Large-Scale Screening of Intact Tomato Seeds for Viability Using Near Infrared Reflectance Spectroscopy (NIRS)
Abstract
:1. Introduction
2. Materials and Methods
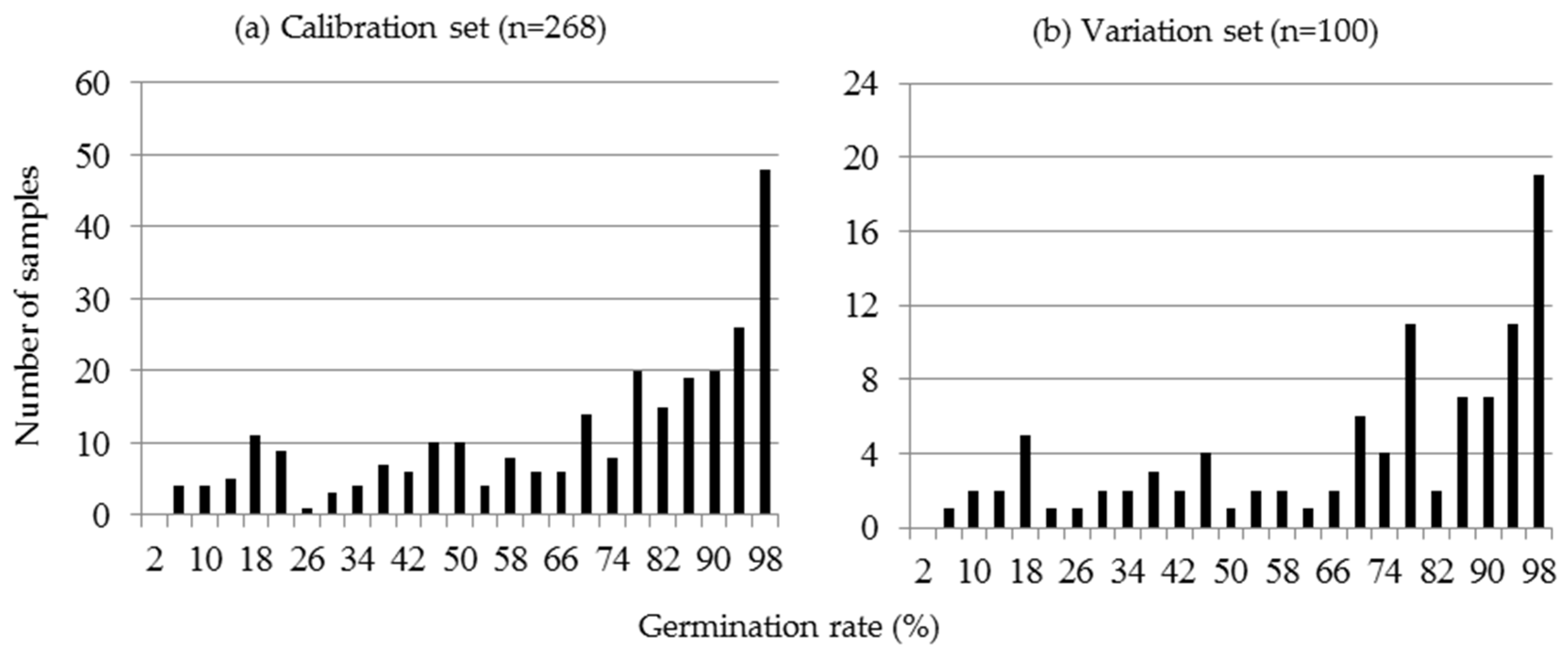
2.1. Preparation of the Calibration Set and Prediction Set by AAT
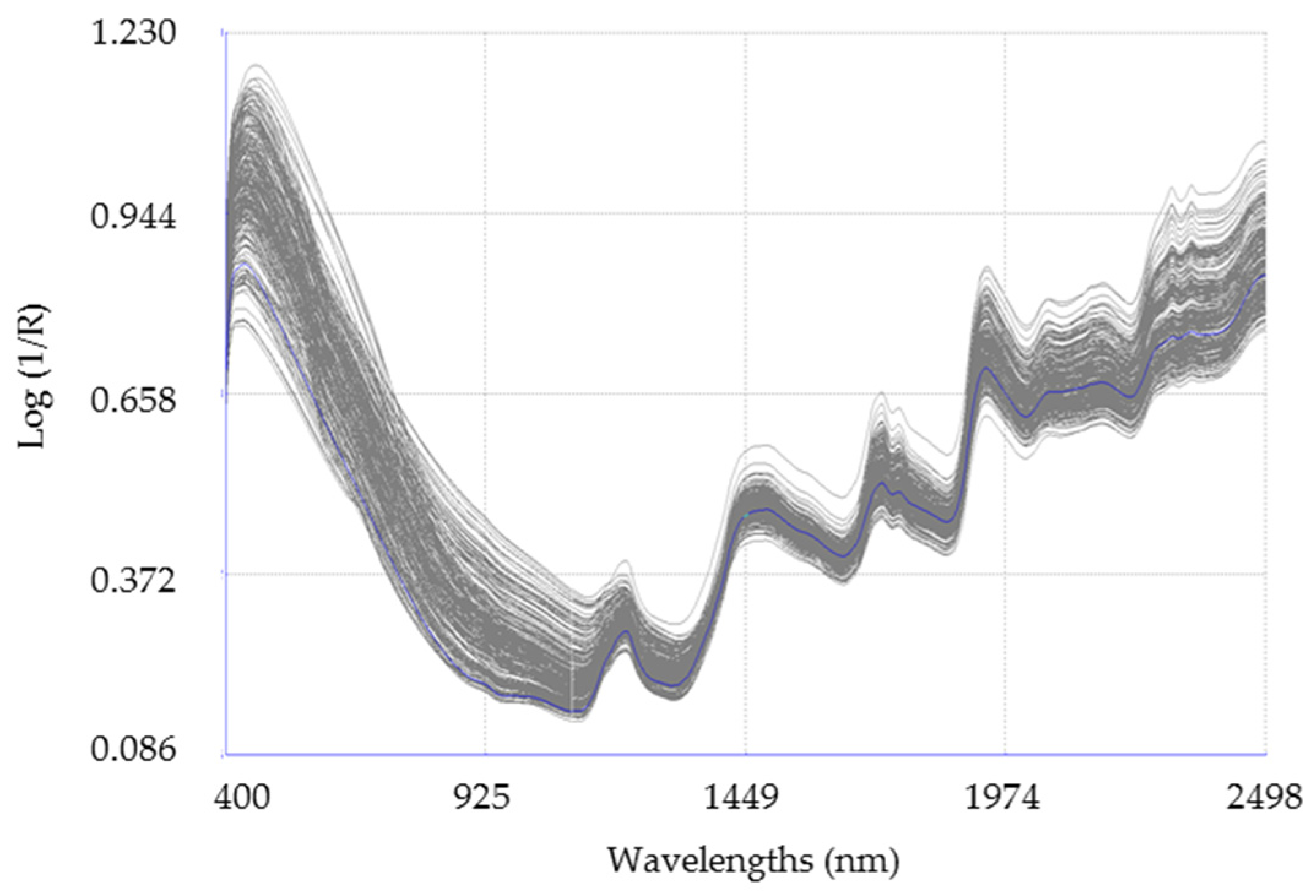
2.2. Collection NIRS Data
2.3. Germination Test
2.4. Development and Cross-Validation of the NIRS Calibration Model
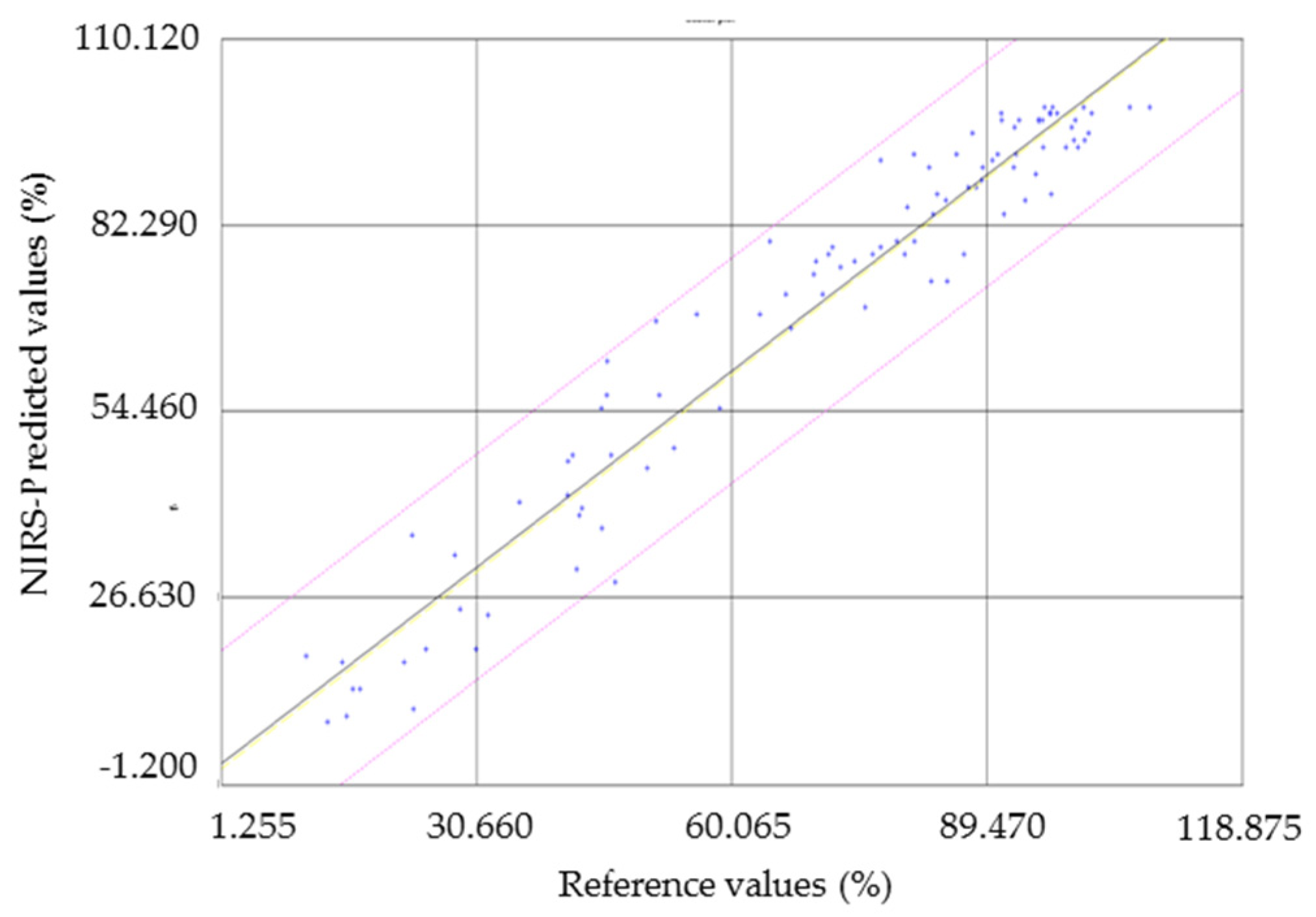
2.5. External Validation of the NIRS Calibration Model
3. Results
3.1. Preparation of the Calibration Set and Prediction Set by AAT
3.2. Development and Cross-Validation of the NIRS Calibration Model
3.3. External Validation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Copeland, L.O.; McDonald, M.B. Seed storage and deterioration. In Principles of Seed Science and Technology; Springer: Boston, MA, USA, 2001; pp. 192–230. [Google Scholar]
- Copeland, L.O.; McDonald, M.B. Seed viability and viability testing. In Principles of Seed Science and Technology; Springer: Boston, MA, USA, 2001; pp. 124–139. [Google Scholar]
- Kusama, T.; Abe, H.; Kawano, S.; Iwamoto, M. Classification of normal and aged soybean seeds by discriminant analysis using principal component scores of near infrared spectra. J. Jpn. Soc. Food Sci. 1997, 44, 569–578. [Google Scholar] [CrossRef]
- Cogdill, R.P.; Hurburgh, C.R.; Rippke, G.R. Single-kernel maize analysis by near-infrared hyperspectral imaging. Trans. ASAE 2004, 47, 311–320. [Google Scholar] [CrossRef]
- Pearson, T.C.; Wicklow, D.T.; Maghirang, E.B.; Xie, F.; Dowell, F.E. Detecting aflatoxin in single corn kernels by transmittance and reflectance spectroscopy. Trans. ASAE 2001, 44, 1247–1254. [Google Scholar] [CrossRef]
- Cozzolino, D.; Kwiatkowski, M.J.; Parker, M.; Cynkar, W.U.; Dambergs, R.G.; Gishen, M.; Herderich, M.J. Prediction of phenolic compounds in red wine fermentations by visible and near infrared spectroscopy. Anal. Chim. Acta 2004, 513, 73–80. [Google Scholar] [CrossRef]
- McClure, W. Review: 204 years of near infrared technology: 1800–2003. J. Near Infrared Spectrosc. 2003, 11, 487–518. [Google Scholar] [CrossRef]
- Kang, W.S. Nondestructive Determination of Seed Viability by Optical Methods. Ph.D. Thesis, Daegu University, Daegu, Korea, 2008. [Google Scholar]
- Juhász, R.; Gergely, S.; Gelencsér, T.; Salgó, A. Relationship between nir spectra and rva parameters during wheat germination. Cereal Chem. J. 2005, 82, 488–493. [Google Scholar] [CrossRef]
- Tigabu, M.; Oden, P.C. Discrimination of viable and empty seeds of pinus patula schiede & deppe with near-infrared spectroscopy. New For. 2003, 25, 163–176. [Google Scholar]
- Min, T.G.; Kang, W.S. Nondestructive classification of viable and nonviable radish (Raphanus sativus L.) seeds using single near infrared spectroscopy. J. Hortic. Environ. Biotechnol. 2008, 49, 42–46. [Google Scholar]
- Olesen, M.H.; Shetty, N.; Gislum, R.; Boelt, B. Classification of viable and non-viable spinach (Spinacia oleracea L.) seeds by single seed near infrared spectroscopy and extended canonical variates analysis. J. Near Infrared Spectrosc. 2011, 19, 171–180. [Google Scholar] [CrossRef]
- Daneshvar, A.; Tigabu, M.; Karimidoost, A.; Oden, P.C. Single seed near infrared spectroscopy discriminates viable and non-viable seeds of juniperus polycarpos. Silva Fenn. 2015, 49, 1–14. [Google Scholar] [CrossRef]
- Williams, P.C.; Sobering, D.C. How Do We Do It: A Brief Summary of the Methods We Use in Developing near Infrared Calibration; Davis, A.M.C., Williams, P.C., Eds.; NIR Publications: Chichester, UK, 1996. [Google Scholar]
- Lin, H.; Ying, Y. Theory and application of near infrared spectroscopy in assessment of fruit quality: A review. Sens. Instrum. Food Qual. Saf. 2009, 3, 130–141. [Google Scholar] [CrossRef]
- Delouche, J.C.; Baskin, C.C. Accelerated aging techniques for predicting the relative storability of seed lots. Seed Sci. Technol. 1973, 1, 427–452. [Google Scholar]
- Copeland, L.O.; McDonald, M.B. Seed vigor and vigor tests. In Principles of Seed Science and Technology; Springer: Boston, MA, USA, 1999; pp. 153–180. [Google Scholar]
- Probert, R.J.; Daws, M.I.; Hay, F.R. Ecological correlates of ex situ seed longevity: A comparative study on 195 species. Ann. Bot. 2009, 104, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Lovigny, Y.; Groot, S.P.; Belghazi, M.; Job, C.; Job, D. Proteome-wide characterization of seed aging in arabidopsis: A comparison between artificial and natural aging protocols. Plant Physiol. 2008, 148, 620–641. [Google Scholar] [CrossRef] [PubMed]
- Agelet, L.E.; Ellis, D.D.; Duvick, S.; Goggi, A.S.; Hurburgh, C.R.; Gardner, C.A. Feasibility of near infrared spectroscopy for analyzing corn kernel damage and viability of soybean and corn kernels. J. Cereal Sci. 2012, 55, 160–165. [Google Scholar] [CrossRef]
- Lestander, T.A.; Oden, P.C. Separation of viable and non-viable filled scots pine seeds by differentiating between drying rates using single seed near infrared transmittance spectroscopy. Seed Sci. Technol. 2002, 30, 383–392. [Google Scholar]
- Lohumi, S.; Mo, C.; Kang, J.-S.; Hong, S.-J.; Cho, B.-K. Nondestructive evaluation for the viability of watermelon (citrullus lanatus) seeds using fourier transform near infrared spectroscopy. J. Biosyst. Eng. 2013, 38, 312–317. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of nir spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
| Regressio Method z | GH y | Math Treatment | N x | Mean w | SD v | Calibration u | Cross-Validation t | |||
|---|---|---|---|---|---|---|---|---|---|---|
| SEC | RSQ | SECV | 1-VR | SD/SECV | ||||||
| MPLS | 10 | 1,4,4,1 | 260 | 70.58 | 27.49 | 6.6119 | 0.9421 | 7.8291 | 0.9187 | 3.51 |
| 3 | 1,4,4,1 | 256 | 70.72 | 27.61 | 6.5012 | 0.9446 | 7.8264 | 0.9194 | 3.53 | |
| 3 | 2,4,4,1 | 255 | 70.93 | 27.23 | 6.5515 | 0.9421 | 8.2729 | 0.9074 | 3.29 | |
| 3 | 2,8,6,1 | 256 | 70.60 | 27.45 | 6.6374 | 0.9416 | 8.0404 | 0.9139 | 3.41 | |
| PLS | 10 | 1,4,4,1 | 260 | 70.06 | 27.68 | 7.7336 | 0.9220 | 8.5802 | 0.9036 | 3.23 |
| 3 | 1,4,4,1 | 259 | 70.20 | 27.65 | 7.7901 | 0.9206 | 8.7277 | 0.9001 | 3.17 | |
| 3 | 2,4,4,1 | 258 | 70.16 | 27.32 | 8.6306 | 0.9002 | 10.175 | 0.8608 | 2.69 | |
| 3 | 2,8,6,1 | 258 | 70.38 | 27.52 | 7.9458 | 0.9167 | 9.2901 | 0.8857 | 2.96 | |
| PCR | 10 | 1,4,4,1 | 262 | 69.87 | 27.71 | 13.0589 | 0.7779 | 13.0699 | 0.7769 | 2.12 |
| 3 | 1,4,4,1 | 261 | 69.76 | 27.70 | 13.0164 | 0.7792 | 13.0323 | 0.7780 | 2.13 | |
| 3 | 2,4,4,1 | 260 | 69.92 | 27.59 | 12.8153 | 0.7843 | 13.1216 | 0.7733 | 2.10 | |
| 3 | 2,8,6,1 | 259 | 69.70 | 27.57 | 14.5249 | 0.7225 | 14.5353 | 0.7212 | 1.90 | |
| GH z | N | Mean (P) | Mean (M) | SD (P) | SD (M) | Bias | Slope | R2 | SEP | SEP (C) | RPD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 100 | 70.89 | 70.26 | 28.12 | 27.46 | 0.626 | 0.992 | 0.939 | 6.93 | 6.57 | 3.96 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-S.; Jeon, Y.-A.; Lee, Y.-Y.; Lee, G.-A.; Raveendar, S.; Ma, K.H. Large-Scale Screening of Intact Tomato Seeds for Viability Using Near Infrared Reflectance Spectroscopy (NIRS). Sustainability 2017, 9, 618. https://doi.org/10.3390/su9040618
Lee H-S, Jeon Y-A, Lee Y-Y, Lee G-A, Raveendar S, Ma KH. Large-Scale Screening of Intact Tomato Seeds for Viability Using Near Infrared Reflectance Spectroscopy (NIRS). Sustainability. 2017; 9(4):618. https://doi.org/10.3390/su9040618
Chicago/Turabian StyleLee, Ho-Sun, Young-Ah Jeon, Young-Yi Lee, Gi-An Lee, Sebastin Raveendar, and Kyung Ho Ma. 2017. "Large-Scale Screening of Intact Tomato Seeds for Viability Using Near Infrared Reflectance Spectroscopy (NIRS)" Sustainability 9, no. 4: 618. https://doi.org/10.3390/su9040618






