Comparison of Satellite-Derived Phytoplankton Size Classes Using In-Situ Measurements in the South China Sea
Abstract
:1. Introduction
2. Materials and Methods
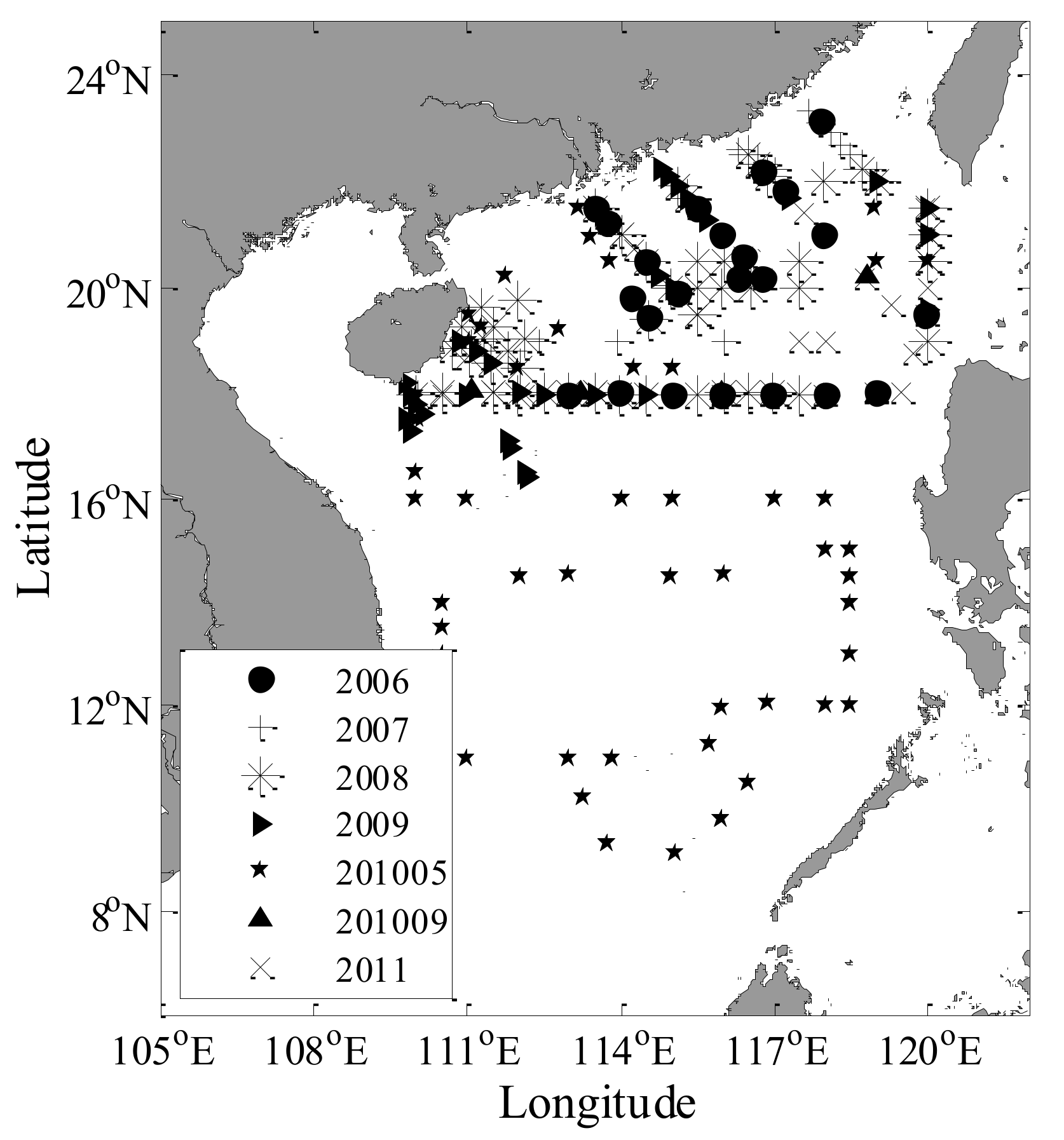
2.1. In-Situ Data
2.1.1. High Performance Liquid Chromatography Datasets
2.1.2. Spectral Absorption of Phytoplankton
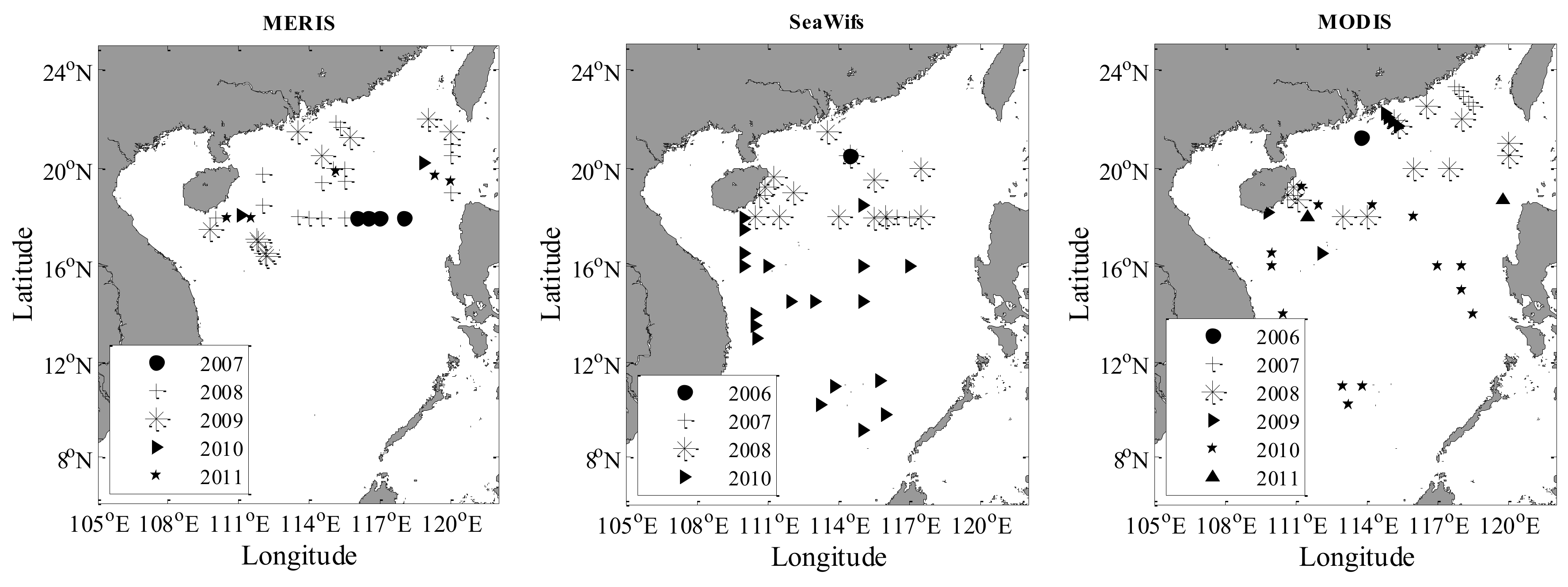
2.2. Satellite-Derived Data
2.2.1. Satellite Products
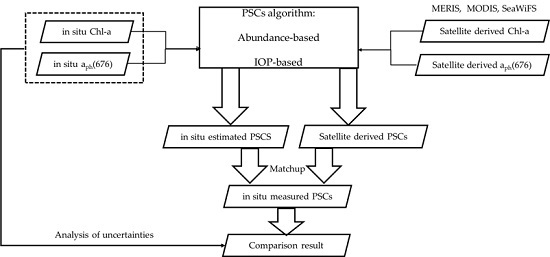
2.2.2. Matching Procedures for Satellite and In-Situ Data
2.3. PSCs Algorithms
2.3.1. Abundance-Based PSCs Algorithm: Uitz2006
2.3.2. Abundance-Based PSCs Algorithm: Brewin2010
2.3.3. Abundance-Based PSCs Algorithm: Hirata2011
2.3.4. IOP-Based PSCs Algorithm: Roy2013
2.4. Statistical Methods
3. Results
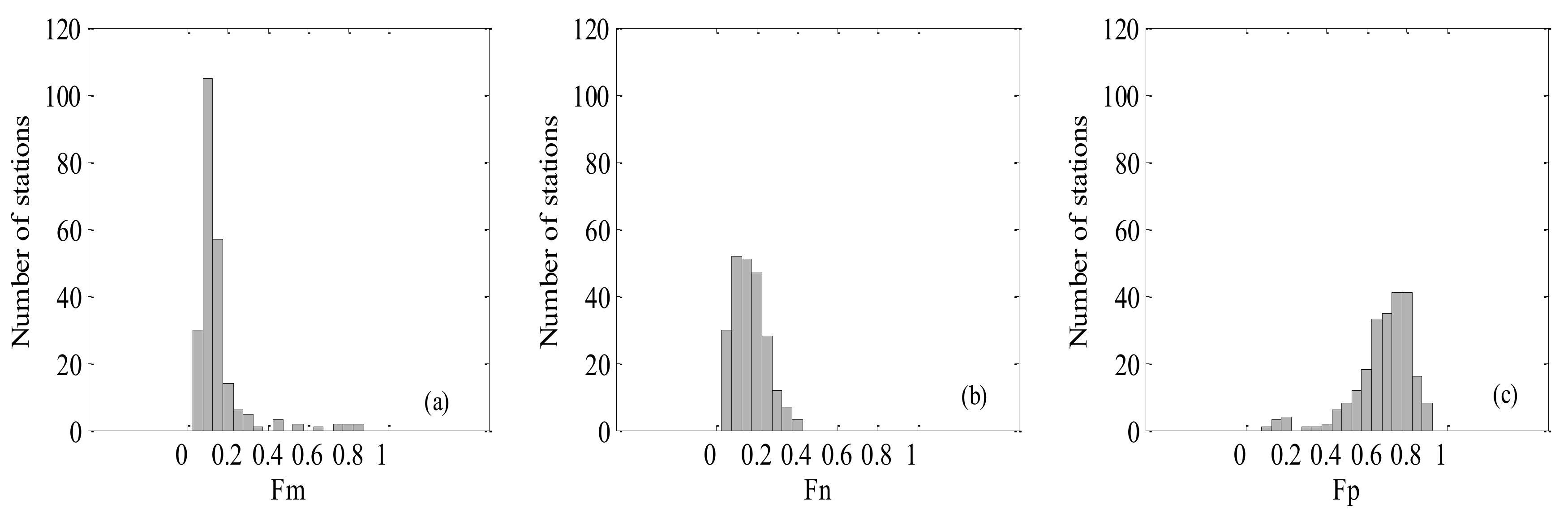
3.1. Statistics of In Situ Datasets and Match-up Data
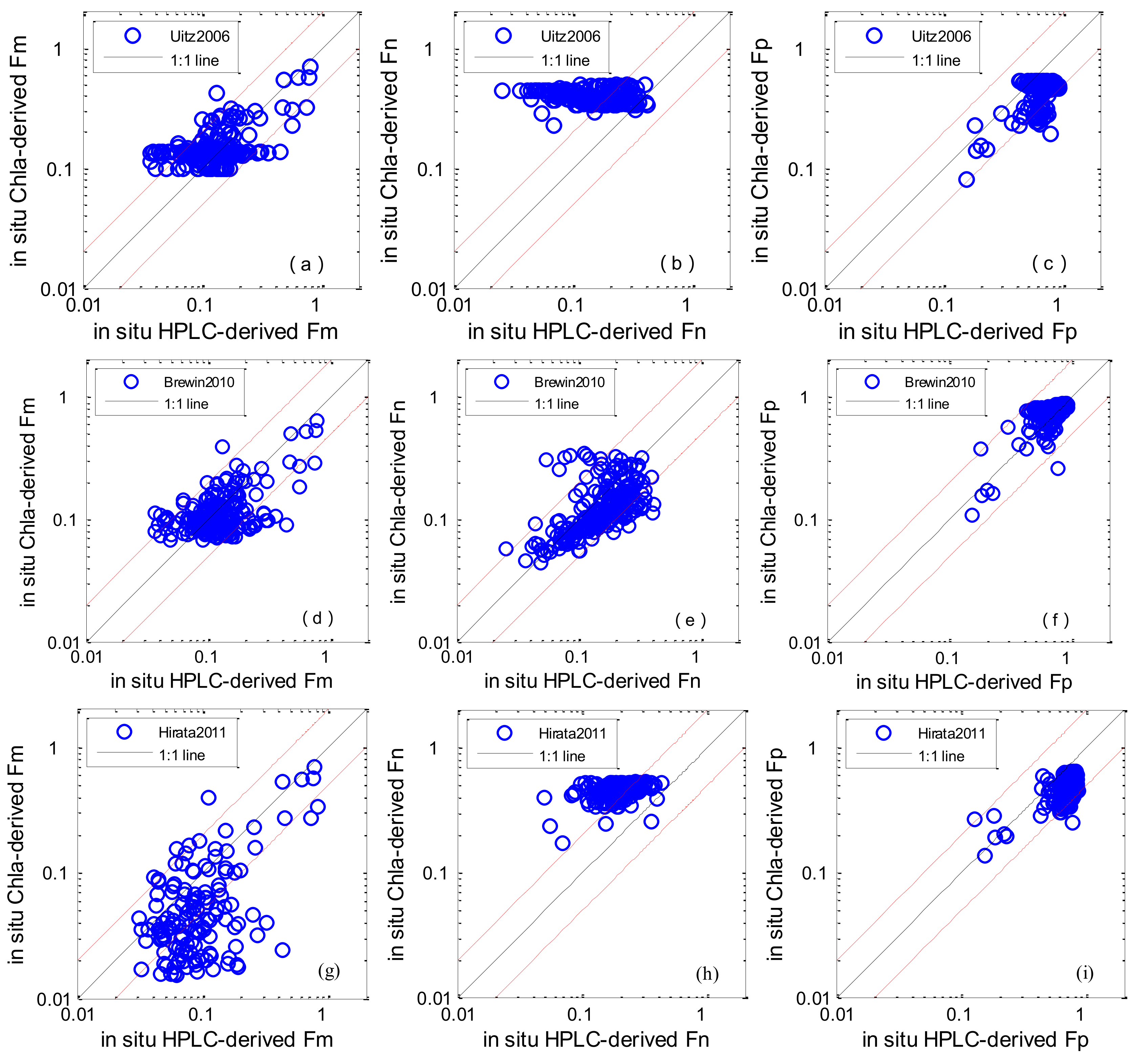
3.2. Algorithm Evaluation Using In-Situ Data
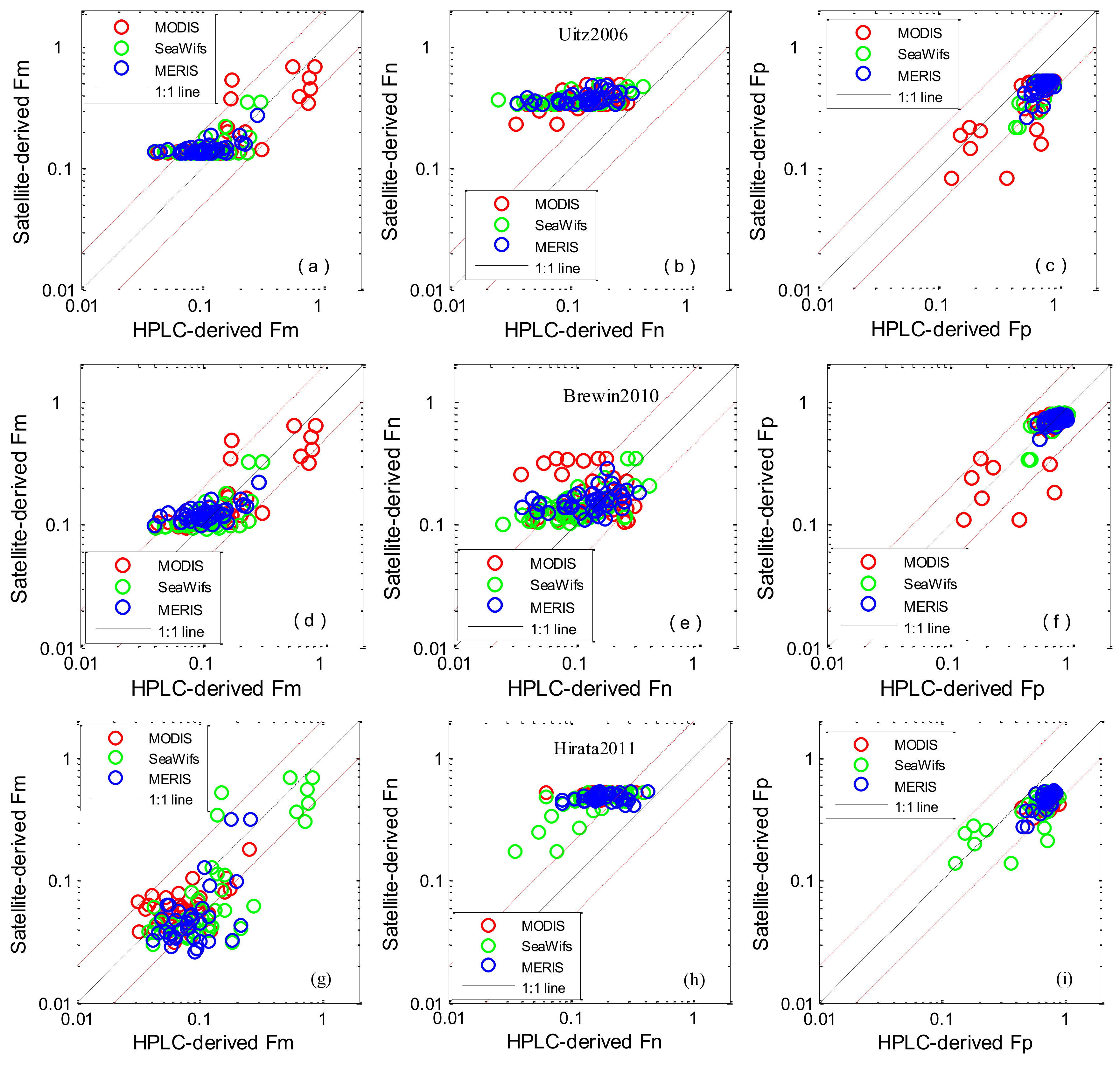
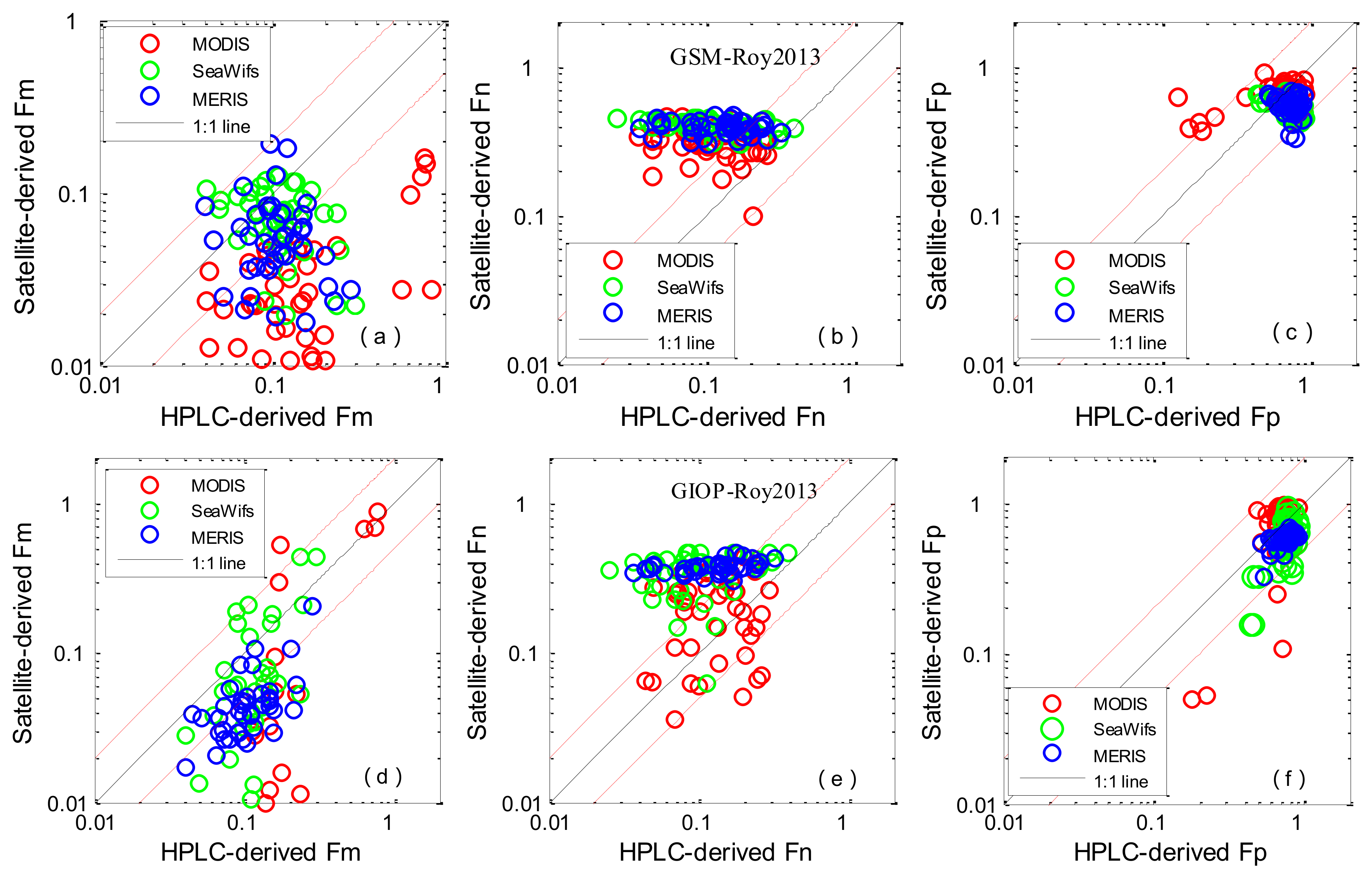
3.3. Algorithm Evaluation Using Satellite Data
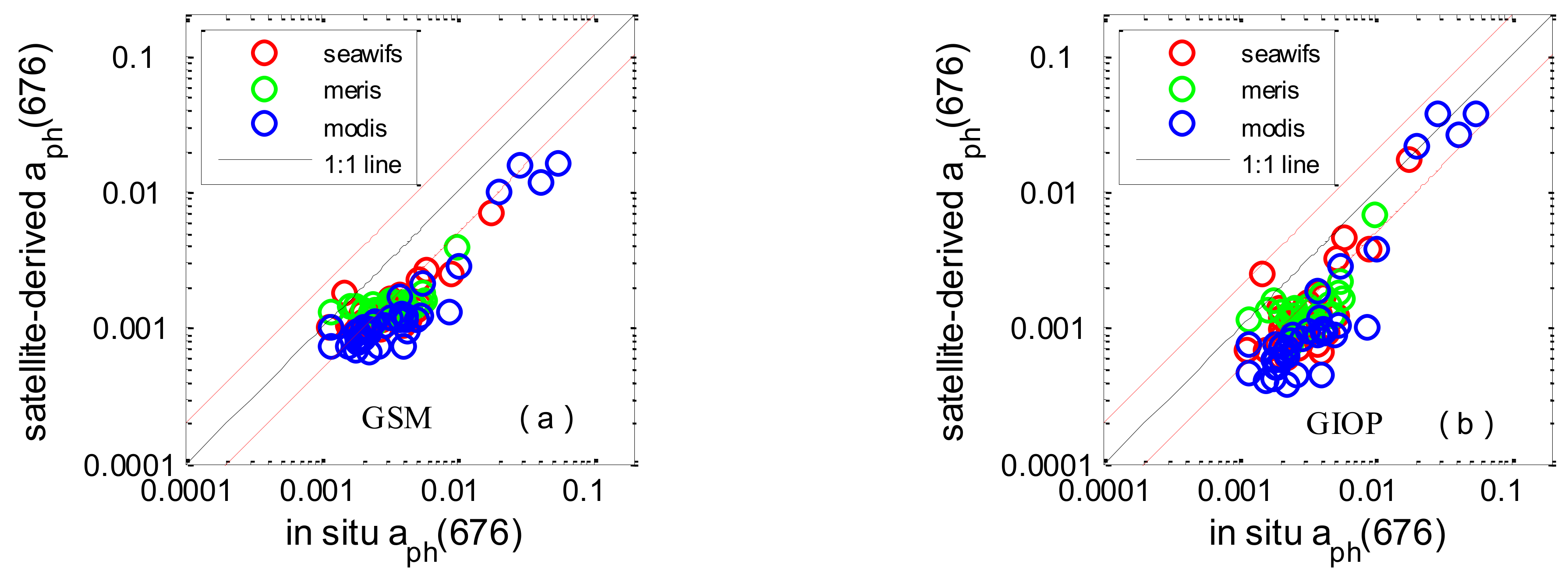
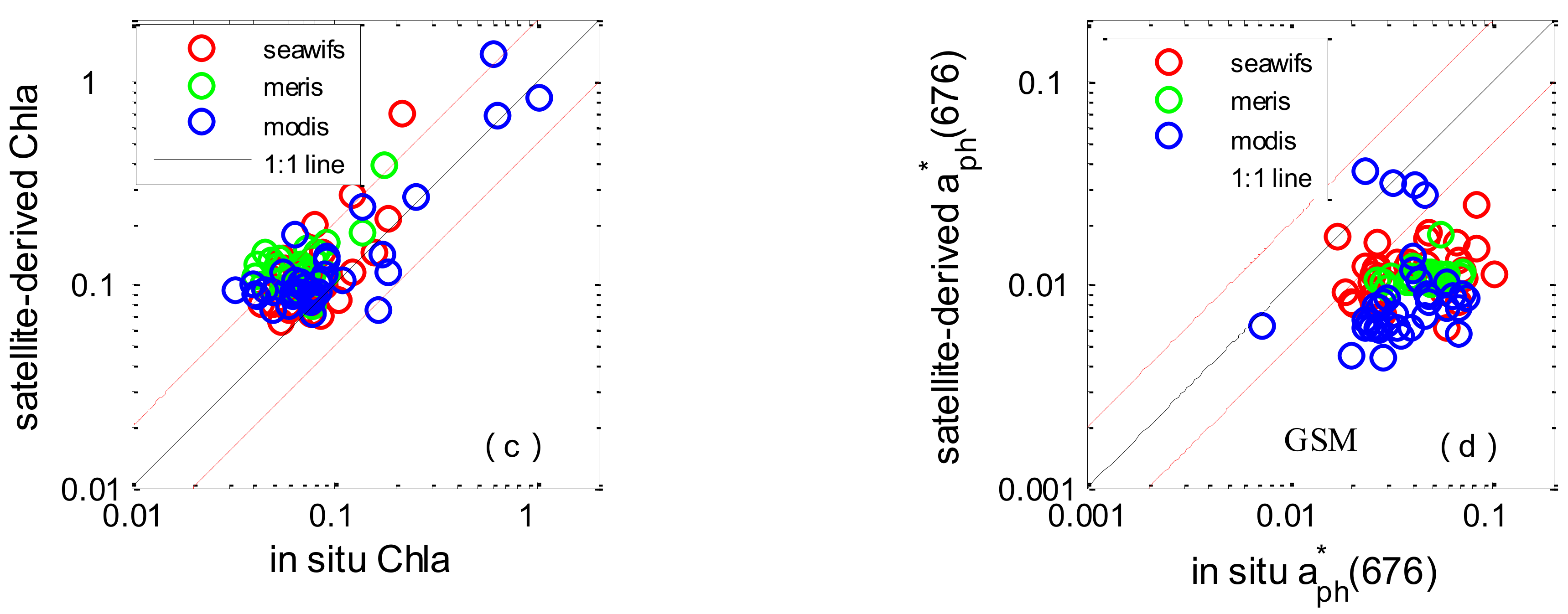
3.4. Chl-a and aph(676) Assessment in the SCS
4. Discussion
5. Conclusions
Acknowledgement
Author Contributions
Conflicts of Interest
References
- Falkowski, P.G.; Barber, R.T.; Smetacek, V. Biogeochemical controls and feedbacks on ocean primary production. Science 1998, 281, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A.; Buesseler, K.O.; Doney, S.C.; Sailley, S.F.; Behrenfeld, M.J.; Boyd, P.W. Global assessment of ocean carbon export by combining satellite observations and food-web models. Glob. Biogeochem. Cycles 2014, 28, 181–196. [Google Scholar] [CrossRef]
- Lin, J.; Cao, W.; Wang, G.; Hu, S. Satellite-observed variability of phytoplankton size classes associated with a cold eddy in the South China Sea. Mar. Pollut. Bull. 2014, 83, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Uitz, J.; Claustre, H.; Morel, A.; Hooker, S.B. Vertical distribution of phytoplankton communities in open ocean: An assessment based on surface chlorophyll. J. Geophys. Res. Ocean. 2006, 111, 275–303. [Google Scholar] [CrossRef]
- Kostadinov, T.S.; Siegel, D.A.; Maritorena, S. Global variability of phytoplankton functional types from space: Assessment via the particle size distribution. Biogeosci. Discuss. 2010, 7, 3239–3257. [Google Scholar] [CrossRef] [Green Version]
- Sieburt, J.M.; Smetacek, V.V.; Lenz, J. Pelagic ecosystem structure: Heterotrophic compartments of the plankton and their relationship to plankton size fractions. Limnol. Oceanogr. 1978, 23, 1256–1263. [Google Scholar] [CrossRef]
- Mcclain, C.R. A decade of satellite ocean color observations. Ann. Rev. Mar. Sci. 2009, 1, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A.; Behrenfeld, M.J.; Maritorena, S.; Mcclain, C.R.; Antoine, D.; Bailey, S.W.; Bontempi, P.S.; Boss, E.S.; Dierssen, H.M.; Doney, S.C. Regional to global assessments of phytoplankton dynamics from the seawifs mission. Remote Sens. Environ. 2013, 135, 77–91. [Google Scholar] [CrossRef]
- Bracher, A.; Bouman, H.A.; Brewin, R.J.W.; Bricaud, A.; Brotas, V.; Ciotti, A.M.; Clementson, L.; Devred, E.; Di Cicco, A.; Dutkiewicz, S.; et al. Obtaining phytoplankton diversity from ocean color: A scientific roadmap for future development. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Kostadinov, T.S.; Cabré, A.; Vedantham, H.; Marinov, I.; Bracher, A.; Brewin, R.J.W.; Bricaud, A.; Hirata, T.; Hirawake, T.; Hardman-Mountford, N.J. Inter-comparison of phytoplankton functional type phenology metrics derived from ocean color algorithms and earth system models. Remote Sens. Environ. 2017, 190, 162–177. [Google Scholar] [CrossRef]
- Mouw, C.B.; Hardman-Mountford, N.J.; Alvain, S.; Bracher, A.; Brewin, R.J.W.; Bricaud, A.; Ciotti, A.M.; Devred, E.; Fujiwara, A.; Hirata, T.; et al. A consumer’s guide to satellite remote sensing of multiple phytoplankton groups in the global ocean. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Brewin, R.J.W.; Hardman-Mountford, N.J.; Lavender, S.J.; Raitsos, D.E.; Hirata, T.; Uitz, J.; Devred, E.; Bricaud, A.; Ciotti, A.; Gentili, B. An intercomparison of bio-optical techniques for detecting dominant phytoplankton size class from satellite remote sensing. Remote Sens. Environ. 2011, 115, 325–339. [Google Scholar] [CrossRef]
- Hirata, T.; Hardman-Mountford, N.J.; Brewin, R.J.W.; Aiken, J.; Barlow, R.; Suzuki, K.; Isada, T.; Howell, E.; Hashioka, T.; Noguchi-Aita, M.; et al. Synoptic relationships between surface chlorophyll-a and diagnostic pigments specific to phytoplankton functional types. Biogeosciences 2011, 8, 311–327. [Google Scholar] [CrossRef] [Green Version]
- Hirata, T.; Hardman-Mountford, N.; Brewin, R.J.W. Comparing satellite-based phytoplankton classification methods. Eos Trans. Am. Geophys. Union 2012, 93, 59–60. [Google Scholar] [CrossRef]
- Roy, S.; Sathyendranath, S.; Bouman, H.; Platt, T. The global distribution of phytoplankton size spectrum and size classes from their light-absorption spectra derived from satellite data. Remote Sens. Environ. 2013, 139, 185–197. [Google Scholar] [CrossRef]
- International Ocean-Colour Coordinating Group (IOCCG). Phytoplankton Functional Types from Space. In Reports of the International Ocean-Colour Coordinating Group No. 15; Sathyendranath, S., Ed.; IOCCG: Dartmouth, NH, USA, 2014. [Google Scholar]
- Chen, Y.L.L.; Chen, H.Y. Seasonal dynamics of primary and new production in the northern South China Sea: The significance of river discharge and nutrient advection. Deep Sea Res. I 2006, 53, 971–986. [Google Scholar] [CrossRef]
- Dai, M.; Zhai, W.; Cai, W.J.; Callahan, J.; Huang, B.; Shang, S.; Huang, T.; Li, X.; Lu, Z.; Chen, W. Effects of an estuarine plume-associated bloom on the carbonate system in the lower reaches of the pearl river estuary and the coastal zone of the northern South China Sea. Cont. Shelf Res. 2008, 28, 1416–1423. [Google Scholar] [CrossRef]
- Gan, J.; Li, L.; Wang, D.; Guo, X. Interaction of a river plume with coastal upwelling in the northeastern South China Sea. Cont. Shelf Res. 2009, 29, 728–740. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, C.; Shang, S.; Müller-Karger, F.E.; Li, Y.; Dai, M.; Huang, B.; Ning, X.; Hong, H. Bridging between seawifs and modis for continuity of chlorophyll-a concentration assessments off southeastern china. Remote Sens. Environ. 2006, 102, 250–263. [Google Scholar] [CrossRef]
- Zhao, W.J.; Wang, G.Q.; Cao, W.X.; Cui, T.W.; Wang, G.F.; Ling, J.F.; Sun, L.; Zhou, W.; Sun, Z.H.; Xu, Z.T.; et al. Assessment of seawifs, modis, and meris ocean colour products in the South China Sea. Int. J. Remote Sens. 2014, 35, 4252–4274. [Google Scholar] [CrossRef]
- Hu, S.B.; Cao, W.X.; Wang, G.F.; Xu, Z.T.; Lin, J.F.; Zhao, W.J.; Yang, Y.Z.; Zhou, W.; Sun, Z.H.; Yao, L.J. Comparison of meris, modis, seawifs-derived particulate organic carbon, and in situ measurements in the South China Sea. Int. J. Remote Sens. 2016, 37, 1585–1600. [Google Scholar] [CrossRef]
- Aiken, J.; Pradhan, Y.; Barlow, R.; Lavender, S.; Poulton, A.; Holligan, P.; Hardman-Mountford, N. Phytoplankton pigments and functional types in the Atlantic Ocean: A decadal assessment, 1995–2005. Deep Sea Res. II 2009, 56, 899–917. [Google Scholar] [CrossRef]
- Vidussi, F.; Claustre, H.; Manca, B.B.; Luchetta, A.; Marty, J.C. Phytoplankton pigment distribution in relation to upper thermocline circulation in the eastern mediterranean sea during winter. J. Geophys. Res. Ocean. 2001, 106, 633–636. [Google Scholar] [CrossRef]
- Brewin, R.J.W.; Sathyendranath, S.; Hirata, T.; Lavender, S.J.; Barciela, R.M.; Hardman-Mountford, N.J. A three-component model of phytoplankton size class for the Atlantic Ocean. Ecol. Model. 2010, 221, 1472–1483. [Google Scholar] [CrossRef]
- Stramski, D.; Reynolds, R.A.; Kaczmarek, S.; Uitz, J.; Zheng, G. Correction of pathlength amplification in the filter-pad technique for measurements of particulate absorption coefficient in the visible spectral region. Appl. Opt. 2015, 54, 6763–6782. [Google Scholar] [CrossRef] [PubMed]
- Kishino, M.; Takahashi, M.; Okami, N.; Ichimura, S. Estimation of the spectral absorption coefficients of phytoplankton in the sea. Bull. Mar. Sci. 1985, 37, 634–642. [Google Scholar]
- Bricaud, A.; Babin, M.; Claustre, H.; Ras, J.; Tièche, F. Light absorption properties and absorption budget of southeast pacific waters. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef]
- Roesler, C.S. Theoretical and experimental approaches to improve the accuracy of particulate absorption coefficients derived from the quantitative filter technique. Limnol. Oceanogr. 1998, 43, 1649–1660. [Google Scholar] [CrossRef]
- Morel, A.; Maritorenal, S. Bio-optical properties of oceanic waters: A reappraisal. J. Geophys. Res. 2001, 106, 7163–7180. [Google Scholar] [CrossRef]
- Bailey, S.W.; Werdell, P.J. A multi-sensor approach for the on-orbit validation of ocean color satellite data products. Remote Sens. Environ. 2006, 102, 12–23. [Google Scholar] [CrossRef]
- Roy, S.; Sathyendranath, S.; Platt, T. Retrieval of phytoplankton size from bio-optical measurements: Theory and applications. J. R. Soc. Interface 2011, 8, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Brewin, R.J.W.; Hirata, T.; Hardman-Mountford, N.J.; Lavender, S.J.; Sathyendranath, S.; Barlow, R. The influence of the Indian Oceandipole on interannual variations in phytoplankton size structure as revealed by earth observation. Deep Sea Res. II 2012, 77, 117–127. [Google Scholar] [CrossRef]
- Brewin, R.J.W.; Sathyendranath, S.; Jackson, T.; Barlow, R.; Brotas, V.; Airs, R.; Lamont, T. Influence of light in the mixed-layer on the parameters of a three-component model of phytoplankton size class. Remote Sens. Environ. 2015, 168, 437–450. [Google Scholar] [CrossRef]
- Maritorena, S.; Siegel, D.A.; Peterson, A.R. Optimization of a semianalytical ocean color model for global-scale applications. Appl. Opt. 2002, 41, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Werdell, P.J.; Franz, B.A.; Bailey, S.W.; Feldman, G.C.; Boss, E.; Brando, V.E.; Dowell, M.; Hirata, T.; Lavender, S.J.; Lee, Z.; et al. Generalized ocean color inversion model for retrieving marine inherent optical properties. Appl. Opt. 2013, 52, 2019–2037. [Google Scholar] [CrossRef] [PubMed]
- Claustre, H.; Hooker, S.B.; Heukelem, L.V.; Berthon, J.F.; Barlow, R.; Ras, J.; Sessions, H.; Targa, C.; Thomas, C.S.; Linde, D.V.D. An intercomparison of hplc phytoplankton pigment methods using in situ samples: Application to remote sensing and database activities. Mar. Chem. 2004, 85, 41–61. [Google Scholar] [CrossRef]
- Lee, Z.; Hu, C.; Arnone, R.; Liu, Z. Impact of sub-pixel variations on ocean color remote sensing products. Opt. Express 2012, 20, 20844–20854. [Google Scholar] [CrossRef] [PubMed]

| Algorithm Publication(s) | Acronym | Input Data | Methodology |
|---|---|---|---|
| Uitz et al., (2006) | Uitz2006 | Chl-a, Ze, Zm | Abundance-based |
| Brewin et al., (2010) | Brewin2010 | Chl-a | Abundance-based |
| Hirata et al., (2011) | Hirata2011 | Chl-a | Abundance-based |
| Roy et al., (2011, 2013) | Roy2013 | aph * (676) | IOP-based |
| Study Regions | (mg m−3) | Sp,n | (mg m−3) | Sp |
|---|---|---|---|---|
| South China Sea (this study) [4] | 0.953 | 0.984 | 0.256 | 3.535 |
| Atlantic Ocean [26] | 0.977 | 0.910 | 0.095 | 7.822 |
| Indian Ocean [34] | 0.937 | 1.033 | 0.170 | 4.804 |
| Global [35] | 0.770 | 1.221 | 0.130 | 6.154 |
| In Situ Data | Median ± SD | Average | Max. | Min. | N |
|---|---|---|---|---|---|
| Fm | 0.12 ± 0.14 | 0.16 | 0.85 | 0.05 | 230 |
| Fn | 0.17 ± 0.09 | 0.16 | 0.40 | 0.03 | 230 |
| Fp | 0.71 ± 0.15 | 0.68 | 0.91 | 0.09 | 230 |
| Chl-a | 0.10 ± 0.43 | 0.20 | 3.25 | 0.03 | 230 |
| Fm * | 0.12 ± 0.15 | 0.17 | 0.83 | 0.05 | 101 |
| Fn * | 0.15 ± 0.08 | 0.16 | 0.39 | 0.03 | 101 |
| Fp * | 0.74 ± 0.16 | 0.70 | 0.91 | 0.13 | 101 |
| Algorithm | MR | SIQR | RMSE (%) | R2 | Slope | N |
|---|---|---|---|---|---|---|
| Fm | ||||||
| Uitz2006 | 1.204 | 0.298 | 32.666 | 0.307 | 0.504 | 227 |
| Brewin2010 | 0.906 | 0.246 | 38.848 | 0.292 | 0.487 | 227 |
| Hirata2011 | 0.612 | 0.339 | 71.525 | 0.205 | 0.599 | 178 |
| Roy2013 | 0.589 | 0.464 | 221.282 | 0.011 | 0.065 | 75 |
| Fn | ||||||
| Uitz2006 | 2.421 | 1.005 | 49.956 | 0.039 | −0.078 | 227 |
| Brewin2010 | 0.778 | 0.187 | 32.090 | 0.355 | 0.368 | 227 |
| Hirata2011 | 2.243 | 0.500 | 51.346 | 0.171 | 0.356 | 178 |
| Roy2013 | 1.675 | 0.676 | 40.215 | 0.000 | 0.083 | 75 |
| Fp | ||||||
| Uitz2006 | 0.647 | 0.081 | 205.294 | 0.418 | 0.332 | 227 |
| Brewin2010 | 1.068 | 0.069 | 44.566 | 0.597 | 0.715 | 227 |
| Hirata2011 | 0.709 | 0.102 | 148.755 | 0.481 | 0.433 | 178 |
| Roy2013 | 0.854 | 0.253 | 238.369 | 0.008 | 0.254 | 75 |
| Algorithm | MR | SIQR | RMSE | R2 | Slope | N |
|---|---|---|---|---|---|---|
| Fm | ||||||
| Uitz2006MODIS | 1.218 | 0.396 | 59.382 | 0.584 | 0.584 | 48 |
| Uitz2006Meris | 1.362 | 0.314 | 18.091 | 0.304 | 0.331 | 41 |
| Uitz2006Seawifs | 1.258 | 0.248 | 18.696 | 0.288 | 0.572 | 42 |
| Uitz2006Average | 1.275 | 0.333 | 38.814 | 0.504 | 0.579 | 131 |
| Brewin2010MODIS | 0.965 | 0.277 | 68.146 | 0.624 | 0.563 | 48 |
| Brewin2010Meris | 1.170 | 0.248 | 15.408 | 0.325 | 0.294 | 41 |
| Brewin2010Seawifs | 1.055 | 0.223 | 16.878 | 0.357 | 0.605 | 42 |
| Brewin2010Average | 1.042 | 0.268 | 43.211 | 0.536 | 0.558 | 131 |
| Hirata2011MODIS | 0.631 | 0.255 | 70.907 | 0.591 | 0.673 | 48 |
| Hirata2011Meris | 0.808 | 0.214 | 21.581 | 0.274 | 0.383 | 41 |
| Hirata2011Seawifs | 0.599 | 0.255 | 35.700 | 0.184 | 0.663 | 42 |
| Hirata2011Average | 0.697 | 0.252 | 48.955 | 0.421 | 0.666 | 131 |
| Fn | ||||||
| Uitz2006MODIS | 2.983 | 1.281 | 51.083 | 0.198 | 0.276 | 48 |
| Uitz2006Meris | 2.612 | 0.904 | 51.448 | 0.183 | 0.272 | 41 |
| Uitz2006Seawifs | 3.759 | 1.408 | 54.118 | 0.237 | 0.299 | 42 |
| Uitz2006Average | 3.028 | 1.256 | 52.188 | 0.197 | 0.286 | 131 |
| Brewin2010MODIS | 1.417 | 0.609 | 32.511 | 0.000 | −0.050 | 48 |
| Brewin2010Meris | 1.105 | 0.441 | 22.535 | 0.122 | 0.182 | 41 |
| Brewin2010Seawifs | 1.373 | 0.538 | 26.131 | 0.347 | 0.406 | 42 |
| Brewin2010Average | 1.306 | 0.524 | 27.661 | 0.083 | 0.199 | 131 |
| Hirata2011MODIS | 2.715 | 0.788 | 55.639 | 0.447 | 0.602 | 48 |
| Hirata2011Meris | 2.685 | 0.320 | 58.472 | 0.043 | 0.092 | 41 |
| Hirata2011Seawifs | 2.897 | 0.527 | 55.838 | 0.024 | 0.054 | 42 |
| Hirata2011Average | 2.755 | 0.497 | 56.604 | 0.287 | 0.336 | 131 |
| Fp | ||||||
| Uitz2006MODIS | 0.668 | 0.075 | 216.880 | 0.608 | 0.536 | 48 |
| Uitz2006Meris | 0.646 | 0.033 | 212.641 | 0.401 | 0.416 | 41 |
| Uitz2006Seawifs | 0.648 | 0.052 | 253.765 | 0.639 | 0.532 | 42 |
| Uitz2006Average | 0.650 | 0.051 | 228.107 | 0.617 | 0.519 | 131 |
| Brewin2010MODIS | 0.980 | 0.117 | 81.580 | 0.612 | 0.810 | 48 |
| Brewin2010Meris | 0.951 | 0.053 | 56.528 | 0.396 | 0.354 | 41 |
| Brewin2010Seawifs | 0.949 | 0.071 | 52.072 | 0.613 | 0.686 | 42 |
| Brewin2010Average | 0.967 | 0.068 | 65.635 | 0.623 | 0.736 | 131 |
| Hirata2011MODIS | 0.650 | 0.077 | 190.522 | 0.600 | 0.414 | 48 |
| Hirata2011Meris | 0.592 | 0.034 | 218.544 | 0.277 | 0.231 | 41 |
| Hirata2011Seawifs | 0.628 | 0.039 | 169.353 | 0.584 | 0.492 | 42 |
| Hirata2011Average | 0.619 | 0.049 | 193.504 | 0.579 | 0.401 | 131 |
| Algorithm | MR | SIQR | RMSE | R2 | Slope | N |
|---|---|---|---|---|---|---|
| Fm | ||||||
| a Roy2013MODIS | 4.804 | 1.507 | 100.427 | 0.225 | −0.272 | 48 |
| a Roy2013MERIS | 4.982 | 1.426 | 72.642 | 0.014 | 0.321 | 41 |
| a Roy2013SeaWiFS | 4.882 | 1.067 | 69.773 | 0.143 | 0.492 | 42 |
| a Roy2013Average | 4.964 | 1.408 | 83.114 | 0.000 | −0.068 | 131 |
| b Roy2013MODIS | 5.567 | 2.564 | 334.090 | 0.520 | −1.189 | 41 |
| b Roy2013MERIS | 5.601 | 1.558 | 74.534 | 0.342 | −0.842 | 39 |
| b Roy2013SeaWiFS | 5.278 | 1.683 | 73.616 | 0.249 | −1.577 | 39 |
| b Roy2013Average | 5.567 | 2.065 | 205.068 | 0.361 | −0.984 | 119 |
| Fn | ||||||
| a Roy2013MODIS | 2.705 | 1.585 | 47.053 | 0.041 | −0.227 | 48 |
| a Roy2013MERIS | 2.864 | 1.055 | 53.279 | 0.010 | −0.084 | 41 |
| a Roy2013SeaWiFS | 4.341 | 1.898 | 58.924 | 0.217 | −0.210 | 42 |
| a Roy2013Average | 3.076 | 1.669 | 53.036 | 0.040 | −0.196 | 131 |
| b Roy2013M°DIS | 1.506 | 0.852 | 42.041 | 0.025 | 0.166 | 41 |
| b Roy2013MERIS | 2.719 | 1.087 | 51.524 | 0.155 | 0.238 | 39 |
| b Roy2013SeaWiFS | 3.568 | 1.701 | 53.018 | 0.028 | 0.372 | 39 |
| b Roy2013Average | 2.496 | 1.185 | 48.992 | 0.004 | 0.195 | 119 |
| Fp | ||||||
| a Roy2013MODIS | 0.032 | 0.019 | 1666.956 | 0.142 | −0.108 | 48 |
| a Roy2013MERIS | 0.072 | 0.023 | 1211.414 | 0.053 | 0.077 | 41 |
| a Roy2013SeaWiFS | 0.092 | 0.023 | 1198.100 | 0.266 | 0.122 | 42 |
| a Roy2013Average | 0.066 | 0.033 | 1392.007 | 0.000 | −0.008 | 131 |
| b Roy2013MODIS | 0.006 | 0.012 | 2934.519 | 0.274 | −0.955 | 41 |
| b Roy2013MERIS | 0.056 | 0.017 | 1360.411 | 0.425 | −0.207 | 39 |
| b Roy2013SeaWiFS | 0.068 | 0.046 | 1924.974 | 0.206 | −0.670 | 39 |
| b Roy2013Average | 0.042 | 0.036 | 2188.129 | 0.099 | −0.724 | 119 |
| Name | MR | SIQR | RMSE | R2 | Slope | N |
|---|---|---|---|---|---|---|
| a aph(676)MODIS | 0.403 | 0.084 | 20.785 | 0.858 | 0.334 | 37 |
| a aph(676)MERIS | 0.485 | 0.096 | 15.168 | 0.475 | 0.242 | 21 |
| a aph(676)SeaWiFS | 0.443 | 0.096 | 16.920 | 0.672 | 0.299 | 37 |
| a aph(676)Average | 0.434 | 0.104 | 18.183 | 0.745 | 0.328 | 95 |
| b aph(676)MODIS | 0.306 | 0.064 | 22.423 | 0.858 | 0.800 | 37 |
| b aph(676)MERIS | 0.448 | 0.104 | 15.152 | 0.453 | 0.513 | 21 |
| b aph(676)SeaWiFS | 0.430 | 0.077 | 17.593 | 0.617 | 0.797 | 37 |
| b aph(676)Average | 0.376 | 0.097 | 19.161 | 0.723 | 0.795 | 95 |
| Chl-aMODIS | 1.305 | 0.228 | 38.896 | 0.785 | 0.556 | 37 |
| Chl-aMERIS | 1.834 | 0.308 | 26.108 | 0.472 | 1.546 | 21 |
| Chl-aSeaWiFS | 1.224 | 0.258 | 20.114 | 0.496 | 1.745 | 37 |
| Chl-aAverage | 1.429 | 0.373 | 24.713 | 0.643 | 0.573 | 95 |
| a * ph(676)MODIS | 0.265 | 0.084 | 44.495 | 0.038 | 0.016 | 37 |
| a * ph(676)MERIS | 0.257 | 0.042 | 48.389 | 0.007 | −0.006 | 21 |
| a * ph(676)SeaWiFS | 0.339 | 0.157 | 43.918 | 0.027 | −0.009 | 37 |
| a * ph(676)Average | 0.265 | 0.103 | 45.165 | 0.004 | 0.005 | 95 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Zhou, W.; Wang, G.; Cao, W.; Xu, Z.; Liu, H.; Wu, G.; Zhao, W. Comparison of Satellite-Derived Phytoplankton Size Classes Using In-Situ Measurements in the South China Sea. Remote Sens. 2018, 10, 526. https://doi.org/10.3390/rs10040526
Hu S, Zhou W, Wang G, Cao W, Xu Z, Liu H, Wu G, Zhao W. Comparison of Satellite-Derived Phytoplankton Size Classes Using In-Situ Measurements in the South China Sea. Remote Sensing. 2018; 10(4):526. https://doi.org/10.3390/rs10040526
Chicago/Turabian StyleHu, Shuibo, Wen Zhou, Guifen Wang, Wenxi Cao, Zhantang Xu, Huizeng Liu, Guofeng Wu, and Wenjing Zhao. 2018. "Comparison of Satellite-Derived Phytoplankton Size Classes Using In-Situ Measurements in the South China Sea" Remote Sensing 10, no. 4: 526. https://doi.org/10.3390/rs10040526