1. Introduction
With a global production of approximately 1.5 × 10
9 cattle and 9.8 × 10
8 pigs [
1], the risk of infection by livestock epidemics, such as foot-and-mouth disease (FMD), tends to increase, threatening national food safety and economy. FMD, a highly transmissible viral disease of cloven-hoofed animals, is considered as one of the most serious and economically significant diseases of livestock animals [
2]. FMD outbreaks have been reported worldwide, including in the United Kingdom, Taiwan, Japan, China, Korea, and so on. Carcasses that are infected or suspected of being infected by livestock infectious diseases have been treated by a wide range of treatment methods including burial, burning, incineration, rendering, and composting, and the choice of carcass treatment method(s) varies depending upon the individual country’s policy and regulation [
3,
4,
5].
Burial is a traditional way to burrow carcasses in graves, trenches or landfills. Burial has the advantage of minimizing the spread of disease and of treating a large amount of carcasses in short times. Its main drawbacks are potential leachate release from decomposed carcasses to the subsurface environment, which can lead to secondary contaminations of the surface water environment and farming land [
5,
6,
7]. Onsite-burning and incineration are regarded as effective methods in destroying infectious agents. However, the uses of onsite burning and incineration have been reduced or often banned due to public concerns about the production of dioxin and odor from incomplete combustion [
8,
9]. In the case of rendering, in which carcasses are crushed into fat, proteinaceous material and water [
10], it requires high energy for treatment and high initial facility costs. Onsite-burning, incineration, and rendering are not good for large scale treatment of infected carcasses. In addition, their products are subject to disposal in landfills designed for that specific purpose or subject to incineration [
11]. To circumvent the problems from the above-mentioned methods, composting is often regarded as an alternative, not only because subsurface environmental contamination can be avoided by its leachate control but also because large amounts of carcasses can be treated [
12,
13]. In addition, auto-thermal reaction during aerobic composting causes the temperature to increase up to 70 °C, sterilizing significant amounts of pathogenic microorganisms in products from composting [
12,
13].
In Korea, large scale FMD outbreaks occurred between 2010 and 2011, and approximately 9.8 million domestic animals were disposed of in approximately 4800 burials [
14]. From some of the burials, groundwater and river were contaminated with leachate. In addition, buried carcasses were often found to be undecomposed even after the legal burial period (3 years) in Korea [
14,
15]. Because the FMD virus was inactivated after the 3-year burial period, the Korean government has considered the novel approach of moving the undecomposed carcasses from the burial sites into other sites for further treatment in order to reuse the land areas used for the burial purposes. To evaluate the feasibility of this novel approach, we have conducted pilot-scale composting (Icheon, Gyeonggi Province, Korea) to biologically stabilize the undecomposed carcasses from a 3-year old burial site [
15]. To enhance the bio-stabilization, a carcass-degrading bacterium (
Corynebacterium glutamicum) and other materials stimulating carcass degradation activities were aerobically mixed with previously buried carcasses using a rotary agitation system. To the best of our knowledge, this was the first trial to evaluate the feasibility of bio-augmented aerobic composting of excavated carcasses from a burial.
In terms of feasible bio-stabilization of excavated carcasses, groundwater protection and plant toxicity are the main considerations. Because dissolved organic matter and nitrogen species such as ammonia are contaminants to groundwater, it is necessary to examine whether bio-augmented composting can effectively eliminate such organic matter and nitrogen contaminants from previously buried carcasses. In addition, if the final product of bio-augmented composting of carcasses is applied as soil, no plant toxicity should be guaranteed. However, little is known about the effects of bio-augmented composting on the reduction of organic matter and nitrogen contaminants and potential plant toxicity.
In addition to the groundwater protection and plant toxicity, bacterial pathogenicity to human beings is an important factor in evaluating the feasibility of composting of carcasses. However, the methodology of assessing bacterial pathogenicity in biologically stabilized carcasses still remains poorly established. Currently, cultivation-based detection of bacterial pathogen indicators is used in assessing the microbial risk of buried carcasses [
16,
17]. However, the current cultivation-dependent detection may not be suitable for evaluating microbial risk in composed carcasses because of the fact that only 5%–10% of the microbes in environmental samples are detectable by cultivation [
16,
17]. To circumvent the methodological limitation, NGS (next generation sequencing) methods sequencing 16S rRNA gene segments in microbial community DNA have been widely used in the area of microbial ecology [
18]. According to other recent studies using pyrosequencing (one of the NGS methods) targeting the bacterial 16S rRNA gene, the detected potential pathogenic bacteria were quite different from the cultivable pathogen indicators currently used in evaluating microbial risk in buried carcasses [
16,
17]. This led us to the need to investigate bacterial community characteristics and dominant potential bacterial pathogens in composted carcasses using NGS.
In this work, we attempted to evaluate the feasibility of bio-augmented aerobic composting of excavated carcasses from a 3-year old burial. For this, the reduction of organic matter and nitrogen contaminants was monitored in pilot-scale bio-augmented aerobic composting, and potential plant toxicity was tested in the final product from bio-augmented aerobic composting. Furthermore, MiSeq (one of the NGS methods) targeting the bacterial 16S rRNA gene was used to investigate the characteristics of bacterial communities and the quantitative information of potentially pathogenic bacteria in the composted carcasses.
2. Materials and Methods
2.1. Environmental Management Research Facility for Carcasses Burial at Pilot Scale
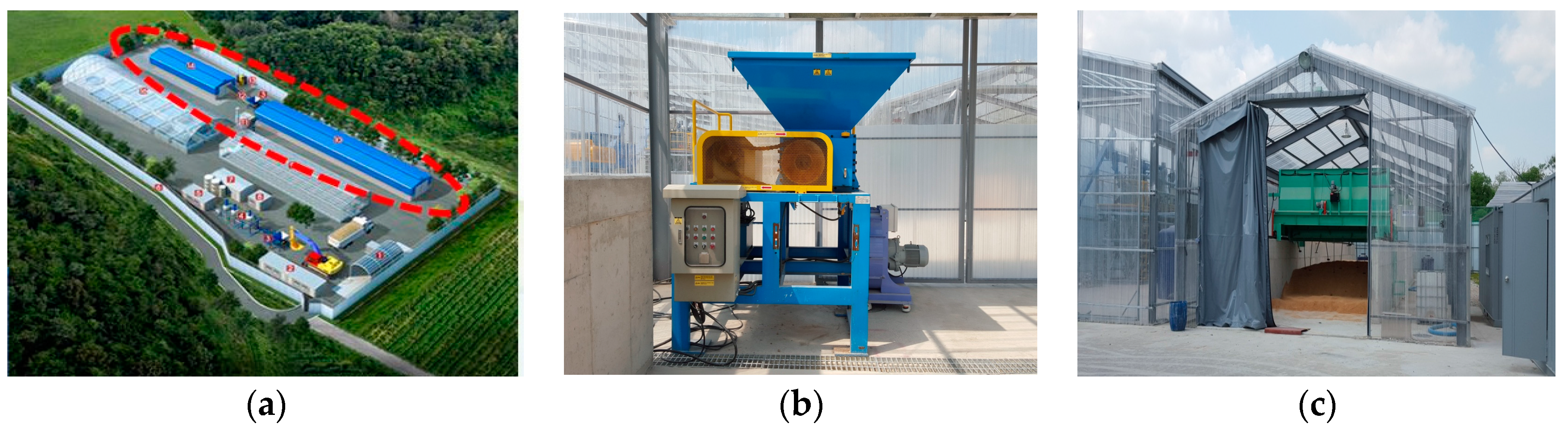
An environmental management research facility for carcass burial was constructed in 2015 in Icheon, Gyeonggi Province, Korea by SAFE research center for environmental management of carcasses burial sites (
Figure 1). It was equipped with a barrier-disinfection facility; fence and dustproof facility; an electricity and water facility; a carcass crushing facility; a carcass–soil separation facility; a carcass storage facility; a carcass bio-degradation evaluation facility; a carcass decommissioning device; and a soil purification facility.
The pretreatment facility has a width of 7.0 m, a length of 32.0 m, a wall height of 1.5 m, and has a volume of 336.0 m3 for storing carcasses excavated from a burial site. The carcass crusher should not be clogged during the crushing process, and the primary goal is to crush carcasses to a maximum size of 10 cm to reduce the load during the rotary rotation of the agitator. The manufacturing goal was to make it easy to break down components of a certain strength, such as bones and horns, and tough components, such as leather and guts. Considering these difficulties, the bio-crusher was designed with a 20 HP (horse-power) crushing motor and could crush 5 tons of carcasses per hour. The bio-degradation evaluation facility with the bio-agitator has a width of 4.75 m, a length of 25.0 m, a wall height of 1.8 m, a total area of 120.0 m2, and an appropriate stirring volume of 180.0 m3. The leachate from carcasses is collected in a reservoir by a discharge frame (an air passage) in the bottom. The rotary bio-agitator can transport and agitate carcasses, control the stirring depth, constantly maintain the rotational speed, and be operated in the forward and reverse directions.
2.2. Information about the Carcass Burial Site
The burial site used in this study was constructed in Gyeonggi Province in January 2011, and 186 cattle infected with FMD were urgently buried. The carcasses from the site were transported and stabilized at the pilot-scale environmental management research facility for carcass burial.
Table 1 shows the details of the site.
2.3. Process and Operating Conditions
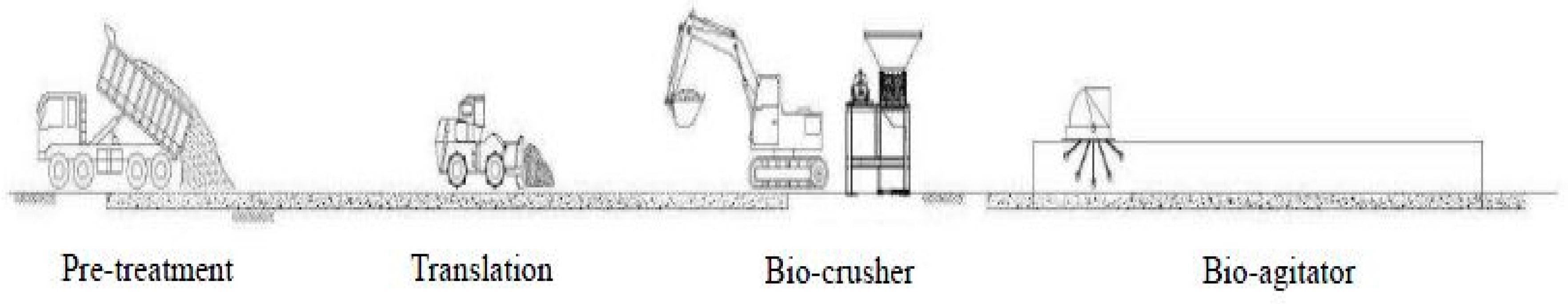
The carcass treatment processes are shown in
Figure 2. Incompletely decomposed carcasses were excavated from the targeted burial site and transported and deposited at the pretreatment facility. The pretreatment facility physically obstructed the transportation of odor and airborne contaminants to the outside during the storage period. The stored carcasses were shredded by the bio-crusher and transported to the bio-degradation evaluation facility. In the bio-degradation evaluation facility, a bio-agitator was used as a large livestock stirrer to maintain aerobic conditions in order to enhance the stabilization of the carcasses.
The operating conditions of the bio-degradation evaluation facility are shown in
Table 2.
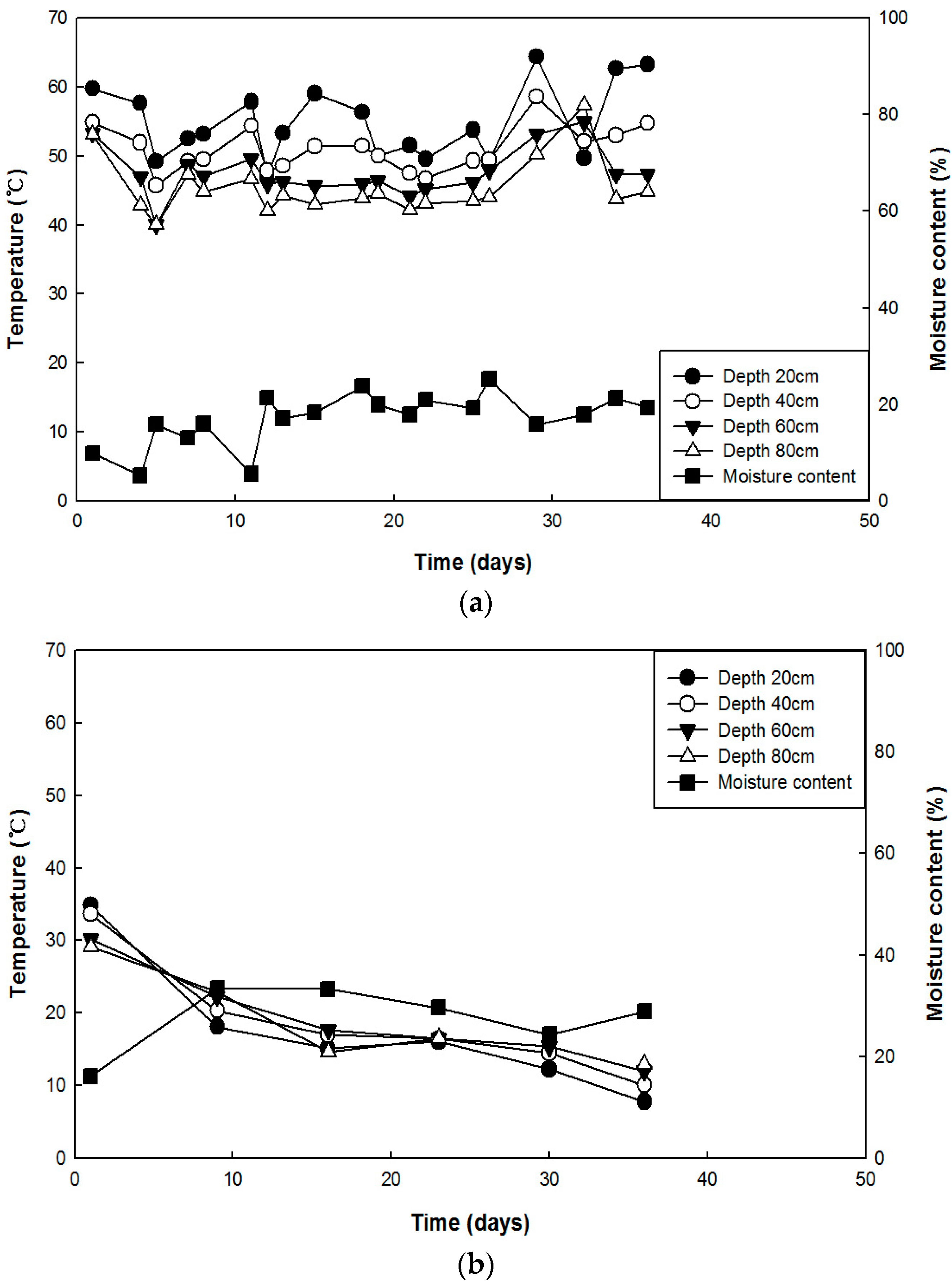
Water was replenished by a water spraying facility, and the moisture content of the carcass pile was maintained at 15%–20% or more before spraying water to reach the appropriate moisture content (40%). Decomposition was promoted by using a water spraying device to maintain the appropriate moisture content of the carcasses, and the bio-degradation performance of the aerobic microorganisms was maximized by the bio-agitator. When composting livestock manure, oxygen deficiency commonly occurs due to compression by self-weight. To prevent this condition and to optimize the activity of the microorganisms, an adequate level of bulking material is needed [
19]. The bulking material was stirred to maintain the proper moisture content and air permeability. In this study, rice hulls were used as the bulking material.
Corynebacterium glutamicum was used as the carcass-degrading microorganism. This microorganism can fertilize soil by producing useful amino acids from the treatment of organic matter, and it is known to be effective for degrading livestock carcasses [
15]. The microorganism was cultivated with molasses (1%) onsite to increase the efficiency, and it was added once a week at a concentration of 3.0 × 10
7–1.0 × 10
8 gene copies/mL to accelerate carcass degradation. The concentration of residual molasses filtered through 0.2 μm membrane filters (Whatman™, Buckinghamshire, UK) after culturing was measured under 1000 mg-COD/L-media (approximately 11.2 mg-COD/kg-carcass) and it was considered to affect to the carcass degradation insignificantly.
2.4. Sampling Carcasses in the Bio-Degradation Evaluation Facility
After rotating carcasses with the bio-agitator in the bio-degradation evaluation facility, more than three carcass samples were collected from each area by dividing the carcass pile into three sections at least once a week. Fresh samples were stored in closed plastic bags in a refrigerator at 4 °C during the experiments. Before analysis, samples were pulverized using a mortar and pestle.
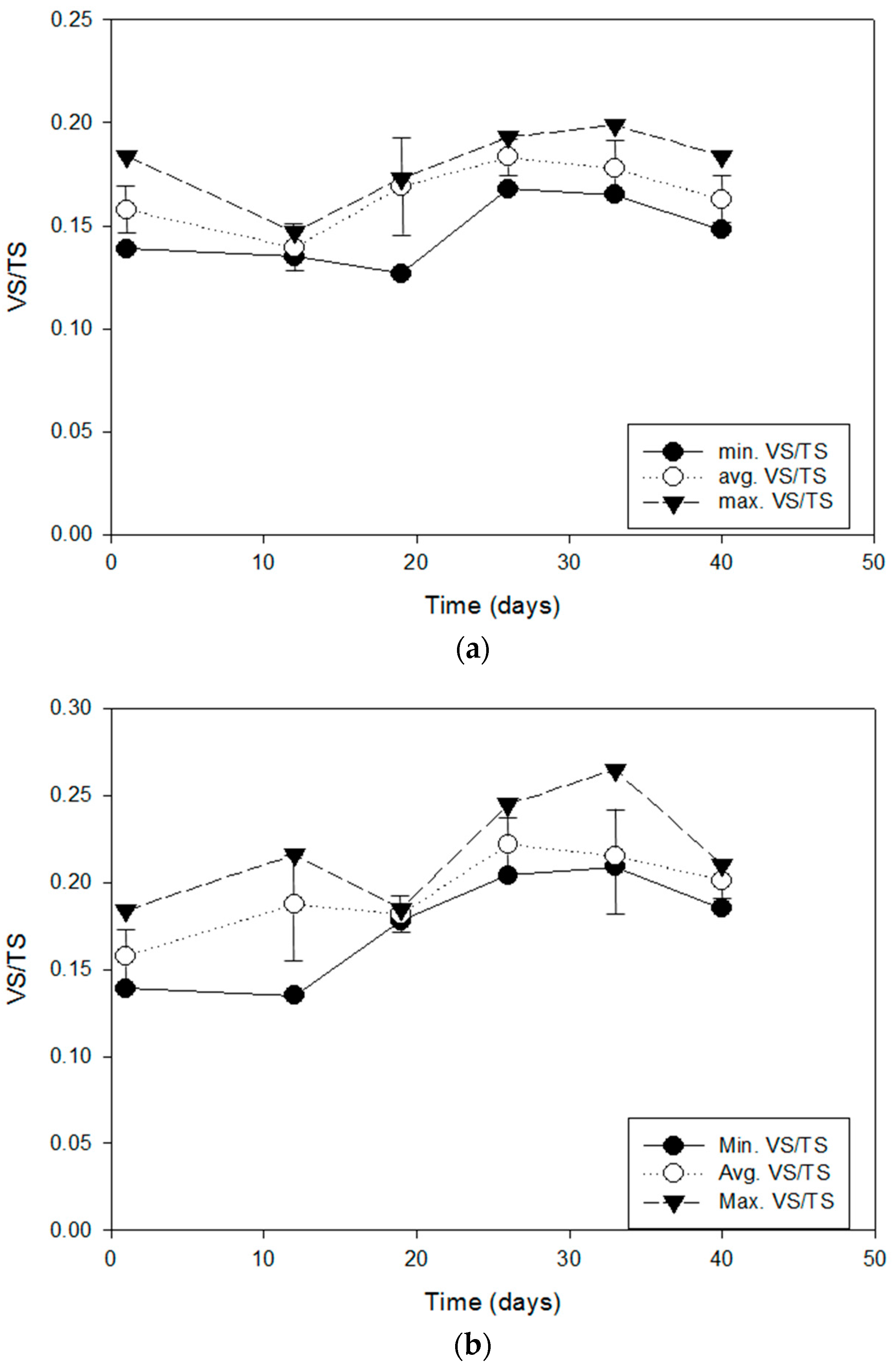
2.5. Total Solids (TS) and Volatile Solids (VS)
The TS and VS of the carcass samples were measured using the United States Environmental Protection Agency (EPA) method 1684. Evaporating dishes were heated at 550 °C for 1 h in an oven, cooled, dried in a desiccator and weighed (W
dish). Samples (25–50 g) pulverized by hand were prepared in triplicate and were placed into the dishes and weighed (W
sample). The dishes were dried at 103 °C to 105 °C for a minimum of 12 h and were then cooled to the balance temperature in a desiccator and weighed (W
total). The evaporating dishes containing dried residues were heated at 550 °C for 2 h, and the residue was cooled in a desiccator and weighed (W
volatile). All measurements were performed in triplicate. The TS and VS were calculated using the following equations:
2.6. Chemical Oxygen Demand (COD) and Inorganic Nitrogen Measurment
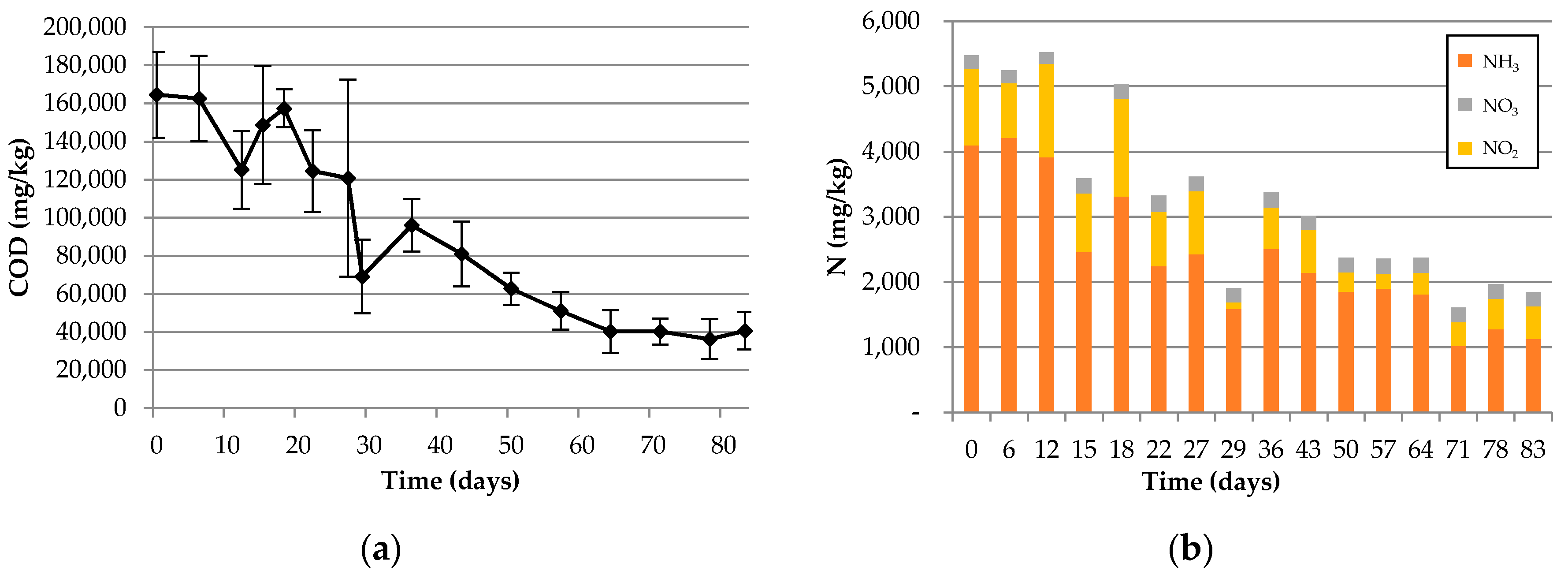
Distilled water (100 mL) was added to tubes with an amount of 10 g from the sample dried at 60 °C during 24 h. The tubes were shaken for 2 h and allowed to stand for a further 30 min to equilibrate and the supernatant was filtered through 0.2 μm membrane filters (Whatman™, Buckinghamshire, UK). The soluble COD of the carcass was measured using a spectrophotometer (DR4000, HACH, Loveland, CO, USA) with HACH COD digestion reagent (HACH, Loveland, CO, USA). Inorganic nitrogen (NO2−, NO3− and NH4+-N) was measured using a previously described method and HACH NO2−, NO3− and NH3 digestion reagents (HACH, Loveland, CO, USA). All measurements were performed in triplicate, and t-tests were used to test for significant differences.
2.7. Germination Test and Organic Matter Measurement
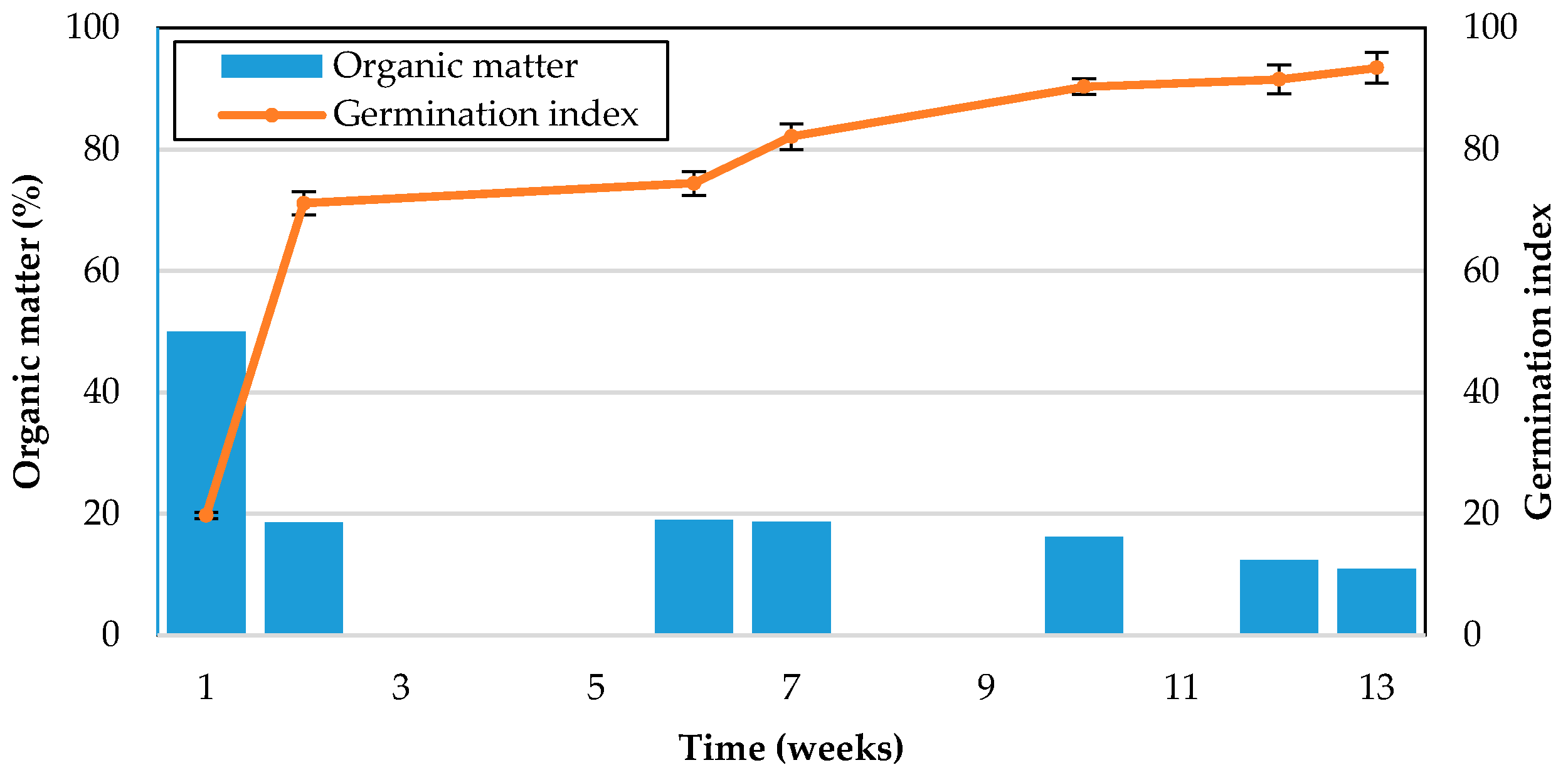
A germination test was performed using white radish seeds. A 10 g sample with a moisture content of 50% was added to 100 mL of distilled water and extracted using a reflux condenser for 2 h. The extract was filtered through a No. 2 filter, and 5 mL of the filtrate was added to an 85 mm diameter petri dish with white radish seeds. The control was performed with 5 mL of distilled water. The number of seeds per petri dish was 30. After 5 days, the germination rate and root length of seedlings were measured. The germination index was calculated using the following equations:
This test was performed by the Foundation of Agriculture Technology Commercialization & Transfer (FACT) in Korea.
The organic matter in the carcass samples was measured by the Tyurin method. The samples were oxidized by K2Cr2O7 under strongly acidic conditions with concentrated sulfuric acid, and the carbon content was measured based on the amount of K2Cr2O7 consumed for oxidation. The amount of organic matter was determined by multiplying the carbon content by 1.724 (the ratio of the carbon content was 57% during soil erosion). The measurement was performed by FACT in Korea.
2.8. DNA Extraction, Quantitative Real-Time PCR Analysis, PCR Amplification and MiSeq Sequencing
Total genomic DNA was extracted from the carcasses at the bio-degradation evaluation facility using a Power Soil DNA Extraction Kit (MoBio, Carlsbad, CA, USA) according to the manufacturer’s protocol. The extracted DNA was stored at −20 °C prior to the subsequent analysis.
Real-time PCR amplification reactions of the 16S rRNA gene were performed using a BIORAD iQ5 (Bio-Rad, Hercules, CA, USA) to quantify the bacteria. The reaction mixture had a total volume of 25 µL: 12.5 µL of iQ5 SYBR Green Supermix (Bio-Rad, Hercules, CA, USA), 10 ng of total DNA, 1 µL of 10 mM 16S rRNA primer [
20,
21] and RNase-free water as the remaining volume. To amplify the 16S rRNA gene from the extracted DNA, 16S amplicon PCR forward and reverse primers targeting the V3-V4 region of the 16S rRNA gene were used [
22].
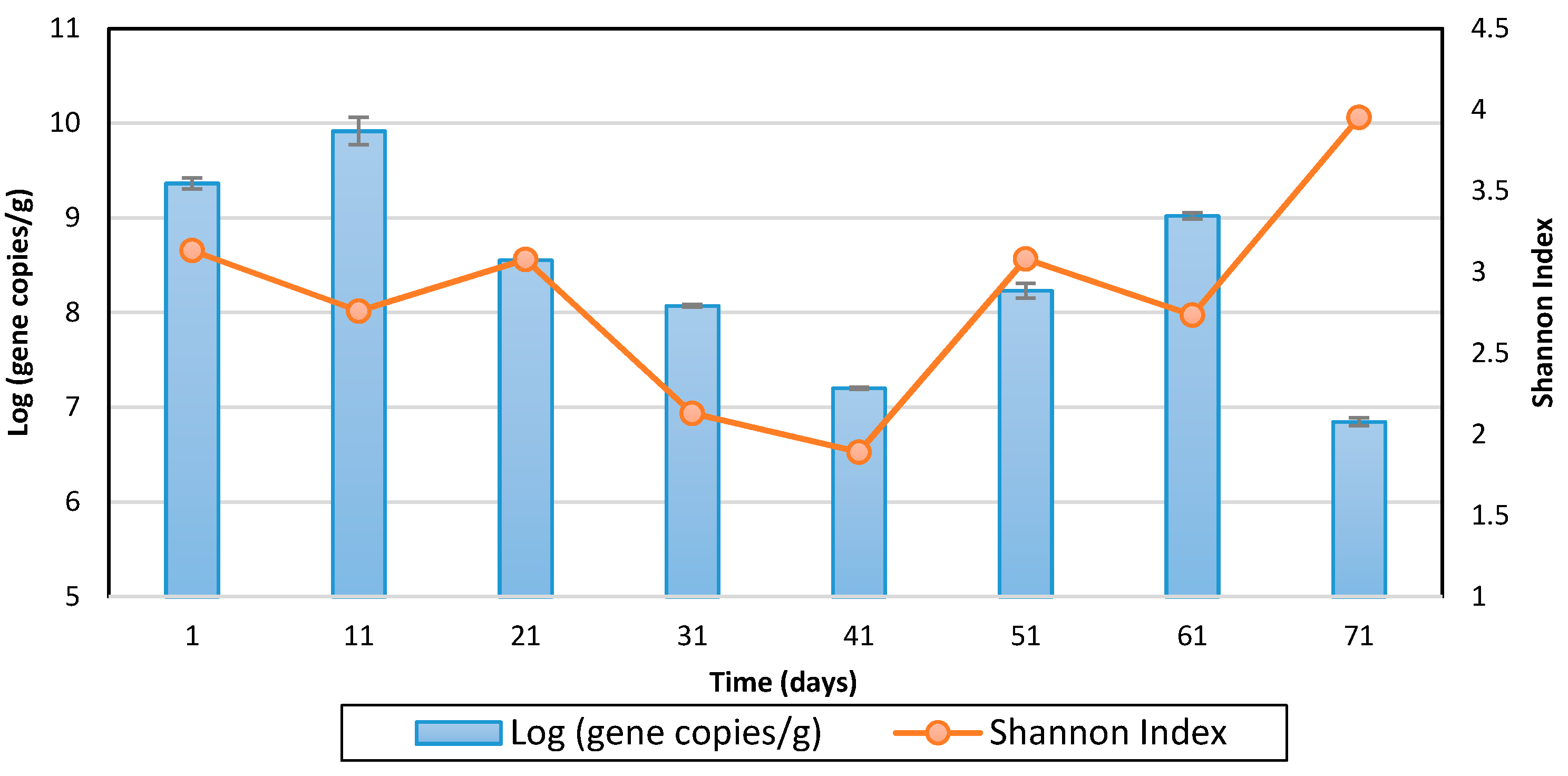
The PCR reaction mixtures contained 2.5 µL of gDNA from the samples, 5 µL of each primer (1 µM) and 12.5 µL of 2 × KAPA Hi Fi HotStart Ready Mix (Invitrogen, Carlsbad, CA, USA). Amplification was performed using a C1000TM thermal cycler (Bio-Rad, Hercules, CA, USA) with the following conditions: an initial denaturation at 94 °C for 1 min; 30 cycles at 94 °C for 1 min, 50 °C for 1 min, and 72 °C for 2 min; and a final extension at 72 °C for 5 min. Gel electrophoresis was performed to confirm the PCR amplification. Amplicon sequencing was performed by Macrogen (Seoul, Korea) using an Illumina MiSeq platform. The Shannon index was used to measure the diversity of the microorganisms of the carcasses. The diversity index was calculated using the following equation [
23]:
2.9. Potentially Pathogenic Bacteria Identification
To identify potential pathogens, the 16S rRNA gene sequences (from the previously mentioned steps) were matched with the US EPA database of potentially pathogenic bacteria using BLAST (blastn, identity ≥97%) [
24]. However, microbiologically, it is not easy to identify whether the bacteria detected by the 16S rRNA gene sequence alone are pathogenic. Nevertheless, if a number of individuals belong to genera known as pathogenic bacteria or if the frequency of the detection of the nucleotide sequence is high, it is presumed that the possibility of detecting pathogenic bacteria is high. Therefore, the pathogenic bacteria detected by the 16S rRNA gene are considered potentially pathogenic bacteria.
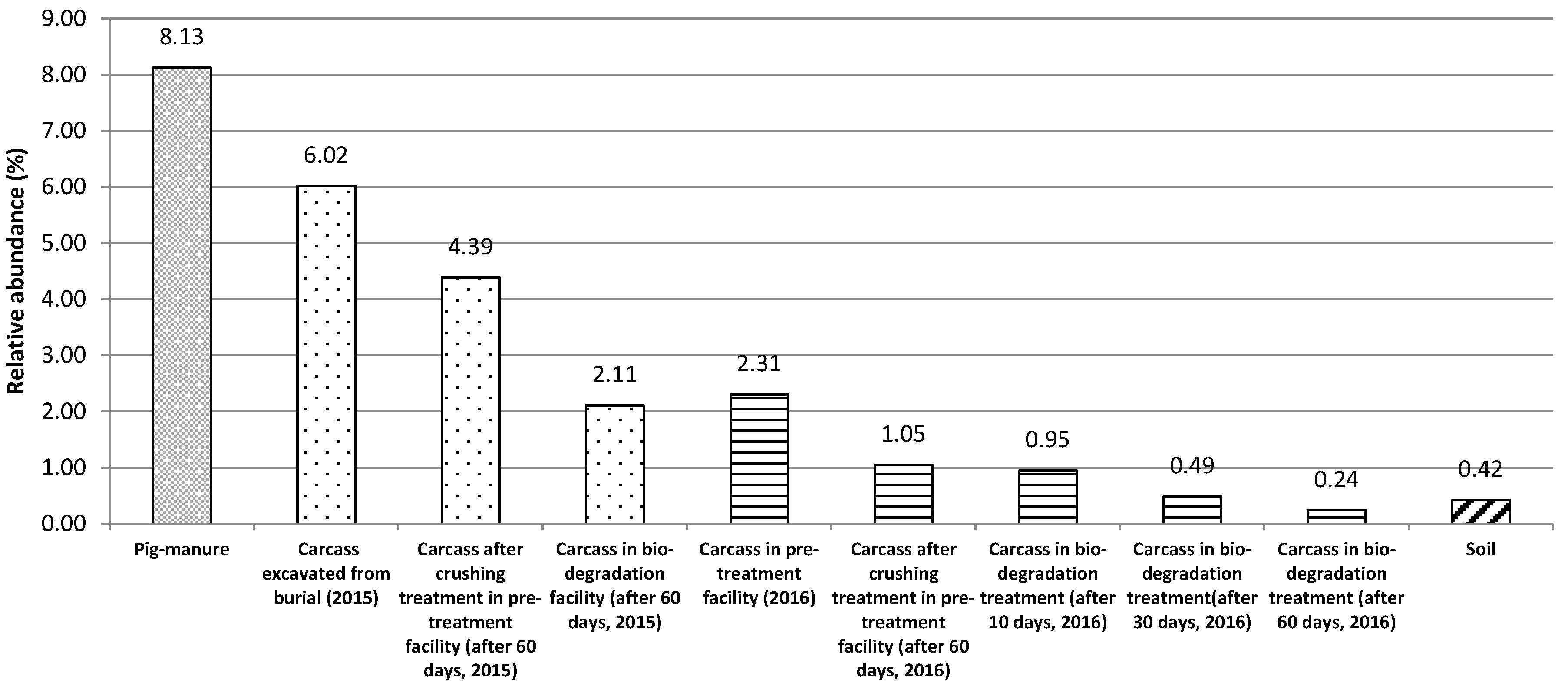
The relative abundance of potential pathogens was calculated as the ratio of the number of sequences matched to the pathogen database and the total bacterial sequence. To compare the potential pathogens of carcasses, pig manure and soil samples were chosen because these two samples are representative of a high and basal level of potential pathogens [
25]. Triplicate pig manure samples were collected from a pig manure treatment facility in Vietnam. The soil samples were collected from soil for landfill construction in Gyeonggi Province, Korea.
4. Discussion
The feasibility of bio-augmented aerobic composting of excavated carcasses was tested at pilot scale in this work. The results of monitoring COD, organic matter, and nitrogen species showed a significant degree of bio-stabilization of previously buried but undecomposed carcasses although VS measurement was not sufficiently sensitive to detect the same bio-stabilization. The increased germination index of the post-composed product compared to the pre-composted carcasses is evidence of a reduction in plant toxicity by bio-augmented composting. These results demonstrated that the novel burial–composting sequential treatment system was successful in stabilizing carcasses to a suitable level at which the final product from the burial–composting sequential treatment can be used for soil applications, such as farming.
The microbiome monitoring using a NGS method revealed that the bio-augmented aerobic composting effectively reduced microbial risk to human health, as indicated by the decreased relative abundance of potential pathogenic bacteria. The following identification of dominant pathogen candidates showed that the NGS-detected Clostridium botulinum, Clostridium tetani, and Enterobacter cloacae were different from the bacterial pathogen indicators currently used for assessing microbial risk in carcasses in Korea [
4]. The findings from the NGS microbiome monitoring provide a basis for improving the accuracy of microbial risk assessment for bio-stabilized product from burial–composting sequential treatment.
If a treatment is urgently required for a large amount of livestock carcasses infected by an epidemic disease, burial seems to be the best option. If sufficient land is available for a long-term burial process, buried carcasses should be kept at the same site(s). However, if a country has a limited land area (as Korea does), burial–composting sequential treatment may be a very economically sound option. Although there could be multiple burial sites, a limited number of designated composting sites can handle the bio-stabilization of excavated carcasses from the multiple burial sites. For that case, it is a pre-requirement to guarantee the deactivation of the infection-causing virus before excavating and transferring carcasses.