1. Introduction
A long breeding cycle and slow rate of genetic improvement are constraining the development of improved sugarcane varieties to meet the rising demand for sucrose and bioenergy around the world. Indeed, in the past few decades, genetic gain in sugarcane yield and sucrose content appears to have stagnated across most sugarcane breeding programs [
1,
2]. The principal objective of breeding programs worldwide is to deliver productive, sustainable, and profitable varieties to maximise the economic returns. Development of new sugarcane varieties is resources intense and time-consuming, taking 12–14 years from crossing to variety release. Typically, sugarcane breeding programs rely on end-of-season yield alone for clonal selections because measuring growth at different stages of crop cycle is not feasible using conventional approaches. High-throughput phenotyping using unmanned aerial vehicles (UAV) and remote sensors provides an opportunity to overcome the phenotyping bottlenecks in traditional breeding programs [
3]. They may bring about a step-change in genetic gain by improving the efficiency and effectiveness of clonal selection systems by shifting the traditional plant phenotyping paradigm that is labour intensive and relatively low accuracy, especially at early stages of selection, to an approach that is more comprehensive, high-throughput, and accurate [
4].
Among the several challenges, one of the main constraints of traditional breeding programs is the low heritability of yield in the early stages of selection and its poor genetic correlation with yield in pure-stand [
5]. In early-stage selection, small plots with one or two rows are typically used to maximise the sampling for population size [
6]. Limitations in estimating true yield potential of clones in the early stages of breeding due to large inter-plot competition have long been recognised [
7,
8]. Results from studies of inter-plot competition demonstrate that large variance associated with between-genotype competition can significantly bias early generation selection in small plots [
5]. Significant competition effects were observed among sugarcane families grown in single-row plots with the suggestion that 10% of families might be overlooked if not accounted for competition effects [
9]. Statistical models to quantify the bias caused by competition in single-row plot trials to improve the efficiency of selection have been suggested [
10,
11]. However, they do not account for all the competition effects, particularly when evaluating the yield of a genetically diverse population with limited replications and do not explain the physical or biological causes. The large residual error variation, compared to genetic variation, associated with limited replications and inter-plot competition in the early stage of sugarcane breeding trials, reduces the accuracy and efficiency of selection.
Sugarcane breeding could be greatly accelerated if yield potential can be quantitatively assessed during early growth prior to the onset of inter-plot competition effects. One approach to improve the prediction of yield potential in early stages of selection is by either complementing or substituting yield measured at harvest with yield-correlated indirect traits measured over multiple time points during the growing season. Rebetzke et al. [
12] considered early-stage plant vigour via larger specific leaf area as an indirect selection target in wheat, and more recently, Kipp et al. [
13] and Duan et al. [
14] reported a high-throughput scoring method for early vigour using visual images that may improve wheat selections at early growth stage. Further, a strong correlation of sugarcane stalk population and yield with normalised difference vegetation index (NDVI) and reflectance at specific wavelengths was reported, suggesting that canopy reflectance measurements at the early growth stage can be used as a screening tool to estimate yield potential [
15]. In maize, combining data from 62 wavebands and vegetation indices measured across multiple times using aerial phenotyping lead to an increase in prediction accuracy compared with using single-time-point data [
16].
The ability to quantitatively assess adaptive mechanisms and traits driven by variable water environments offers significant value for selection programs [
17,
18]. In sugarcane, the importance of specific physiological traits, such as stomatal conductance [
18], canopy temperature [
19], and transpiration efficiency [
20,
21], from a breeding perspective have been discussed in the past [
22]. Extensive measurements of canopy conductance, the product of leaf area and stomatal conductance, in sugarcane made during the early growth stage (2–6 months) had a range of genetic correlation (
rg: −0.29 to 0.94) with average cane yield in multi-row plots across multiple environments [
18]. The broad-sense heritability of canopy conductance was relatively high (H
2b: 0.8 to 0.9, in some environments), which makes it a good yield predictor in early-stage sugarcane breeding trials when plot yield from small plots itself has low heritability and poor genetic correlation with yield in pure-stand conditions. Canopy temperature, a surrogate trait for canopy conductance, has been previously monitored in sugarcane, and it showed a significant genotypic variation and a strong negative genetic correlation with biomass [
19,
23].
Selection based on a linear combination of indirect traits that are less affected by inter-plot competition effects and that have high covariance with cane yield will likely improve prediction of the true genetic value of clones in early-stage selections. This is because cane yield estimation in small plots in early-stage selection trials can be biased and prone to inter-plot competition effects, as described above. A selection index is designed to put together information in an optimal way to enable selection of individuals likely to have the highest overall performance for the traits of interest. Implications of applying a multi-traits indirect selection index have been studied and reviewed extensively in cereals [
24]. In sugarcane, Yang et al. [
25] provided a theoretical assessment of applying a multi-trait selection system in conventional breeding in China and predicted their value across the production chain. However, optimal selection indices based on indirect traits are not widely applied in breeding programs, partly due to the practical difficulties in measuring multiple traits from a large number of clones in large-scale breeding programs.
In recent years, rapid advances in field-based high-throughput phenotyping have enabled precise and rapid measurement of multiple traits in breeding programs that would otherwise be impractical using traditional approaches [
26]. Unmanned aerial vehicles fitted with remote sensors are affordable and are becoming an increasingly crucial component of plant phenotyping for crop production and crop improvement [
27]. The operational cost of a UAV is much lower than manned aircraft, satellites, or manual data collection, and it allows information collection from all crop stages, unlike ground vehicles that are inoperable after 3–4 months of crop growth in sugarcane. To our knowledge, little or no previous assessment has been done on the potential use of UAV-based high-throughput phenotyping for improving sugarcane clone selection in breeding and related activities.
The objective of this study was to evaluate the potential of indirect traits, viz. canopy height, canopy cover, NDVI, and canopy temperature, measured using UAV-based high-throughput phenotyping to assess clonal performance in early-stage sugarcane selection trials. We demonstrate that high-throughput UAV-based field phenotyping is a potentially effective strategy to overcome certain barriers of genetic gain in conventional sugarcane breeding.
2. Materials and Methods
In this study, multiple sensors mounted on a UAV were used to monitor an early stage single-row experiment and a multi-row experiment representative of pure-stand conditions on multiple occasions for the indirect traits canopy height, canopy cover, NDVI, and canopy temperature. Heritability of the indirect traits were estimated in both experiments, and genetic correlation of the indirect traits with single-row and multi-row yield was estimated. An indirect selection index was developed using phenotypic variance-covariance of the traits estimated in the single-row experiment, and genetic covariance of indirect traits with the trait of economic value, i.e., pure-stand yield, in the multi-row experiment. The clones that were likely to be selected using the indirect selection index and the single-row yield-based selection were compared. Finally, correlated response to selection for pure-stand yield based on indirect selection index and single-row yield were estimated. In addition, indirect traits and yield of the potential selections were compared with known commercially-grown standard cultivars to investigate their relative value in environments with different water availability. These are detailed in the following sections.
2.1. Single-Row Experiment
This experiment was carried out within the Sugar Research Australia (SRA) regional breeding program in Burdekin, Queensland, Australia (19°34.0926′ S 147°19.3842′ E). A stage 2 variety selection trial, termed clonal assessment trial, was established with 2134 clones in August 2016. The clones were vegetatively propagated and planted using a whole stick planter in 6 m single-row plots with a row-spacing of 1.52 m and plots within a row separated by 0.5 m. Two replicates of each clone were in a randomised complete block design. Regional sugar industry best management practices were followed for irrigation, fertiliser applications, and weed and pest control. The experiment received 0.5 ML/ha of water at each irrigation event through furrows at approximately three-week intervals. To obtain cane yield, each single-row plot was harvested (single-row yield) with a commercial harvester and weighed using an electronic weigh bin in September 2017. The harvested crop was ratooned (regrown from the crop stubble for the next crop cycle) twice. Weather data for the growing period was obtained from a ‘Bureau of Meteorology’ weather station approx. 8 km away from the field experiment (
longpaddock.qld.gov.au/silo/).
2.2. Multi-Row Experiment
A four-row field experiment was established in July 2017 with a population of 40 genetically diverse genotypes containing both commercial and unselected clones in 3 replicates in a randomised block design; an unselected clone and 4 commercial genotypes were in common with the single-row experiment. Each experimental plot was 11 m long and 4 rows wide with a row spacing of 1.52 m and plot spacing of 0.5 m. This experiment was also located in Burdekin (19°35.9485′ S 147°18.0538′ E) and was maintained like the single-row experiment. In July 2018, the middle two rows of each plot were mechanically harvested to estimate pure-stand yield [
7].
2.3. Ground-Truthing
A sub-population of 75 clones in two replications (150 plots) within the single-row experiment was used for comparing manual measurements of plant height, stalk number, and early biomass with UAV estimates. Manual and UAV data were collected simultaneously from the sub-population in February 2017, approximately six months after planting.
Early biomass was determined by harvesting a sub-sample of 6 stalks from each plot, partitioned to leaf and stem portions and dried in an oven at 60 °C until constant weight. The product of total leaf and stem dry weights and the number of stalks per plot was calculated to estimate early biomass per plot.
Stalk number, an important yield determining trait in sugarcane [
28], was manually counted in each 6 m plot of the sub-population. Plant height was measured manually using a ruler in a sub-sample of 4 plants per plot in the sub-population.
2.4. UAV Mission
A customised quadcopter (DJI Matrice 100) with a flight time of 15 min and a payload capacity of approx. 1.2 kg was used. Date and time, height, and ground sampling distance (GSD) of each UAV flight is shown in
Table 1. Sixteen flights were conducted in the single-row experiment from November 2016 to December 2018, involving a plant crop and two ratoon crops. In the multi-row experiment, three flights were conducted at the 4- and 6-months stage in the plant crop, which provided data for estimating early-stage indirect traits genetic correlation with the pure-stand yield.
Multispectral images were captured using a five narrow-band camera (MicaSense, Inc., USA); the bands centred at 475 nm, 560 nm, 668 nm, 717 nm, and 840 nm corresponding to the blue, green, red, red edge, and near-infrared (NIR) regions, respectively. Images of a calibration panel captured before and after each flight along with a sun irradiance sensor mounted on the UAV were used for calibrating multispectral images. Thermal images were captured using a FLIR TAU2 640 (13 mm) camera that captures images in the long-wave infrared region (7.5 µm to 13.5 µm) at a resolution of 640 × 512 pixels. The camera was integrated with a capture board (TeAx ThermalCapture, Germany) that records and stores temperature and location data onto a removable flash memory card on-board the UAV as well as perform automatic flat-field corrections. The UAV was customised to carry two cameras at a time; therefore, in each flight, visual and either multispectral or thermal images were captured simultaneously.
The UAV was programmed to fly autonomously along a pre-defined single grid path controlled by a ground control station. The UAV mission plan was optimised by varying flight height and speed in order to capture the experimental area within the 15 min flight time, with a GSD less than 5 and 8 cm for the multispectral and thermal camera, respectively, and a minimum overlap of 80% in both X and Y directions. The multispectral and visual cameras captured images at one-second intervals while the thermal camera acquired video at a rate of 9 Hz.
To geo-rectify the images, 6 highly visible ground control points (GCP) were placed in the corners of the experimental block. Coordinates of the GCP were obtained using a global navigation satellite systems (GNSS) receiver connected to a correction network (HxGN SmartNet, Australia) for receiving horizontal and vertical position accuracy of less than 2 cm.
2.5. Image Processing
Individual thermal images were extracted from the thermal video after applying a thermal drift compensation and non-uniformity correction following manufacture instructions (TeAX Technology, Germany). Thermal, multispectral, and visual images were triangulated and mosaicked using a photogrammetric software (Pix4dMapper, Switzerland) based on the structure from motion algorithm to generate an orthomosaic of the experimental field. The orthomosaic images were geo-rectified using the GCP captured within the experimental block.
A digital terrain model (DTM) of bare earth was generated from visual imagery of the first flight after planting, and a digital surface model (DSM) was generated for each flight from visual imagery in Pix4dMapper. Canopy height at each flight was estimated as the difference in DSM to the average DTM.
Canopy cover was estimated from the visual orthomosaic by classifying the orthomosaic to vegetation, soil, and other background using the support vector machine (SVM) algorithm in ENVI 5.5 (Exelis Visual Information Solutions, Boulder, Colorado). In this method, vegetation and background pixels on the visual orthomosaic were specified manually to train the algorithm, and subsequently, all pixels in the orthomosaic were segmented into vegetation and background.
Spectral index NDVI was calculated from the 5 band multispectral images using the NIR and red band reflectance, as shown in Equation (1).
To estimate canopy temperature, the temperature of pixels corresponding to the classified vegetation pixels were extracted from the thermal orthomosaic, and subsequently, Otsu thresholding was applied to remove any mixed pixels (pixels with both vegetation and background) [
29].
Individual plot boundaries were identified on the orthomosaic by segmenting the experimental field into regularly shaped plot polygons along with a buffer around the plots to minimise neighbouring plot edge effects based on a supplied field experimental design, similar to the method described in Haghighattalab et al. [
30]. Mean and other zonal statistics from each plot polygon were extracted from the underlying orthomosaic. The segmentation and zonal statistics operations were performed in R [
31].
2.6. Statistical Analysis
Variance and covariance of all traits were estimated using restricted maximum likelihood (REML) method in the lme4 package in R [
32]. The following statistical model was used to partition the variance components:
where
Yij is the observed value of the
ith genotype in
jth block;
μ is the mean of observations;
gi is the effect of the
ith genotype;
bj is the effect of
jth block, and
eij is experimental error. Best linear unbiased predictors (BLUP) were estimated for each trait and genotype from the model in Equation (2) using the ‘ranef’ (random effect) command in the lme4 package [
32].
The genetic coefficient of variation (GCV %) was calculated as:
where
σg is the square root of genetic variance, and
is the mean.
Genetic and error variances were estimated, and broad-sense heritability (H
2b) from the genotype plot means was estimated for each of the measurements, as described by Fehr [
33]:
where
σ2g and
σ2e are genetic and error variances, and
nr is the number of replications.
Genetic correlations between traits were estimated using the method in Kempthorne [
34]:
where
Covg(xy) is the genetic covariance of the two traits (
x and
y), and
σ2gx and
σ2gy are the genetic variances of the two traits,
x and
y, respectively.
2.7. Selection Methods
Two sets of clones were selected from the single-row experiment based on: 1) single-row yield and 2) indirect selection index. The indirect selection index (
Si) was developed based on the selection index theory, as described in Wei et al. [
35].
where
Xi is the phenotypic values of traits estimated in the single-row experiment, and
bi is the index coefficients of traits that were estimated by the following equation in matrix form.
where
P−1 is an inverse matrix of the phenotypic variance-covariance of indirect traits in the single-row experiment, and
G is a genetic covariance matrix between the indirect traits and pure-stand yield in the multi-row experiment. To construct
G, genetic correlation of indirect traits under pure-stand conditions with yield in pure-stand were obtained from the multi-row experiment, while, Jackson and McRae [
5] had previously determined 0.49 as the genetic correlation of single-row yield with pure-stand yield.
2.8. Response to Selection
The efficiency of indirect selection (
CRx/
Rx) was estimated according to Falconer and Mackay [
36]. Expected response to selection (
Rx) is the genetic improvement of a trait in response to selection upon that trait itself:
where
h2x and
σx are the heritability, and phenotypic standard deviation for the trait, respectively.
Correlated response (
CRx) is the genetic improvement of a target trait in response to selection of an indirectly associated trait:
where
hx and
hy are the square roots of the heritability of the target and indirect trait, respectively,
rgxy is the genetic correlation between the two traits, and
σx is the phenotypic standard deviation for the target trait. In the estimation of these parameters, the selection differential (
i) was considered unity and ignored.
Subsequently, relative efficiency of indirect selection (C
Rx/Rx) was estimated as:
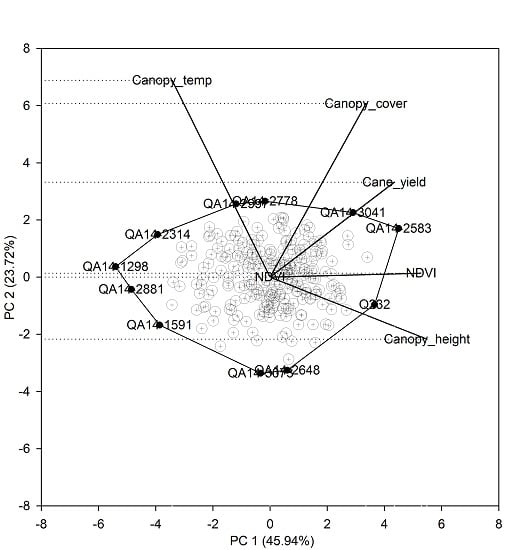
2.9. Principal Component Analysis
Principal component analysis (PCA) was performed for the top 10% of clones (250) selected using the single-row yield based selection. PCA was performed using the ‘princomp’ function in R [
31], and a PCA biplot was generated in SigmaPlot (Systat Software Inc.). A correlation matrix of the indirect traits (canopy height, canopy cover, NDVI, and canopy temperature) and single-row yield were entered in the analysis. A PCA biplot was constructed by plotting the PC1 score against the PC2 score for each clone and trait. The aim of the analysis was to examine the relationship among the indirect traits and single-row yield of clones to evaluate their relative growth and transpiration characteristics.
4. Discussion
This paper describes a cost-effective high throughput phenotyping system that can be used to evaluate sugarcane clone performance in early stage selection trials. The high heritability of the indirect traits and their strong genetic correlation with yield in single-row and in pure-stands indicate the potential of UAV-based high-throughput phenotyping in improving the prediction of the true genetic value of clones and selection accuracy in early stage breeding trials.
Canopy height and canopy cover estimated using UAV were significantly correlated with the ground (manual) measurements of plant height and stalk number, respectively. Predictive ability of UAV and photogrammetric assisted DSM for canopy height has been reported previously [
37,
38]. In this study, we have also demonstrated that UAV-derived canopy cover determined from visual images using the machine learning algorithm SVM is a good predictor of stalk population during the early stage of growth. In addition, canopy height and canopy cover were significantly correlated to biomass at the six-month stage. Manual phenotyping for these traits in large breeding trials is cost and labour intensive and impeded by the destructive sampling requirement for traits, such as biomass. Sugarcane breeders have for long relied on end-of-season yield measurements because, unlike cereal crops, accessing sugarcane plots after the initial stages of growth is very difficult. Therefore, high-throughput phenotyping using UAVs is a cost and time-effective system that enables sugarcane breeders to gather extensive time-series data for comprehensive clone characterisation.
In the early stages of selection, a large number of clones are evaluated in small single-row plots to increase the genetic variation and optimise resources. The inter-plot competition effect in such small single-row plots is substantially high [
7]. These confounding effects on cane yield, besides genetic effects, result in low accuracy of clone selection in early stages of breeding [
5]. Therefore, sugarcane breeders often combine visual assessment of clones with cane yield for early-stage clone selection. Given the high selection intensity and considerable resources devoted to early-stage selection, an accurate and efficient selection system is important to realise genetic gains. We hypothesised that selection based on a linear combination of indirect traits in single-row plots would have a higher genetic correlation with cane yield in pure-stand than selection based on cane yield alone in single-row plots. The broad-sense heritability estimates of the indirect traits were generally high, and in some days of measurements, the heritability was higher than cane yield itself in single-row plots. This indicates that the UAV-based assessment of indirect traits is repeatable across different environments (measurement days). Genetic correlation between the indirect traits and single-row yield was high on individual days and when averaged across the different days of measurement. This result is in contrast to a previous study by Zhao et al. [
39], who reported spectral reflectance indices based on hand-held measurements showed no correlation with yield in sugarcane. Capturing spectral reflectance using UAV at the whole canopy-level rather than at individual leaf-level may have been a contributing factor for the high trait-yield correlation observed in this study, as individual leaf spot measurements do not always represent whole-canopy dynamics, and scaling-up from leaf to whole-canopy can be difficult in a population with different canopy architecture. The high heritability and strong genetic correlation of indirect traits with the single-row yield indicate the possibility of replacing or complementing yield measurement in single-row plots with UAV-assisted phenotyping. Furthermore, to estimate the true yield potential of clones, breeders typically rely on testing the clones across several target production environments over multiple years [
6]. However, in early stages of selection, this is not achievable due to resource restriction, such as the land requirement for testing a large number of clones and limited availability of seed material of the clones. Therefore, breeders are compelled to restrict testing early generations in small plots within single environments, which increases the probability of erroneous selections. Although this is a long-accepted risk in breeding programs, current innovations in field-based high throughput phenotyping offer new avenues to address this limitation. Acquiring repeatable non-destructive measurements over a temporal timescale throughout the cropping cycle will help improve phenotype accuracy and help achieve higher genetic gain compared to a single timepoint measurement of yield at the end of season [
3].
The indirect traits in single-row plots had higher relative efficiency of selection for pure-stand yield than yield measurements in single-row plots (
Table 9). This indicates selection for indirect traits might be a better approach than selection for cane yield in single-row plots. Sugarcane crop undergoes several distinct stages of growth during a cropping season; the grand growth stage is characterised by tiller number stabilisation and stalk elongation before the crop reaches the maturation phase [
40]. During the grand growth phase, sugarcane canopy develops rapidly with most clones typically achieving full canopy closure within 7–8 months after planting [
19]. The declining ability of spectral indices, such as NDVI to discriminate between different clones, is widely reported in other crops as the canopy closes and its spectral reflectance saturates [
41]. We observed a similar phenomenon in this study, where genetic variance and heritability of NDVI was higher in the early stages of growth and declined as the crop reaches canopy closure. Canopy development during the grand growth phase is likely a physiological function of differences in radiation use efficiency and early vigour of clones [
42]. Therefore, UAV-assisted assessment during the early stages of growth could capture critical plant growth processes that differentiate clone vigour and growth before the crop attains physiological maturity.
Genetic correlation between traits may be dependent on the genetic material under study [
36]. To construct the selection index, we estimated the phenotypic variance-covariance matrix in an early-stage clonal selection trial with 2134 clones; whereas the genotypic variance-covariance matrix was estimated from a multi-row field experiment with 40 unrelated clones. The 40 genotypes were of a random population, with a mixture of selected commercial cultivars and unselected clones. The testing environment may have an additional influence on the observed genetic correlation between indirect traits and pure-stand yield. However, the multi-row field experiment was maintained according to the Australian sugar industry best practise standards in terms of nutrient and water application, and pest and disease control. Therefore, the multi-row experiment provided us with a realistic representation of pure-stand yield, and a similar level of genetic correlation between the indirect traits and pure-stand yield is expected with a different set of population. Nevertheless, the genetic correlation between the indirect traits and pure-stand yield will need further validation using a random, genetically diverse, and larger set of population under a range of growing environments before the indirect selection index can be implemented in breeding programs.
We identified significant differences between the selected populations based on the indirect selection index and single-row yield. The variations suggest that the performance of clones before the onset of inter-plot competition could be different from that of the final yield. However, as noted earlier and in other studies [
5], cane yield measurements in single-row plots can be associated with high error variances and, by itself, is not a good indicator of yield potential of clones. This is further supported by the higher genetic correlation of the indirect traits with pure-stand yield than with single-row yield in this study. While we reported the expected response to selection based on the indirect traits, further research will focus on estimating realised genetic gains based on selection for the indirect traits. This involves yield assessment of populations selected in early stage trials using the indirect selection index and single-row yield in pure-stand conditions across several target production environments.
A negative correlation of sugarcane yield with canopy conductance [
18] and its surrogate canopy temperature [
19] has been reported previously; however, a range of genetic correlations from −0.29 to 0.94 was reported [
18]. In this study, cane yield and canopy temperature showed a moderate negative genetic correlation and generally low heritability for canopy temperature. The strong influence of environment and measurement conditions on canopy temperature is evident from this and other studies. While high heritability of canopy temperature has been reported previously in other crops, they have relied largely upon cooled thermal cameras that can only be carried aboard manned aircraft [
43]. Issues associated with lightweight uncooled thermal cameras, such as thermal drift and non-uniformities in images have been reported extensively along with statistical models for correction [
44]. However, despite the statistical corrections, it seems difficult to achieve the accuracy required to measure small temperature differences in crop canopies. Uncooled cameras with higher sensitivity and accuracy; and a better understanding of genotype x environment interactions is needed before applying canopy temperature as a selection criterion in breeding programs. In addition, in this study, a strong negative correlation was observed between canopy temperature and plant height (
Table 6), which is consistent with previous observations in cereal crops [
45]. Canopy height and architecture complexity greatly influence the coupling of canopy and the atmosphere, with taller architecturally complex canopies tending to be better coupled to the atmosphere [
46]. The influence on canopy architecture on sugarcane canopy temperature is not well understood and needs further attention before applying canopy temperature as an indirect selection trait.
Efficient breeding and selection programs are essential for both irrigated and rainfed production environments where water and/or energy restrictions and erratic rainfall patterns are constraining crop productivity. Previous research has shown that physiological traits, such as canopy conductance, canopy temperature, and transpiration efficiency, are potentially important for improving productivity in both irrigated and rainfed environments [
18,
43,
47]. However, progress in using physiological traits in selection systems has mostly been hindered by a lack of cost-effective phenotyping tools. In this context, the clone-by-trait biplot relationship (
Figure 3) in this study provides valuable insight into the relationship between yield and physiological traits such as canopy temperature. For example, unknown test clones can be compared with known standard varieties for yield attributes and canopy temperature. In this instance, the biplot indicated that the clone QA14-2583 and QA14-3041 were as productive and possibly less water-consuming as the standard ones. The Northern region sugarcane breeding program in Australia is strategically focussed on selecting clones for high input conditions, and information regarding water use of clones is generally not generated or considered due to practical difficulties. However, given the low genotype x environment interaction for yield due to water conditions [
20], identification of test clones with contrasting transpiration characteristics and with higher water use efficiency in early stages will aid selection of potential varieties specific for either irrigated, rainfed, or both environments.
Breeding programs worldwide are exploring the possibility of implementing high-throughput phenotyping as an integral component of variety selection process. In this study, we demonstrated a UAV-based high-throughput phenotyping approach for capturing valuable phenotypes in early stages of sugarcane breeding trials that could be used for improving clonal selections. Our future efforts will be focussed on (i) estimating realised genetic gains using indirect traits-based selection index, (ii) estimating environmental interactions and trait coefficients for the indirect selection index in different target production environments, and (iii) facilitating the implementation of high-throughput phenotyping-based clonal selection in the Australian sugarcane breeding programme.