From Technical Efficiency to Economic Efficiency: Development of Aza-Friedel–Crafts Reaction Using Phosphoric Acid Immobilized in Glycerol as a Sustainable Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
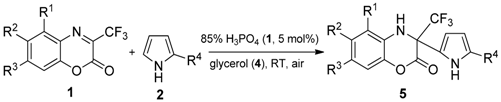
2.2. General Sustainable Procedure for Environmentally Friendly and Atom Economical Synthesis of Dihydrobenzoxazinones

2.3. Method for Biological Activity Study
3. Results and Discussion
3.1. Sustainable and Practical Methodology
3.2. The Catalytic System Recycling
3.3. Biological Activity Study
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trost, B.M. The atom economy—A search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.L.Y.; Smith, R.L.; Poliakoff, M. Principles of green chemistry: Productively. Green Chem. 2005, 7, 761–762. [Google Scholar] [CrossRef]
- Bandini, M.; Melloni, A.; Umani-Ronchi, A. New catalytic approaches in the stereoselective Friedel–Crafts alkylation reaction. Angew. Chem. Int. Ed. 2004, 43, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, T.B.; Jorgensen, K.A. Catalytic asymmetric Friedel−Crafts alkylation reactions—Copper showed the way. Chem. Rev. 2008, 108, 2903–2915. [Google Scholar] [CrossRef] [PubMed]
- You, S.L.; Cai, Q.; Zeng, M. Chiral Brønsted acid catalyzed Friedel–Crafts alkylation reactions. Chem. Soc. Rev. 2009, 38, 2190–2201. [Google Scholar] [CrossRef] [PubMed]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multicomponent reactions: advanced tools for sustainable organic synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Gu, Y.; Jérôme, F. Glycerol as a sustainable solvent for green chemistry. Green Chem. 2010, 12, 1127–1138. [Google Scholar] [CrossRef]
- Gu, Y.; Barrault, J.; Jérôme, F. Glycerol as an efficient promoting medium for organic reactions. Adv. Synth. Catal. 2008, 350, 2007–2012. [Google Scholar] [CrossRef]
- Tagliapietra, S.; Orio, L.; Palmisano, G.; Penoni, A.; Gravotto, G. Catalysis in glycerol: a survey of recent advances. Chem. Pap. 2015, 69, 1519–1531. [Google Scholar] [CrossRef]
- Wolfson, A.; Dlugy, C.; Shotland, Y. Glycerol as a green solvent for high product yields and selectivities. Env. Chem. Lett. 2007, 5, 67–71. [Google Scholar] [CrossRef]
- Ying, A.; Zhang, Q.; Li, H.; Shen, G.; Gong, W.; He, M. An environmentally benign protocol: Catalyst-free Michael addition of aromatic amines to α,β-unsaturated ketones in glycerol. Res. Chem. Inter. 2013, 39, 517–525. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Laird, T. Green chemistry is good process chemistry. Org. Process. Res. Dev. 2012, 16, 1–2. [Google Scholar] [CrossRef]
- Dunn, P.J. The importance of green chemistry in process research and development. Chem. Soc. Rev. 2012, 41, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Augé, J.; Scherrmann, M.C. Determination of the global material economy (GME) of synthesis sequences—A green chemistry metric to evaluate the greenness of products. New J. Chem. 2012, 36, 1091–1098. [Google Scholar] [CrossRef]
- Mohamed El-said, M.; Akio, T.; Nobuo, I. Trifluoropyruvic acid hydrate in heterocyclic synthesis, part II: Synthesis of trifluomethylated benzoxazine, benzothiazine, and benzoxazole derivatives. Heterocycles 1986, 24, 593–599. [Google Scholar]
- Wen, J.; Zhang, R.; Chen, S.; Zhang, J.; Yu, X. Direct arylation of arene and N-heteroarenes with diaryliodonium salts without the use of transition metal catalyst. J. Org. Chem. 2012, 77, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.; García-Álvarez, J. Glycerol: a biorenewable solvent for base-free Cu(I)-catalyzed 1,3-dipolar cycloaddition of azides with terminal and 1-iodoalkynes. Highly efficient transformations and catalyst recycling. Green Chem. 2014, 16, 3515–3521. [Google Scholar] [CrossRef]
- Wu, P.L.; Hsu, Y.L.; Zao, C.W.; Damu, A.G.; Wu, T.S. Constituents of vittaria anguste-elongata and their biological activities. J. Nat. Prod. 2005, 68, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Bellina, F.; Rossi, R. Synthesis and biological activity of pyrrole, pyrroline and pyrrolidine derivatives with two aryl groups on adjacent positions. Tetrahedron 2006, 62, 7213–7256. [Google Scholar] [CrossRef]
- Múüller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Wang, Y.; Jin, E.; Lin, X. Organocatalytic asymmetric synthesis of dihydrobenzoxazinones bearing trifluoromethylated quaternary stereocenters. J. Org. Chem. 2016, 81, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Rohle, D.; Popovici-Muller, J.; Palaskas, N.; Turcan, S.; Grommes, C.; Campos, C.; Tsoi, J.; Clark, O.; Oldrini, B.; Komisopoulou, E.; et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 2013, 340, 626–630. [Google Scholar] [CrossRef] [PubMed]
- García-ÁA, I.; Corrales, G.; Doncel-Pérez, E.; Muñoz, A.; Nieto-Sampedro, M.; Fernández-Mayoralas, A. Design and synthesis of glycoside inhibitors of glioma and melanoma growth. J. Med. Chem. 2007, 50, 364–373. [Google Scholar]

| Entry | 1 | R1 | R2 | R3 | 2 | R4 | Product | Crude Yield (%) | Isolated Yield (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1a | H | H | H | 2a | H | 5a | 99 | 98 |
| 2 | 1b | H | F | H | 2a | H | 5b | 97 | 97 |
| 3 | 1c | H | Cl | H | 2a | H | 5c | 97 | 96 |
| 4 | 1d | H | H | Br | 2a | H | 5d | 95 | 93 |
| 5 | 1e | H | Me | H | 2a | H | 5e | 99 | 97 |
| 6 | 1f | H | H | Me | 2a | H | 5f | 95 | 93 |
| 7 | 1g | Me | H | H | 2a | H | 5g | 94 | 94 |
| 8 | 1a | H | H | H | 2b | Ph | 5h | 96 | 94 |
| 9 | 1b | H | F | H | 2b | Ph | 5i | 95 | 93 |
| 10 | 1c | H | Cl | H | 2b | Ph | 5j | 96 | 95 |
| 11 | 1e | H | Me | H | 2b | Ph | 5k | 97 | 96 |
| 12 | 1f | H | H | Me | 2b | Ph | 5l | 97 | 95 |
| 13 | 1a | H | H | H | 2c | p-ClC6H4 | 5m | 98 | 95 |
| 14 | 1b | H | F | H | 2c | p-ClC6H4 | 5n | 96 | 93 |
| 15 | 1e | H | Me | H | 2c | p-ClC6H4 | 5o | 95 | 94 |
| 16 | 1f | H | H | Me | 2c | p-ClC6H4 | 5p | 95 | 92 |
| 17 | 1a | H | H | H | 2d | p-MeC6H4 | 5q | 99 | 95 |
| 18 | 1b | H | F | H | 2d | p-MeC6H4 | 5r | 98 | 97 |
| 19 | 1f | H | H | Me | 2d | p-MeC6H4 | 5s | 99 | 96 |

| Cycle | Time (h) | Crude yield | Cycle | Time (h) | Crude Yield |
|---|---|---|---|---|---|
| 1 | 24 | 99 | 6 | 24 | 97 |
| 2 | 24 | 98 | 7 | 24 | 98 |
| 3 | 24 | 99 | 8 | 24 | 99 |
| 4 | 24 | 99 | 9 | 24 | 98 |
| 5 | 24 | 98 | 10 | 24 | 99 (96) b |
| Compound | IC50 (μM) a | Compound | IC50 (μM) a | ||
|---|---|---|---|---|---|
| C6 | B16BL6 | C6 | B16BL6 | ||
| 5a | n.a. | 92.4 | 5k | 46.4 | 58.1 |
| 5b | 169.1 | 154.1 | 5l | 39.5 | 41.6 |
| 5c | 115.6 | n.a. | 5m | 15.4 | 63.2 |
| 5d | 232.5 | n.a. | 5n | 8.9 | 29.8 |
| 5e | 20.9 | n.a. | 5o | 18.7 | 24.2 |
| 5f | 81.0 | 100.2 | 5p | 19.4 | 25.7 |
| 5g | 149.2 | 70.9 | 5q | 42.3 | 68.4 |
| 5h | 127.7 | 193.3 | 5r | 44.2 | 37.4 |
| 5i | 58.3 | 75.7 | 5s | 19.6 | 90.9 |
| 5j | 42.2 | 94.3 | adriamycin | 18.8 | 96.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, L.; Rahman, A. From Technical Efficiency to Economic Efficiency: Development of Aza-Friedel–Crafts Reaction Using Phosphoric Acid Immobilized in Glycerol as a Sustainable Approach. Sustainability 2017, 9, 1176. https://doi.org/10.3390/su9071176
Tan L, Rahman A. From Technical Efficiency to Economic Efficiency: Development of Aza-Friedel–Crafts Reaction Using Phosphoric Acid Immobilized in Glycerol as a Sustainable Approach. Sustainability. 2017; 9(7):1176. https://doi.org/10.3390/su9071176
Chicago/Turabian StyleTan, Lan, and Abdul Rahman. 2017. "From Technical Efficiency to Economic Efficiency: Development of Aza-Friedel–Crafts Reaction Using Phosphoric Acid Immobilized in Glycerol as a Sustainable Approach" Sustainability 9, no. 7: 1176. https://doi.org/10.3390/su9071176




