High-Spatial Resolution Monitoring of Phycocyanin and Chlorophyll-a Using Airborne Hyperspectral Imagery
Abstract
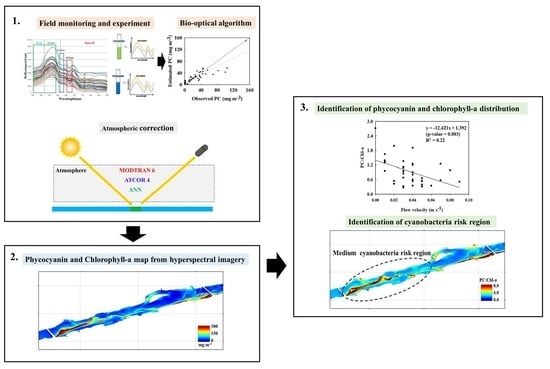
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Remote Sensing of PC and Chl-a Pigment
2.2.1. Water Sampling and Experimental Work
2.2.2. Field Optical and Hyperspectral Reflectance Data
2.2.3. Atmospheric Correction
2.2.4. Bio-Optical Algorithms for Determination of PC and Chl-a Concentration
AOP Algorithm
IOP Algorithm
2.3. Performance Evaluation
3. Results
3.1. Algal Variation in the Baekje Weir
3.2. Performance of Atmospheric Correction Techniques
3.3. Performance of the Bio-Optical Algorithm
3.4. PC and Chl-a Distribution Map
4. Discussion
4.1. Variation in Algae in the Baekje Reservoir
4.2. Atmospheric Correction Performance
4.3. Bio-Optical Algorithm Application
4.4. Spatial Distribution Map of Algal Concentration
5. Conclusions
- The cyanobacteria bloom on 12 and 24 August 2016 occurred as the PC:Chl-a value was greater than 0.5. A succession of algal species from cyanobacteria to diatoms was then observed on 20 September and 14 October 2016.
- MODTRAN 6 provided reasonable atmospheric correction performance compared to that of ATCOR 4. However, the accuracy was low in certain regions of the reflectance spectra ( < 500 nm and > 700 nm). This was mainly because of insufficient atmospheric observations during the campaigns.
- The most accurate atmospheric correction by MODTRAN 6, compared to ATCOR 4 and ANN, contributed to improving the performance of the bio-optical algorithms in terms of the estimation of PC and Chl-a concentration. The ANN model was found to require large quantities of input data to achieve accurate simulation results.
- The spatial distribution of a high PC:Chl-a value was derived using the flow velocity of less than 0.06 m s−1. This study directly proved that the influence factor of the dominant PC bloom was a long water retention time.
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chiswell, R.K.; Shaw, G.R.; Eaglesham, G.; Smith, M.J.; Norris, R.L.; Seawright, A.A.; Moore, M.R. Stability of cylindrospermopsin, the toxin from the cyanobacterium, cylindrospermopsis raciborskii: Effect of ph, temperature, and sunlight on decomposition. Environ. Toxicol. Int. J. 1999, 14, 155–161. [Google Scholar] [CrossRef]
- Gerard, C.; Poullain, V. Variation in the response of the invasive species potamopyrgus antipodarum (smith) to natural (cyanobacterial toxin) and anthropogenic (herbicide atrazine) stressors. Environ. Pollut. 2005, 138, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Shurin, J.B.; Dodson, S.I. Sublethal toxic effects of cyanobacteria and nonylphenol on environmental sex determination and development in daphnia. Environ. Toxicol. Chem. 1997, 16, 1269–1276. [Google Scholar] [CrossRef]
- Wagner, C.; Adrian, R. Cyanobacteria dominance: Quantifying the effects of climate change. Limnol. Oceanogr. 2009, 54, 2460–2468. [Google Scholar] [CrossRef] [Green Version]
- Zanchett, G.; Oliveira, E.C. Cyanobacteria and cyanotoxins: From impacts on aquatic ecosystems and human health to anticarcinogenic effects. Toxins 2013, 5, 1896–1917. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wang, C.; Wang, P.; Qian, J.; Wang, X. How physiological and physical processes contribute to the phenology of cyanobacterial blooms in large shallow lakes: A new euler-lagrangian coupled model. Water Res. 2018, 140, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Park, H.S.; Chung, S.W. Relationship of the thermal stratification and critical flow velocity near the baekje weir in geum river. J. Korean Soc. Water Envrion. 2017, 33, 449–459. [Google Scholar]
- Park, Y.; Pyo, J.; Kwon, Y.S.; Cha, Y.; Lee, H.; Kang, T.; Cho, K.H. Evaluating physico-chemical influences on cyanobacterial blooms using hyperspectral images in inland water, korea. Water Res. 2017, 126, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Romo, S.; Soria, J.; Fernandez, F.; Ouahid, Y.; Barón-Solá, Á. Water residence time and the dynamics of toxic cyanobacteria. Freshw. Biol. 2013, 58, 513–522. [Google Scholar] [CrossRef]
- Ju, H.; Choi, I.; Yoon, J.; Lee, J.; Lim, B.; Lee, S. Analysis of cyanobacterial growth pattern in bekjae weir during recent 3 years. Korean Soc. Water Enviorn. 2016, 2016, 562–563. [Google Scholar]
- Yoon, J.; Ju, H.; Yoon, H.; Lee, J.; Lee, J.; Lee, S. Effect of hydrological conditions on distribution characteristics of algal concentration in the geum river main stream. Korean Soc. Water Environ. 2015, 2015, 631–632. [Google Scholar]
- Cheung, M.Y.; Liang, S.; Lee, J. Toxin-producing cyanobacteria in freshwater: A review of the problems, impact on drinking water safety, and efforts for protecting public health. J. Microbiol. 2013, 51, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, J.-M.; Mazumder, A. Health and environmental policy issues in Canada: The role of watershed management in sustaining clean drinking water quality at surface sources. J. Environ. Manag. 2003, 68, 273–286. [Google Scholar] [CrossRef]
- Brando, V.E.; Dekker, A.G. Satellite hyperspectral remote sensing for estimating estuarine and coastal water quality. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1378–1387. [Google Scholar] [CrossRef]
- Brezonik, P.; Menken, K.D.; Bauer, M. Landsat-based remote sensing of lake water quality characteristics, including chlorophyll and colored dissolved organic matter (CDOM). Lake Reserv. Manag. 2005, 21, 373–382. [Google Scholar] [CrossRef]
- Dekker, A.G. Detection of Optical Water Quality Parameters for Eutrophic Waters by High Resolution Remote Sensing. Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 1993. [Google Scholar]
- Hilton, J. Airborne remote sensing for freshwater and estuarine monitoring. Water Res. 1984, 18, 1195–1223. [Google Scholar] [CrossRef]
- Kallio, K.; Kutser, T.; Hannonen, T.; Koponen, S.; Pulliainen, J.; Vepsäläinen, J.; Pyhälahti, T. Retrieval of water quality from airborne imaging spectrometry of various lake types in different seasons. Sci. Total Environ. 2001, 268, 59–77. [Google Scholar] [CrossRef]
- Kiefer, I.; Odermatt, D.; Anneville, O.; Wüest, A.; Bouffard, D. Application of remote sensing for the optimization of in-situ sampling for monitoring of phytoplankton abundance in a large lake. Sci. Total Environ. 2015, 527, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Jang, E.; Im, J.; Baek, S.H.; Park, Y.; Cho, K.H. Developing data-driven models for quantifying cochlodinium polykrikoides using the geostationary ocean color imager (GOCI). Int. J. Remote Sens. 2018, 39, 68–83. [Google Scholar] [CrossRef]
- Matthews, M.W.; Bernard, S.; Winter, K. Remote sensing of cyanobacteria-dominant algal blooms and water quality parameters in zeekoevlei, a small hypertrophic lake, using meris. Remote Sens. Environ. 2010, 114, 2070–2087. [Google Scholar] [CrossRef]
- Page, B.P.; Kumar, A.; Mishra, D.R. A novel cross-satellite based assessment of the spatio-temporal development of a cyanobacterial harmful algal bloom. Int. J. Appl. Earth Obs. Geoinf. 2018, 66, 69–81. [Google Scholar] [CrossRef]
- Ritchie, J.C.; Zimba, P.V.; Everitt, J.H. Remote sensing techniques to assess water quality. Photogramm. Eng. Remote Sens. 2003, 69, 695–704. [Google Scholar] [CrossRef]
- Song, K.; Li, L.; Tedesco, L.P.; Li, S.; Hall, B.E.; Du, J. Remote quantification of phycocyanin in potable water sources through an adaptive model. ISPRS J. Photogramm. Remote Sens. 2014, 95, 68–80. [Google Scholar] [CrossRef]
- Tyler, A.; Svab, E.; Preston, T.; Présing, M.; Kovács, W. Remote sensing of the water quality of shallow lakes: A mixture modelling approach to quantifying phytoplankton in water characterized by high-suspended sediment. Int. J. Remote Sens. 2006, 27, 1521–1537. [Google Scholar] [CrossRef]
- Zhou, L.G.; Roberts, D.A.; Ma, W.C.; Zhang, H.; Tang, L. Estimation of higher chlorophylla concentrations using field spectral measurement and hj-1a hyperspectral satellite data in dianshan lake, china. ISPRS J. Photogramm. Remote Sens. 2014, 88, 41–47. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Dall’Olmo, G.; Moses, W.; Rundquist, D.C.; Barrow, T.; Fisher, T.R.; Gurlin, D.; Holz, J. A simple semi-analytical model for remote estimation of chlorophyll-a in turbid waters: Validation. Remote Sens. Environ. 2008, 112, 3582–3593. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Song, K.; Li, Y.; Tedesco, L.P.; Shi, K.; Li, Z. An inversion model for deriving inherent optical properties of inland waters: Establishment, validation and application. Remote Sens. Environ. 2013, 135, 150–166. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Song, K. Remote sensing of freshwater cyanobacteria: An extended iop inversion model of inland waters (IIMIW) for partitioning absorption coefficient and estimating phycocyanin. Remote Sens. Environ. 2015, 157, 9–23. [Google Scholar] [CrossRef]
- Simis, S.G.; Peters, S.W.; Gons, H.J. Remote sensing of the cyanobacterial pigment phycocyanin in turbid inland water. Limnol. Oceanogr. 2005, 50, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Simis, S.G.; Ruiz-Verdú, A.; Domínguez-Gómez, J.A.; Peña-Martinez, R.; Peters, S.W.; Gons, H.J. Influence of phytoplankton pigment composition on remote sensing of cyanobacterial biomass. Remote Sens. Environ. 2007, 106, 414–427. [Google Scholar] [CrossRef]
- Hunter, P.D.; Tyler, A.N.; Carvalho, L.; Codd, G.A.; Maberly, S.C. Hyperspectral remote sensing of cyanobacterial pigments as indicators for cell populations and toxins in eutrophic lakes. Remote Sens. Environ. 2010, 114, 2705–2718. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, B.; Yang, W.; Yu, G.; Oyama, Y.; Yoshimura, K.; Fukushima, T. A hybrid algorithm for estimating the chlorophyll-a concentration across different trophic states in asian inland waters. ISPRS J. Photogramm. Remote Sens. 2015, 102, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Mishra, D.R.; Schluchter, W.M. A novel algorithm for predicting phycocyanin concentrations in cyanobacteria: A proximal hyperspectral remote sensing approach. Remote Sens. 2009, 1, 758–775. [Google Scholar] [CrossRef]
- Vincent, R.K.; Qin, X.; McKay, R.M.L.; Miner, J.; Czajkowski, K.; Savino, J.; Bridgeman, T. Phycocyanin detection from landsat tm data for mapping cyanobacterial blooms in Lake Erie. Remote Sens. Environ. 2004, 89, 381–392. [Google Scholar] [CrossRef]
- Duan, H.; Ma, R.; Hu, C. Evaluation of remote sensing algorithms for cyanobacterial pigment retrievals during spring bloom formation in several lakes of East China. Remote Sens. Environ. 2012, 126, 126–135. [Google Scholar] [CrossRef]
- Pyo, J.; Pachepsky, Y.; Baek, S.-S.; Kwon, Y.; Kim, M.; Lee, H.; Park, S.; Cha, Y.; Ha, R.; Nam, G. Optimizing semi-analytical algorithms for estimating chlorophyll-a and phycocyanin concentrations in inland waters in Korea. Remote Sens. 2017, 9, 542. [Google Scholar] [CrossRef]
- Watanabe, F.; Mishra, D.R.; Astuti, I.; Rodrigues, T.; Alcântara, E.; Imai, N.N.; Barbosa, C. Parametrization and calibration of a quasi-analytical algorithm for tropical eutrophic waters. ISPRS J. Photogramm. Remote Sens. 2016, 121, 28–47. [Google Scholar] [CrossRef]
- Roessner, S.; Segl, K.; Heiden, U.; Kaufmann, H. Automated differentiation of urban surfaces based on airborne hyperspectral imagery. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1525–1532. [Google Scholar] [CrossRef]
- Segl, K.; Roessner, S.; Heiden, U.; Kaufmann, H. Fusion of spectral and shape features for identification of urban surface cover types using reflective and thermal hyperspectral data. ISPRS J. Photogramm. Remote Sens. 2003, 58, 99–112. [Google Scholar] [CrossRef]
- Selige, T.; Böhner, J.; Schmidhalter, U. High resolution topsoil mapping using hyperspectral image and field data in multivariate regression modeling procedures. Geoderma 2006, 136, 235–244. [Google Scholar] [CrossRef]
- Shippert, P. Introduction to hyperspectral image analysis. Online J. Space Commun. 2003, 28, 1–13. [Google Scholar]
- Wang, F.; Gao, J.; Zha, Y. Hyperspectral sensing of heavy metals in soil and vegetation: Feasibility and challenges. ISPRS J. Photogramm. Remote Sens. 2018, 136, 73–84. [Google Scholar] [CrossRef]
- Richter, R. Atmospheric correction of dais hyperspectral image data. Comput. Geosci. 1996, 22, 785–793. [Google Scholar] [CrossRef]
- Thiemann, S.; Kaufmann, H. Lake water quality monitoring using hyperspectral airborne data—A semiempirical multisensor and multitemporal approach for the mecklenburg lake district, germany. Remote Sens. Environ. 2002, 81, 228–237. [Google Scholar] [CrossRef]
- Berk, A.; Conforti, P.; Kennett, R.; Perkins, T.; Hawes, F.; van den Bosch, J. Modtran® 6: A major upgrade of the modtran® radiative transfer code. In Proceedings of the 2014 6th Workshop on Hyperspectral Image and Signal Processing: Evolution in Remote Sensing (WHISPERS), Lausanne, Switzerland, 24–27 June 2014; pp. 1–4. [Google Scholar]
- Giardino, C.; Brando, V.E.; Dekker, A.G.; Strömbeck, N.; Candiani, G. Assessment of water quality in Lake Garda (Italy) using hyperion. Remote Sens. Environ. 2007, 109, 183–195. [Google Scholar] [CrossRef]
- Goyens, C.; Jamet, C.; Schroeder, T. Evaluation of four atmospheric correction algorithms for modis-aqua images over contrasted coastal waters. Remote Sens. Environ. 2013, 131, 63–75. [Google Scholar] [CrossRef]
- Schroeder, T.; Fischer, J.; Schaale, M.; Fell, F. Artificial-Neural-Network-Based Atmospheric Correction Algorithm: Application to Meris data. In Ocean Remote Sensing and Applications; International Society for Optics and Photonics: Bellingham, WA, USA, 2003; pp. 124–133. [Google Scholar]
- Schroeder, T.; Behnert, I.; Schaale, M.; Fischer, J.; Doerffer, R. Atmospheric correction algorithm for meris above case-2 waters. Int. J. Remote Sens. 2007, 28, 1469–1486. [Google Scholar] [CrossRef]
- Bernstein, L.S.; Adler-Golden, S.M.; Sundberg, R.L.; Levine, R.Y.; Perkins, T.C.; Berk, A.; Ratkowski, A.J.; Felde, G.; Hoke, M.L. Validation of the quick atmospheric correction (QUAC) algorithm for vnir-swir multi-and hyperspectral imagery. In Algorithms and Technologies for Multispectral, Hyperspectral, and Ultraspectral Imagery XI; International Society for Optics and Photonics: Bellingham, WA, USA, 2005; pp. 668–679. [Google Scholar]
- Gao, B.-C.; Montes, M.J.; Davis, C.O.; Goetz, A.F. Atmospheric correction algorithms for hyperspectral remote sensing data of land and ocean. Remote Sens. Environ. 2009, 113, S17–S24. [Google Scholar] [CrossRef]
- Jaelani, L.M.; Matsushita, B.; Yang, W.; Fukushima, T. An improved atmospheric correction algorithm for applying meris data to very turbid inland waters. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 128–141. [Google Scholar] [CrossRef]
- Pons, X.; Pesquer, L.; Cristóbal, J.; González-Guerrero, O. Automatic and improved radiometric correction of landsat imagery using reference values from modis surface reflectance images. Int. J. Appl. Earth Obs. Geoinf. 2014, 33, 243–254. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, E.; Paul, M.; Tamm, A.; Caesar, J.; Büdel, B.; Escribano, P.; Hill, J.; Weber, B. Biomass assessment of microbial surface communities by means of hyperspectral remote sensing data. Sci. Total Environ. 2017, 586, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, R.M.; Grenzdoerffer, G.; Bill, R.; Schubert, H.; Bachmann, M.; Lennartz, B. Determination of chlorophyll content of small water bodies (kettle holes) using hyperspectral airborne data. Int. J. Appl. Earth Obs. Geoinf. 2011, 13, 912–921. [Google Scholar] [CrossRef]
- Jupp, D.L.; Kirk, J.T.; Harris, G.P. Detection, identification and mapping of cyanobacteria—Using remote sensing to measure the optical quality of turbid inland waters. Mar. Freshw. Res. 1994, 45, 801–828. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Trujillo, J.; de la Orden, M.S.; Hernández-Clemente, R. Hyperspectral and multispectral satellite sensors for mapping chlorophyll content in a mediterranean pinus sylvestris l. Plantation. Int. J. Appl. Earth Obs. Geoinf. 2014, 26, 88–96. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, Y.; Li, Y.; Li, L.; Lv, H.; Liu, X. Remote estimation of cyanobacteria-dominance in inland waters. Water Res. 2015, 68, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, P. Caas: An atmospheric correction algorithm for the remote sensing of complex waters. In Annales Geophysicae; Copernicus GmbH: Göttingen, Germany, 2012; p. 203. [Google Scholar]
- Duan, H.; Tao, M.; Loiselle, S.A.; Zhao, W.; Cao, Z.; Ma, R.; Tang, X. Modis observations of cyanobacterial risks in a eutrophic lake: Implications for long-term safety evaluation in drinking-water source. Water Res. 2017, 122, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, L.-L.; Li, J.; Chen, M.; Zhang, Y.-W. Mechanism of the influence of hydrodynamics on microcystis aeruginosa, a dominant bloom species in reservoirs. Sci. Total Environ. 2018, 636, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.U.; Gopalaswamy, G.; Raghu, R. A simple method for efficient extraction and purification of c-phycocyanin from spirulina platensis geitler. Indian J. Exp. Biol. 2005, 43, 277–279. [Google Scholar] [PubMed]
- Arnon, D.I.; McSwain, B.D.; Tsujimoto, H.Y.; Wada, K. Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim. Biophys. Acta Bioenerg. 1974, 357, 231–245. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Association, A.P.H.; Association, A.W.W.; Federation, W.P.C.; Federation, W.E. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1915; Volume 2. [Google Scholar]
- Richter, R.; Schläpfer, D. Geo-atmospheric processing of airborne imaging spectrometry data. Part 2: Atmospheric/topographic correction. Int. J. Remote Sens. 2002, 23, 2631–2649. [Google Scholar] [CrossRef]
- Tuominen, J.; Lipping, T. Atmospheric correction of hyperspectral data using combined empirical and model based method. In Proceedings of the 7th European association of remote sensing laboratories SIG-imaging spectroscopy workshop, Edinburgh, UK, 11–13 April 2011. [Google Scholar]
- Ruddick, K.G.; Gons, H.J.; Rijkeboer, M.; Tilstone, G. Optical remote sensing of chlorophyll a in case 2 waters by use of an adaptive two-band algorithm with optimal error properties. Appl. Opt. 2001, 40, 3575–3585. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Cherkauer, K.A.; Chaubey, I. Using hyperspectral data to quantify water-quality parameters in the wabash river and its tributaries, indiana. Int. J. Remote Sens. 2015, 36, 5466–5484. [Google Scholar] [CrossRef]
- Olmanson, L.G.; Brezonik, P.L.; Bauer, M.E. Airborne hyperspectral remote sensing to assess spatial distribution of water quality characteristics in large rivers: The mississippi river and its tributaries in minnesota. Remote Sens. Environ. 2013, 130, 254–265. [Google Scholar] [CrossRef]
- Buiteveld, H.; Hakvoort, J.; Donze, M. Optical properties of pure water. In Ocean Optics XII; International Society for Optics and Photonics: Bellingham, WA, USA, 1994; pp. 174–184. [Google Scholar]
- Adler-Golden, S.M.; Matthew, M.W.; Bernstein, L.S.; Levine, R.Y.; Berk, A.; Richtsmeier, S.C.; Acharya, P.K.; Anderson, G.P.; Felde, J.W.; Gardner, J. Atmospheric correction for shortwave spectral imagery based on modtran4. In Imaging Spectrometry V; International Society for Optics and Photonics: Bellingham, WA, USA, 1999; pp. 61–70. [Google Scholar]
- Hadjimitsis, D.G.; Clayton, C.; Hope, V. An assessment of the effectiveness of atmospheric correction algorithms through the remote sensing of some reservoirs. Int. J. Remote Sens. 2004, 25, 3651–3674. [Google Scholar] [CrossRef]
- Gitelson, A.; Garbuzov, G.; Szilagyi, F.; Mittenzwey, K.; Karnieli, A.; Kaiser, A. Quantitative remote sensing methods for real-time monitoring of inland waters quality. Int. J. Remote Sens. 1993, 14, 1269–1295. [Google Scholar] [CrossRef]
- Chen, W.-T.; Zhang, Z.; Wang, Y.-X.; Wen, X.-P. Atmospheric Correction of spot5 Land Surface Imagery. In Proceedings of the 2009 2nd International Congress on Image and Signal Processing, Tianjin, China, 17–19 October 2009; pp. 1–5. [Google Scholar]
- Kaufman, Y.J. Atmospheric effects on remote sensing of surface reflectance. In Remote Sensing: Critical Review of Technology; International Society for Optics and Photonics: Bellingham, WA, USA, 1984; pp. 20–34. [Google Scholar]
- Kaufman, Y.; Wald, A.; Remer, L.; Gao, B.-C.; Li, R.-R.; Flynn, L. The Modis 2.1-μm Channel-Correlation with Visible Reflectancefor Use in Remote Sensing of Aerosol Geoscience and Remote Sensing; IEEE: Piscataway, NJ, USA, 1997; Volume 35. [Google Scholar]
- Kutser, T.; Metsamaa, L.; Dekker, A.G. Influence of the vertical distribution of cyanobacteria in the water column on the remote sensing signal. Estuar. Coast. Shelf Sci. 2008, 78, 649–654. [Google Scholar] [CrossRef]
- Bricaud, A.; Babin, M.; Morel, A.; Claustre, H. Variability in the chlorophyll-specific absorption coefficients of natural phytoplankton: Analysis and parameterization. J. Geophys. Res. Oceans 1995, 100, 13321–13332. [Google Scholar] [CrossRef]
- Garver, S.A.; Siegel, D.A.; Greg, M.B. Variability in near-surface particulate absorption spectra: What can a satellite ocean color imager see? Limnol. Oceanogr. 1994, 39, 1349–1367. [Google Scholar] [CrossRef] [Green Version]
- Yentsch, C.S.; Phinney, D.A. A bridge between ocean optics and microbial ecology. Limnol. Oceanogr. 1989, 34, 1694–1705. [Google Scholar] [CrossRef] [Green Version]
- Cromar, N.J.; Fallowfield, H.J. Effect of nutrient loading and retention time on performance of high rate algal ponds. J. Appl. Phycol. 1997, 9, 301–309. [Google Scholar] [CrossRef]
- Soares, M.C.S.; Marinho, M.M.; Huszar, V.L.; Branco, C.W.; Azevedo, S.M. The effects of water retention time and watershed features on the limnology of two tropical reservoirs in Brazil. Lakes Reserv. Res. Manag. 2008, 13, 257–269. [Google Scholar] [CrossRef]
- Soares, M.C.S.; Marinho, M.M.; Azevedo, S.M.; Branco, C.W.; Huszar, V.L. Eutrophication and retention time affecting spatial heterogeneity in a tropical reservoir. Limnol.-Ecol. Manag. Inland Waters 2012, 42, 197–203. [Google Scholar] [CrossRef]
- Anctil, F.; Michel, C.; Perrin, C.; Andreassian, V. A soil moisture index as an auxiliary ann input for stream flow forecasting. J. Hydrol. 2004, 286, 155–167. [Google Scholar] [CrossRef]
















| Date | Point | Airborne Campaign | Min/Max PC Concentration * | Min/Max Chl-a Concentration * | Min/Max PC:Chl-a | Min/Max TSS Concentration ** |
|---|---|---|---|---|---|---|
| 12 August 2016 | 18 | Implemented | 6.25/150.90 | 14.19/111.40 | 0.32/1.91 | 6.27/40.14 |
| 24 August 2016 | 19 | Implemented | 12.48/100.00 | 25.95/61.44 | 0.28/2.72 | 10.13/23.33 |
| 20 September 2016 | 17 | Implemented | 0.83/1.64 | 11.85/60.88 | 0.025/0.089 | 11.47/19.33 |
| 14 October 2016 | 20 | Implemented | 0.19/0.88 | 13.74/46.17 | 0.0062/0.047 | 13.60/19.60 |
| Reflectance Error (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 August 2016 | 24 August 2016 | 20 September 2016 | 14 October 2016 | |||||||||
| Band | MOD * | ATC ** | ANN | MOD * | ATC ** | ANN | MOD * | ATC ** | ANN | MOD * | ATC ** | ANN |
| 439 nm | 16.50 | 99.97 | 18.74 | 13.19 | 99.95 | 21.21 | 20.40 | 99.96 | 34.43 | 141.23 | 99.67 | 190.50 |
| 443 nm | 15.54 | 99.97 | 18.20 | 13.09 | 99.95 | 21.54 | 15.51 | 99.96 | 31.41 | 115.49 | 99.66 | 151.80 |
| 534 nm | 5.91 | 99.97 | 12.40 | 8.31 | 99.92 | 8.73 | 3.12 | 99.94 | 11.00 | 26.40 | 99.63 | 35.47 |
| 599 nm | 8.03 | 99.97 | 11.78 | 9.64 | 99.94 | 10.85 | 3.17 | 99.92 | 9.22 | 22.27 | 99.72 | 25.72 |
| 618 nm | 7.49 | 99.97 | 12.69 | 9.91 | 99.95 | 11.75 | 2.95 | 99.94 | 8.25 | 23.50 | 99.78 | 28.81 |
| 622 nm | 7.26 | 99.97 | 13.47 | 9.65 | 99.95 | 12.74 | 2.58 | 99.94 | 8.62 | 23.76 | 99.79 | 30.19 |
| 627 nm | 7.75 | 99.97 | 13.36 | 9.70 | 99.95 | 11.56 | 3.26 | 99.94 | 12.53 | 24.30 | 99.80 | 33.91 |
| 660 nm | 9.16 | 99.97 | 14.73 | 10.39 | 99.96 | 11.15 | 2.96 | 99.96 | 12.00 | 29.70 | 99.86 | 40.24 |
| 674 nm | 8.23 | 99.97 | 13.09 | 10.99 | 99.96 | 13.22 | 6.07 | 99.97 | 10.79 | 36.34 | 99.88 | 52.74 |
| 708 nm | 7.25 | 99.97 | 13.86 | 7.15 | 99.94 | 10.16 | 3.04 | 99.95 | 10.23 | 26.65 | 99.87 | 28.21 |
| 755 nm | 21.47 | 99.97 | 23.30 | 9.62 | 99.97 | 15.83 | 6.63 | 99.99 | 16.14 | 277.45 | 99.95 | 373.29 |
| 779 nm | 22.10 | 99.96 | 23.15 | 9.23 | 99.97 | 19.76 | 5.48 | 99.98 | 16.96 | 373.08 | 99.95 | 508.48 |
| PC | MODTRAN 6 | ATCOR 4 | ANN | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | Bias | R2 | RMSE | Bias | R2 | RMSE | Bias | |
| Band (2) | 0.75 | 14.57 | −0.0025 | 0.68 | 18.33 | 2.48 | 0.57 | 18.51 | 0.95 |
| Band (3) | 0.68 | 15.97 | 0.0673 | 0.62 | 18.25 | 2.14 | 0.55 | 18.88 | 0.49 |
| Li | 0.76 | 20.13 | 14.26 | 0.34 | 60.74 | 17.06 | 0.56 | 25.69 | 16.97 |
| Simis | 0.77 | 14.90 | 2.56 | 0.50 | 22.68 | 11.00 | 0.37 | 23.18 | 4.57 |
| Chl-a | R2 | RMSE | Bias | R2 | RMSE | Bias | R2 | RMSE | Bias |
| Band (2) | 0.49 | 12.24 | −5.44 | 0.29 | 13.91 | −4.94 | 0.46 | 13.03 | −6.54 |
| Band (3) | 0.51 | 11.03 | −3.01 | 0.25 | 13.63 | −2.90 | 0.56 | 11.09 | −4.35 |
| Li | 0.53 | 10.62 | −1.71 | 0.025 | 156.73 | 150.82 | 0.52 | 10.90 | −2.30 |
| Simis | 0.53 | 10.88 | −1.20 | 0.29 | 13.17 | −2.08 | 0.53 | 11.19 | −1.96 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyo, J.C.; Ligaray, M.; Kwon, Y.S.; Ahn, M.-H.; Kim, K.; Lee, H.; Kang, T.; Cho, S.B.; Park, Y.; Cho, K.H. High-Spatial Resolution Monitoring of Phycocyanin and Chlorophyll-a Using Airborne Hyperspectral Imagery. Remote Sens. 2018, 10, 1180. https://doi.org/10.3390/rs10081180
Pyo JC, Ligaray M, Kwon YS, Ahn M-H, Kim K, Lee H, Kang T, Cho SB, Park Y, Cho KH. High-Spatial Resolution Monitoring of Phycocyanin and Chlorophyll-a Using Airborne Hyperspectral Imagery. Remote Sensing. 2018; 10(8):1180. https://doi.org/10.3390/rs10081180
Chicago/Turabian StylePyo, Jong Cheol, Mayzonee Ligaray, Yong Sung Kwon, Myoung-Hwan Ahn, Kyunghyun Kim, Hyuk Lee, Taegu Kang, Seong Been Cho, Yongeun Park, and Kyung Hwa Cho. 2018. "High-Spatial Resolution Monitoring of Phycocyanin and Chlorophyll-a Using Airborne Hyperspectral Imagery" Remote Sensing 10, no. 8: 1180. https://doi.org/10.3390/rs10081180