxia Sun. 2017. "Binary Component Sorption of Cadmium, and Copper Ions onto Yangtze River Sediments with Different Particle Sizes" Sustainability 9, no. 11: 2089. https://doi.org/10.3390/su9112089




