Climate Effects on Vertical Forest Phenology of Fagus sylvatica L., Sensed by Sentinel-2, Time Lapse Camera, and Visual Ground Observations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Hourly Time Lapse Camera Images
2.2.1. Sampling
2.2.2. Pre-Processing
2.3. Ground Observations
2.3.1. Sampling
2.3.2. Pre-Processing
2.4. UAV Data
2.5. Sentinel-2 Data
2.5.1. Data Retrieval
2.5.2. Pre-Processing
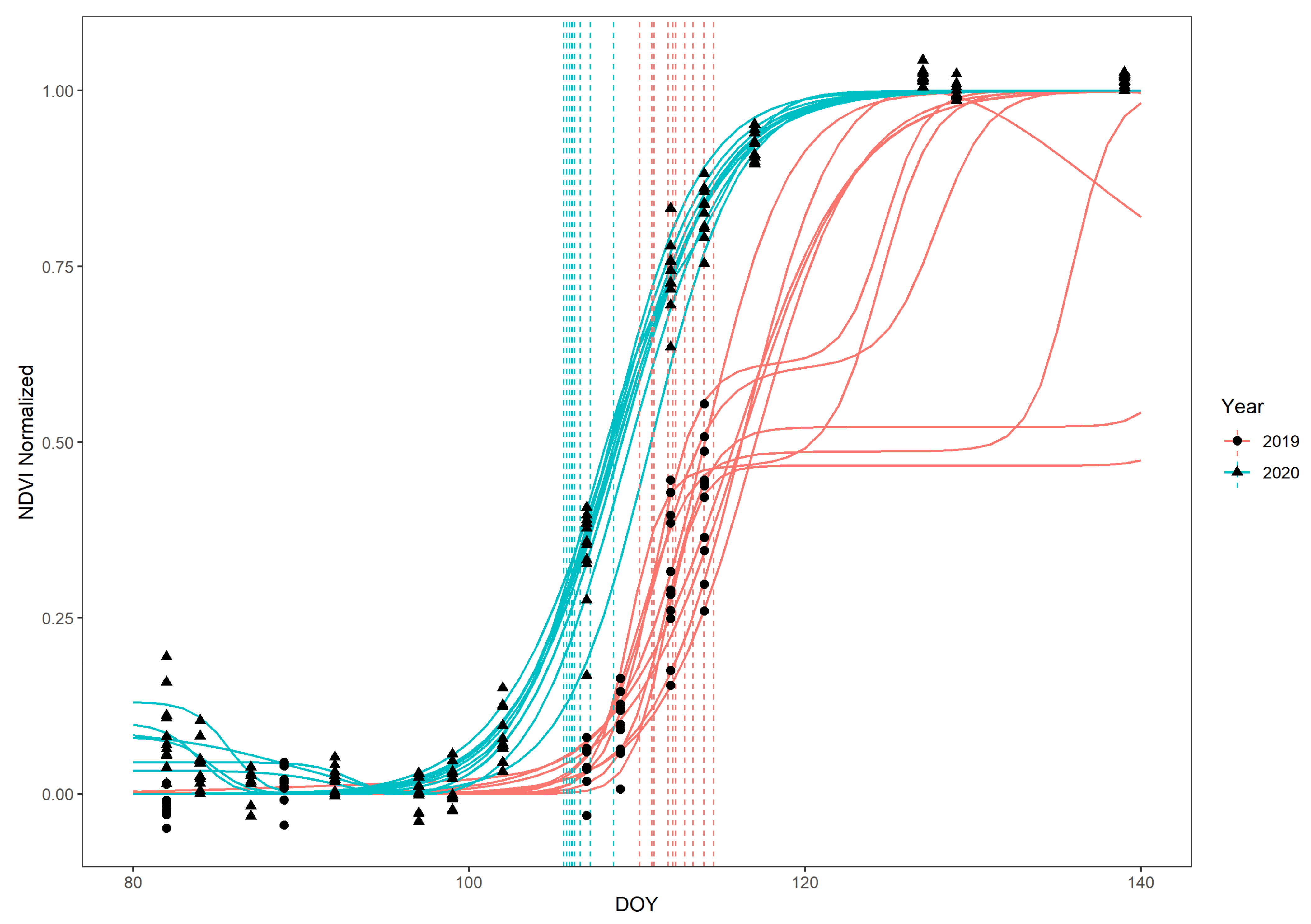
- Data set 1 was generated for the forest stands in which ground observations were also conducted. There, we selected the individuals we did ground observations for, and, additionally, we selected two more random locations per forest stand, selecting all F. sylvatica individuals that fell in an angle-count sample as was done by the German forest inventory “Bundeswaldinventur” [67]. This resulted in a total of 92 trees spread out over the nine quadrants (Table 1). We imported the UAV-acquired RGB orthophotos as a tile layer in ESRI ArcGIS Online, which we enabled for offline use. Then, using the ESRI ArcGIS Collector application on a tablet in the field, we identified all 92 selected trees (ground phenology + additional individuals) on the orthophoto tile layer and manually drew the polygons of the crowns from the selected trees in a separate layer. After this, we extracted the NDVI for each crown at each DOY that had an acquired cloud-free Sentinel-2 image, using bilinear interpolation among the cells where each crown overlapped.
- Data set 2 was generated for quadrant-wide beech forests. We masked the quadrant-wide cloud-free Sentinel-2 NDVI time series by a shapefile, including all forests owned by the Bavarian State Forest agency in the quadrant in which F. sylvatica was the dominant species, according to the Bavarian State Forest agency. This forest type was present in 7 out of the 9 quadrants.
2.6. Environmental Variables
2.6.1. Tree and Canopy Height
2.6.2. Air Temperature
2.6.3. Global Radiation
2.6.4. Canopy Temperature
2.7. Statistical Analysis
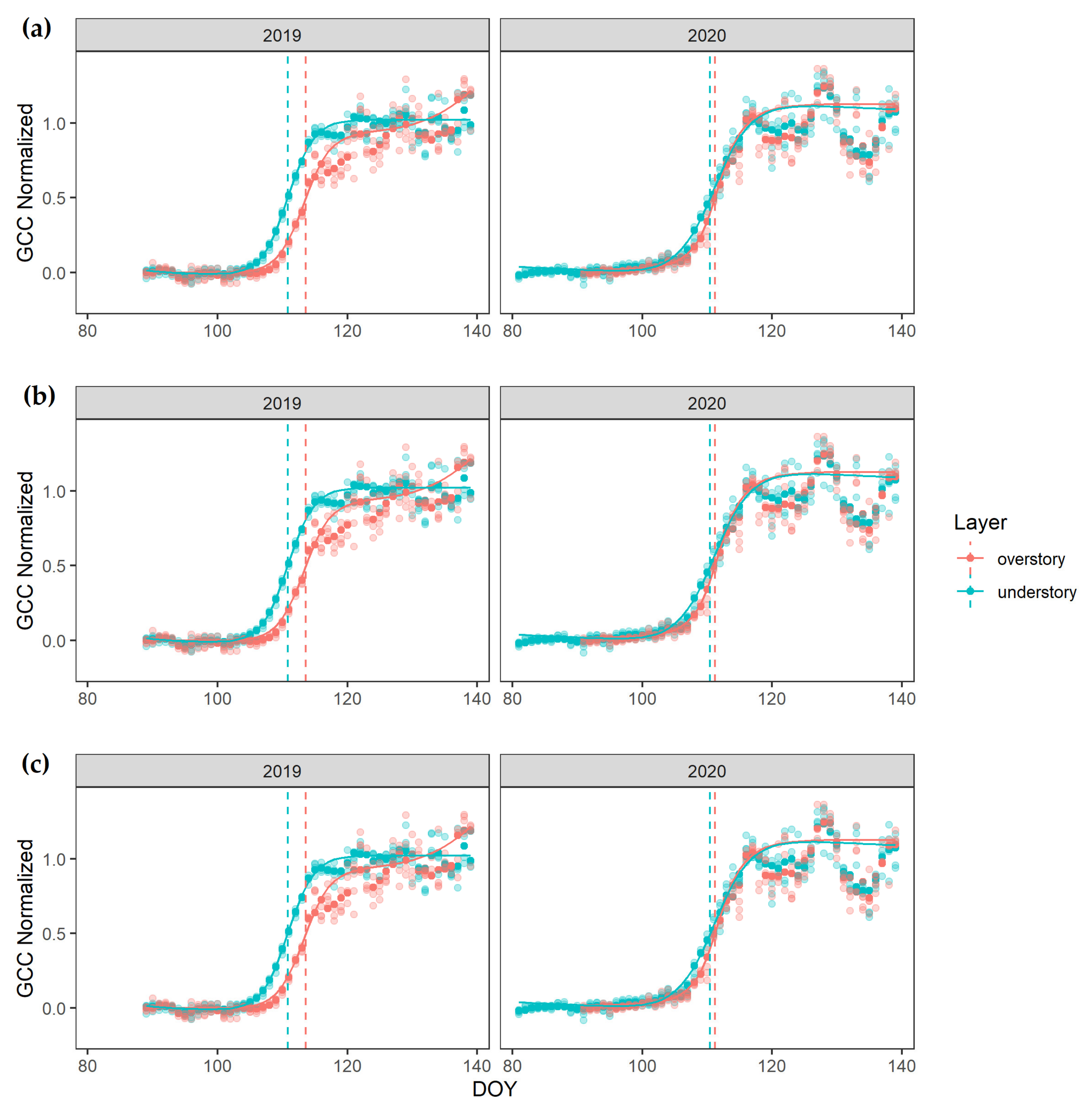
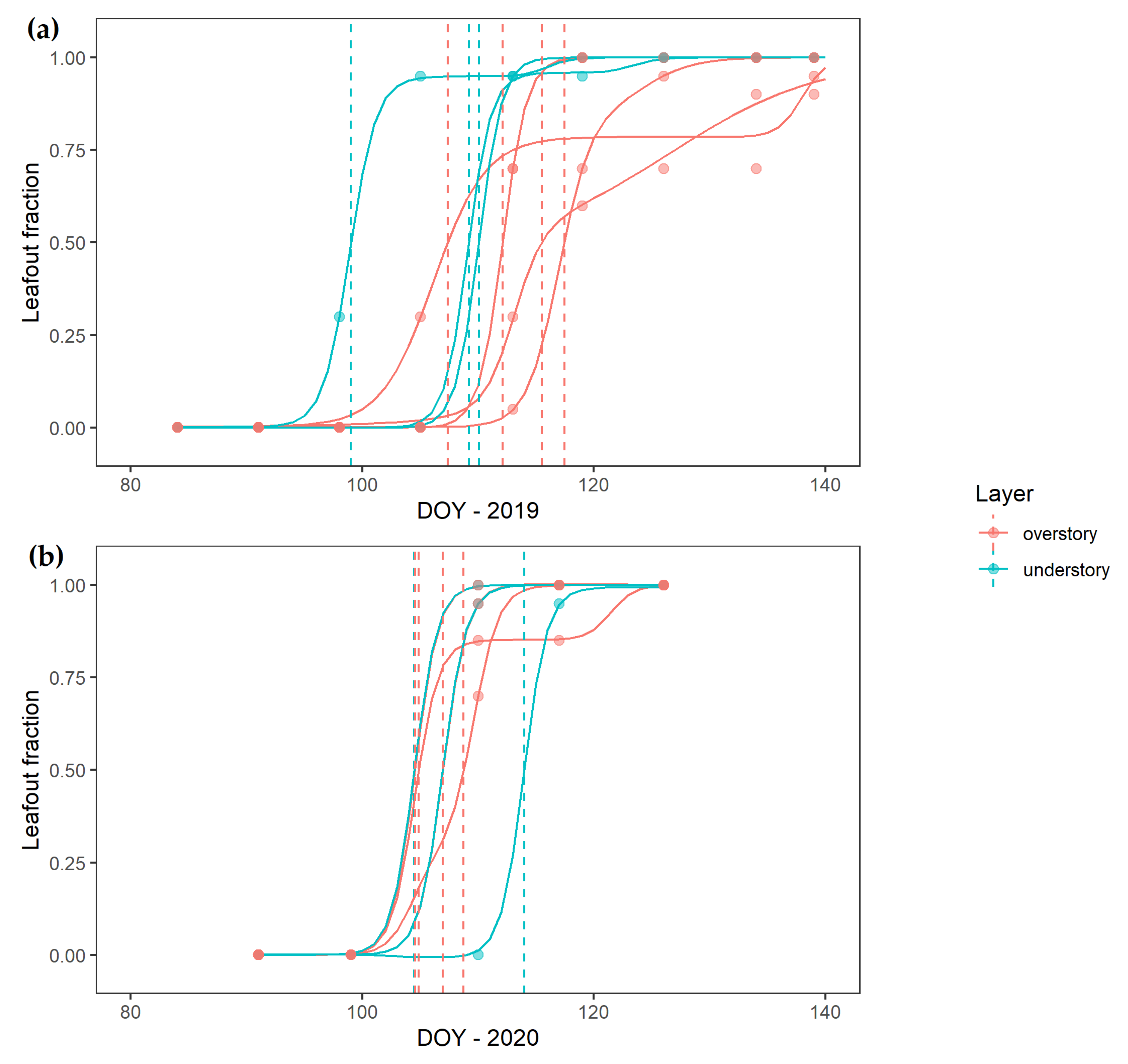
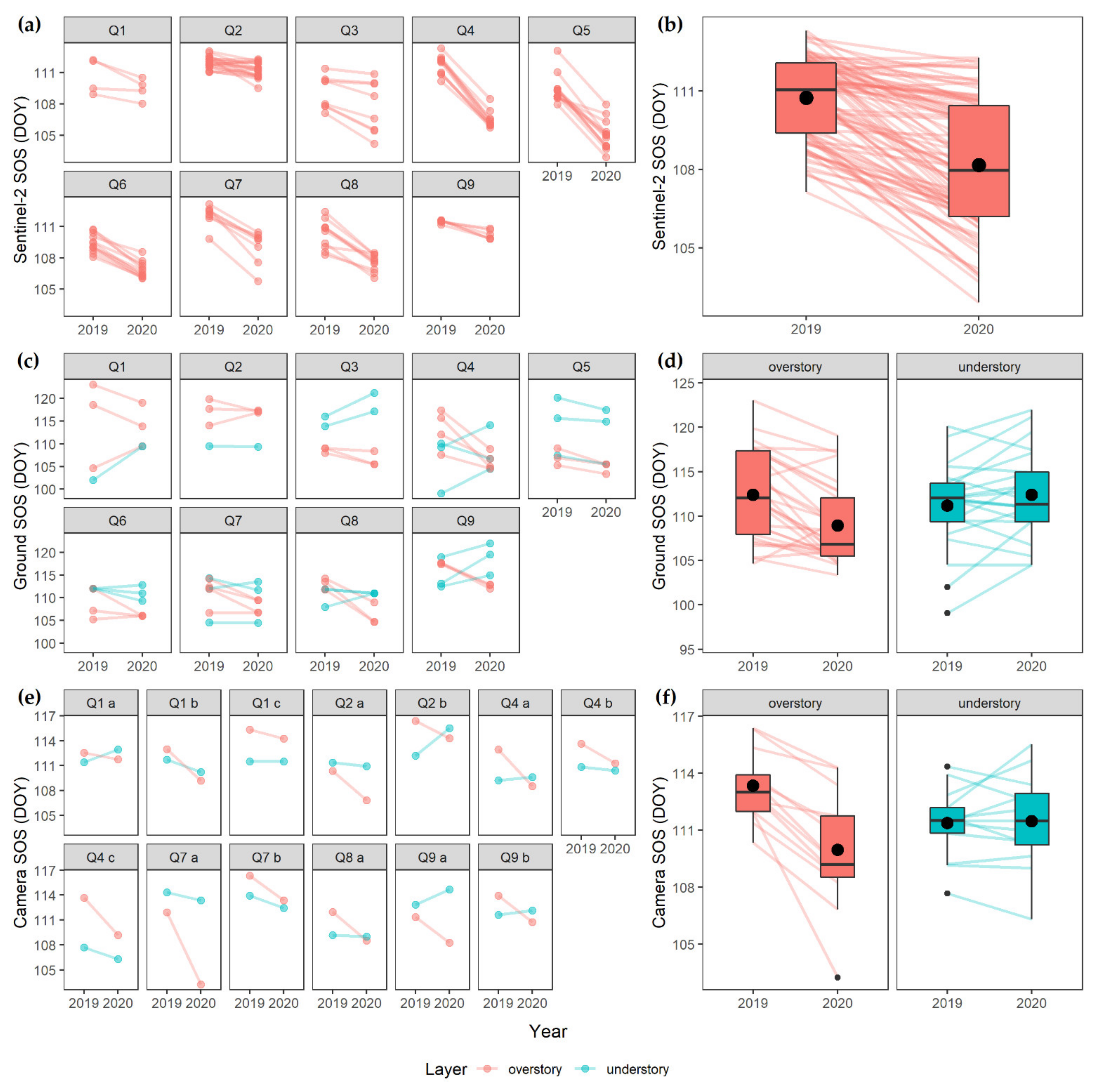
3. Results
4. Discussion
4.1. Over- and Understory Phenology Response to Climatic Drivers
4.2. Vertical Mismatch
4.3. Ecological Consequences and Forest Management
4.4. Methodological Consequences
4.5. Future Research Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.G.; Briede, A. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Cannell, M.; Smith, R. Climatic warming, spring budburst and forest damage on trees. J. Appl. Ecol. 1986, 23, 177–191. [Google Scholar] [CrossRef]
- Gu, L.; Hanson, P.J.; Post, W.M.; Kaiser, D.P.; Yang, B.; Nemani, R.; Pallardy, S.G.; Meyers, T. The 2007 eastern US spring freeze: Increased cold damage in a warming world? BioScience 2008, 58, 253–262. [Google Scholar] [CrossRef]
- Plard, F.; Gaillard, J.-M.; Coulson, T.; Hewison, A.J.M.; Delorme, D.; Warnant, C.; Bonenfant, C. Mismatch Between Birth Date and Vegetation Phenology Slows the Demography of Roe Deer. PLoS Biol. 2014, 12, e1001828. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.S.; Zohner, C.M. Climate Change and Phenological Mismatch in Trophic Interactions Among Plants, Insects, and Vertebrates. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 165–182. [Google Scholar] [CrossRef]
- Thackeray, S.J.; Sparks, T.H.; Frederiksen, M.; Burthe, S.; Bacon, P.J.; Bell, J.R.; Botham, M.S.; Brereton, T.M.; Bright, P.W.; Carvalho, L. Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob. Chang. Biol. 2010, 16, 3304–3313. [Google Scholar] [CrossRef] [Green Version]
- Visser, M.E.; Van Noordwijk, A.J.; Tinbergen, J.M.; Lessells, C.M. Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. B Boil. Sci. 1998, 265, 1867–1870. [Google Scholar] [CrossRef] [Green Version]
- Voigt, W.; Perner, J.; Davis, A.J.; Eggers, T.; Schumacher, J.; Bährmann, R.; Fabian, B.; Heinrich, W.; Köhler, G.; Lichter, D. Trophic levels are differentially sensitive to climate. Ecology 2003, 84, 2444–2453. [Google Scholar] [CrossRef] [Green Version]
- Heberling, J.M.; McDonough MacKenzie, C.; Fridley, J.D.; Kalisz, S.; Primack, R.B. Phenological mismatch with trees reduces wildflower carbon budgets. Ecol. Lett. 2019, 22, 616–623. [Google Scholar] [CrossRef]
- Landuyt, D.; De Lombaerde, E.; Perring, M.P.; Hertzog, L.R.; Ampoorter, E.; Maes, S.L.; De Frenne, P.; Ma, S.; Proesmans, W.; Blondeel, H. The functional role of temperate forest understorey vegetation in a changing world. Glob. Chang. Biol. 2019, 25, 3625–3641. [Google Scholar] [CrossRef]
- Post, E.; Pedersen, C.; Wilmers, C.C.; Forchhammer, M.C. Warming, plant phenology and the spatial dimension of trophic mismatch for large herbivores. Proc. R. Soc. B Boil. Sci. 2008, 275, 2005–2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Tang, Y.; Chen, J. Plant phenological synchrony increases under rapid within-spring warming. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zohner, C.M.; Mo, L.; Renner, S.S. Global warming reduces leaf-out and flowering synchrony among individuals. eLife 2018, 7, e40214. [Google Scholar] [CrossRef]
- Chen, L.; Huang, J.-G.; Ma, Q.; Hänninen, H.; Rossi, S.; Piao, S.; Bergeron, Y. Spring phenology at different altitudes is becoming more uniform under global warming in Europe. Glob. Chang. Biol. 2018, 24, 3969–3975. [Google Scholar] [CrossRef]
- Vitasse, Y.; Signarbieux, C.; Fu, Y.H. Global warming leads to more uniform spring phenology across elevations. Proc. Natl. Acad. Sci. USA 2018, 115, 1004–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, A.; Yu, R. Temperate deciduous shrub phenology: The overlooked forest layer. Int. J. Biometeorol. 2019, 65, 1–13. [Google Scholar] [CrossRef]
- Ulyshen, M.D. Arthropod vertical stratification in temperate deciduous forests: Implications for conservation-oriented management. For. Ecol. Manag. 2011, 261, 1479–1489. [Google Scholar] [CrossRef]
- Kwit, M.C.; Rigg, L.S.; Goldblum, D. Sugar maple seedling carbon assimilation at the northern limit of its range: The importance of seasonal light. Can. J. For. Res. 2010, 40, 385–393. [Google Scholar] [CrossRef]
- Augspurger, C.K.; Bartlett, E.A. Differences in leaf phenology between juvenile and adult trees in a temperate deciduous forest. Tree Physiol. 2003, 23, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; O’Keefe, J. Phenological differences between understory and overstory. In Phenology of Ecosystem Processes; Springer: New York, NY, USA, 2009; pp. 87–117. [Google Scholar]
- Vitasse, Y. Ontogenic changes rather than difference in temperature cause understory trees to leaf out earlier. New Phytol. 2013, 198, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Piao, S.; Op de Beeck, M.; Cong, N.; Zhao, H.; Zhang, Y.; Menzel, A.; Janssens, I.A. Recent spring phenology shifts in western C entral E urope based on multiscale observations. Glob. Ecol. Biogeogr. 2014, 23, 1255–1263. [Google Scholar] [CrossRef]
- Gill, D.S.; Amthor, J.S.; Bormann, F.H. Leaf phenology, photosynthesis, and the persistence of saplings and shrubs in a mature northern hardwood forest. Tree Physiol. 1998, 18, 281–289. [Google Scholar] [CrossRef]
- Hertel, C.; Leuchner, M.; Menzel, A. Vertical variability of spectral ratios in a mature mixed forest stand. Agric. For. Meteorol. 2011, 151, 1096–1105. [Google Scholar] [CrossRef]
- Jolly, W.M.; Nemani, R.; Running, S.W. Enhancement of understory productivity by asynchronous phenology with overstory competitors in a temperate deciduous forest. Tree Physiol. 2004, 24, 1069–1071. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.H.; Allen, B.P.; Sharitz, R.R. Why do early-emerging tree seedlings have survival advantages?: A test using Acer rubrum (Aceraceae). Am. J. Bot. 1997, 84, 1714–1718. [Google Scholar] [CrossRef]
- Seiwa, K. Advantages of early germination for growth and survival of seedlings of Acer mono under different overstorey phenologies in deciduous broad-leaved forests. J. Ecol. 1998, 86, 219–228. [Google Scholar] [CrossRef]
- De Frenne, P.; Zellweger, F.; Rodriguez-Sanchez, F.; Scheffers, B.R.; Hylander, K.; Luoto, M.; Vellend, M.; Verheyen, K.; Lenoir, J. Global buffering of temperatures under forest canopies. Nat. Ecol. Evol. 2019, 3, 744–749. [Google Scholar] [CrossRef]
- Bolte, A.; Czajkowski, T.; Cocozza, C.; Tognetti, R.; De Miguel, M.; Pšidová, E.; Ditmarová, Ĺ.; Dinca, L.; Delzon, S.; Cochard, H. Desiccation and mortality dynamics in seedlings of different European beech (Fagus sylvatica L.) populations under extreme drought conditions. Front. Plant Sci. 2016, 7, 751. [Google Scholar] [CrossRef] [Green Version]
- Jacques, M.H.; Lapointe, L.; Rice, K.; Montgomery, R.A.; Stefanski, A.; Reich, P.B. Responses of two understory herbs, Maianthemum canadense and Eurybia macrophylla, to experimental forest warming: Early emergence is the key to enhanced reproductive output. Am. J. Bot. 2015, 102, 1610–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitasse, Y.; Baumgarten, F.; Zohner, C.M.; Kaewthongrach, R.; Fu, Y.H.; Walde, M.G.; Moser, B. Impact of microclimatic conditions and resource availability on spring and autumn phenology of temperate tree seedlings. New Phytol. 2021, 232, 537–550. [Google Scholar] [CrossRef]
- Bennie, J.; Huntley, B.; Wiltshire, A.; Hill, M.O.; Baxter, R. Slope, aspect and climate: Spatially explicit and implicit models of topographic microclimate in chalk grassland. Ecol. Model. 2008, 216, 47–59. [Google Scholar] [CrossRef]
- Myneni, R.B.; Keeling, C.; Tucker, C.J.; Asrar, G.; Nemani, R.R. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 1997, 386, 698–702. [Google Scholar] [CrossRef]
- White, M.A.; Thornton, P.E.; Running, S.W. A continental phenology model for monitoring vegetation responses to interannual climatic variability. Glob. Biogeochem. Cycles 1997, 11, 217–234. [Google Scholar] [CrossRef]
- Ahl, D.E.; Gower, S.T.; Burrows, S.N.; Shabanov, N.V.; Myneni, R.B.; Knyazikhin, Y. Monitoring spring canopy phenology of a deciduous broadleaf forest using MODIS. Remote Sens. Environ. 2006, 104, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, H.M.; Eklundh, L.; Kuusk, A.; Nilson, T. Impact of understory vegetation on forest canopy reflectance and remotely sensed LAI estimates. Remote Sens. Environ. 2006, 103, 408–418. [Google Scholar] [CrossRef]
- Tuanmu, M.-N.; Viña, A.; Bearer, S.; Xu, W.; Ouyang, Z.; Zhang, H.; Liu, J. Mapping understory vegetation using phenological characteristics derived from remotely sensed data. Remote Sens. Environ. 2010, 114, 1833–1844. [Google Scholar] [CrossRef]
- Ryu, Y.; Lee, G.; Jeon, S.; Song, Y.; Kimm, H. Monitoring multi-layer canopy spring phenology of temperate deciduous and evergreen forests using low-cost spectral sensors. Remote Sens. Environ. 2014, 149, 227–238. [Google Scholar] [CrossRef]
- Rankine, C.; Sánchez-Azofeifa, G.; Guzmán, J.A.; Espirito-Santo, M.; Sharp, I. Comparing MODIS and near-surface vegetation indexes for monitoring tropical dry forest phenology along a successional gradient using optical phenology towers. Environ. Res. Lett. 2017, 12, 105007. [Google Scholar] [CrossRef] [Green Version]
- Pisek, J.; Chen, J.M.; Kobayashi, H.; Rautiainen, M.; Schaepman, M.E.; Karnieli, A.; Sprinstin, M.; Ryu, Y.; Nikopensius, M.; Raabe, K. Retrieval of seasonal dynamics of forest understory reflectance from semiarid to boreal forests using MODIS BRDF data. J. Geophys. Res. Biogeosci. 2016, 121, 855–863. [Google Scholar] [CrossRef] [Green Version]
- Misra, G.; Buras, A.; Heurich, M.; Asam, S.; Menzel, A. LiDAR derived topography and forest stand characteristics largely explain the spatial variability observed in MODIS land surface phenology. Remote Sens. Environ. 2018, 218, 231–244. [Google Scholar] [CrossRef]
- Rodriguez-Galiano, V.; Dash, J.; Atkinson, P.M. Intercomparison of satellite sensor land surface phenology and ground phenology in Europe. Geophys. Res. Lett. 2015, 42, 2253–2260. [Google Scholar] [CrossRef] [Green Version]
- White, M.A.; de Beurs, K.M.; Didan, K.; Inouye, D.W.; Richardson, A.D.; Jensen, O.P.; O’Keefe, J.; Zhang, G.; Nemani, R.R.; van Leeuwen, W.J. Intercomparison, interpretation, and assessment of spring phenology in North America estimated from remote sensing for 1982–2006. Glob. Chang. Biol. 2009, 15, 2335–2359. [Google Scholar] [CrossRef]
- Keenan, T.; Darby, B.; Felts, E.; Sonnentag, O.; Friedl, M.; Hufkens, K.; O’Keefe, J.; Klosterman, S.; Munger, J.W.; Toomey, M. Tracking forest phenology and seasonal physiology using digital repeat photography: A critical assessment. Ecol. Appl. 2014, 24, 1478–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klosterman, S.; Richardson, A.D. Observing spring and fall phenology in a deciduous forest with aerial drone imagery. Sensors 2017, 17, 2852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klosterman, S.; Melaas, E.; Wang, J.; Martinez, A.; Frederick, S.; O’Keefe, J.; Orwig, D.A.; Wang, Z.; Sun, Q.; Schaaf, C. Fine-scale perspectives on landscape phenology from unmanned aerial vehicle (UAV) photography. Agric. For. Meteorol. 2018, 248, 397–407. [Google Scholar] [CrossRef]
- Vrieling, A.; Meroni, M.; Darvishzadeh, R.; Skidmore, A.K.; Wang, T.; Zurita-Milla, R.; Oosterbeek, K.; O’Connor, B.; Paganini, M. Vegetation phenology from Sentinel-2 and field cameras for a Dutch barrier island. Remote Sens. Environ. 2018, 215, 517–529. [Google Scholar] [CrossRef]
- Hufkens, K.; Friedl, M.; Sonnentag, O.; Braswell, B.H.; Milliman, T.; Richardson, A.D. Linking near-surface and satellite remote sensing measurements of deciduous broadleaf forest phenology. Remote Sens. Environ. 2012, 117, 307–321. [Google Scholar] [CrossRef]
- Liu, N.; Garcia, M.; Singh, A.; Clare, J.D.; Stenglein, J.L.; Zuckerberg, B.; Kruger, E.L.; Townsend, P.A. Trail camera networks provide insights into satellite-derived phenology for ecological studies. Int. J. Appl. Earth Obs. Geoinf. 2021, 97, 102291. [Google Scholar] [CrossRef]
- Redlich, S.; Zhang, J.; Benjamin, C.; Dhillon, M.S.; Englmeier, J.; Ewald, J.; Fricke, U.; Ganuza, C.; Haensel, M.; Hovestadt, T. Disentangling effects of climate and land use on biodiversity and ecosystem services-a multi-scale experimental design. bioRxiv 2021. [Google Scholar] [CrossRef]
- Gressler, E.; Jochner, S.; Capdevielle-Vargas, R.M.; Morellato, L.P.C.; Menzel, A. Vertical variation in autumn leaf phenology of Fagus sylvatica L. in southern Germany. Agric. For. Meteorol. 2015, 201, 176–186. [Google Scholar] [CrossRef]
- Ammer, C. Impact of ungulates on structure and dynamics of natural regeneration of mixed mountain forests in the Bavarian Alps. For. Ecol. Manag. 1996, 88, 43–53. [Google Scholar] [CrossRef]
- Motta, R. Impact of wild ungulates on forest regeneration and tree composition of mountain forests in the Western Italian Alps. For. Ecol. Manag. 1996, 88, 93–98. [Google Scholar] [CrossRef]
- Putman, R.; Moore, N. Impact of deer in lowland Britain on agriculture, forestry and conservation habitats. Mammal Rev. 1998, 28, 141–164. [Google Scholar] [CrossRef]
- Sonnentag, O.; Hufkens, K.; Teshera-Sterne, C.; Young, A.M.; Friedl, M.; Braswell, B.H.; Milliman, T.; O’Keefe, J.; Richardson, A.D. Digital repeat photography for phenological research in forest ecosystems. Agric. For. Meteorol. 2012, 152, 159–177. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hufkens, K.; Milliman, T.; Aubrecht, D.M.; Chen, M.; Gray, J.M.; Johnston, M.R.; Keenan, T.F.; Klosterman, S.T.; Kosmala, M. Tracking vegetation phenology across diverse North American biomes using PhenoCam imagery. Sci. Data 2018, 5, 1–24. [Google Scholar] [CrossRef]
- Beck, P.S.; Atzberger, C.; Høgda, K.A.; Johansen, B.; Skidmore, A.K. Improved monitoring of vegetation dynamics at very high latitudes: A new method using MODIS NDVI. Remote Sens. Environ. 2006, 100, 321–334. [Google Scholar] [CrossRef]
- Filippa, G.; Cremonese, E.; Migliavacca, M.; Galvagno, M.; Forkel, M.; Wingate, L.; Tomelleri, E.; Di Cella, U.M.; Richardson, A.D. Phenopix: AR package for image-based vegetation phenology. Agric. For. Meteorol. 2016, 220, 141–150. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics: Updated Edition; John Wiley and Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Meier, U. (Ed.) BBCH-Monograph: Growth Stages of Mono-and Dicotyledonous Plants, 2nd ed.; Technical Report; Federal Biological Research Centre for Agriculture and Forestry: Bonn, Germany, 2001. [Google Scholar]
- Lange, M.; Doktor, D. Phenex: Auxiliary Functions for Phenological Data Analysis, R Package Version 1.4-5. 2017. Available online: https://rdrr.io/cran/phenex/ (accessed on 4 October 2021).
- Fischer, A. A model for the seasonal variations of vegetation indices in coarse resolution data and its inversion to extract crop parameters. Remote Sens. Environ. 1994, 48, 220–230. [Google Scholar] [CrossRef]
- White, K.; Pontius, J.; Schaberg, P. Remote sensing of spring phenology in northeastern forests: A comparison of methods, field metrics and sources of uncertainty. Remote Sens. Environ. 2014, 148, 97–107. [Google Scholar] [CrossRef]
- Ranghetti, L.; Boschetti, M.; Nutini, F.; Busetto, L. “sen2r”: An R toolbox for automatically downloading and preprocessing Sentinel-2 satellite data. Comput. Geosci. 2020, 139, 104473. [Google Scholar] [CrossRef]
- Malingreau, J. The vegetation index and the study of vegetation dynamics. In Applications of Remote Sensing to Agrometeorology; Springer: New York, NY, USA, 1989; pp. 285–303. [Google Scholar]
- Riedel, T.; Hennig, P.; Kroiher, F.; Polley, H.; Schmitz, F.; Schwitzgebel, F. Die dritte Bundeswaldinventur (BWI 2012). In Inventur-und Auswertemethoden; Johann Heinrich von Thünen Institute: Braunschweig, Germany, 2017. [Google Scholar]
- Zhang, X.; Wang, J.; Gao, F.; Liu, Y.; Schaaf, C.; Friedl, M.; Yu, Y.; Jayavelu, S.; Gray, J.; Liu, L. Exploration of scaling effects on coarse resolution land surface phenology. Remote Sens. Environ. 2017, 190, 318–330. [Google Scholar] [CrossRef] [Green Version]
- Misra, G.; Buras, A.; Menzel, A. Effects of different methods on the comparison between land surface and ground phenology—A methodological case study from south-western Germany. Remote Sens. 2016, 8, 753. [Google Scholar] [CrossRef] [Green Version]
- Roussel, J.-R.; Auty, D.; Coops, N.C.; Tompalski, P.; Goodbody, T.R.; Meador, A.S.; Bourdon, J.-F.; de Boissieu, F.; Achim, A. lidR: An R package for analysis of Airborne Laser Scanning (ALS) data. Remote Sens. Environ. 2020, 251, 112061. [Google Scholar] [CrossRef]
- DWD Climate Data Center (CDC). Annual Grids of Monthly Averaged Daily Air Temperature (2 m) Over Germany; Version v1.0; DWD: Offenbach am Main, Germany, 2020. [Google Scholar]
- Fu, P.; Rich, P.M. Design and implementation of the Solar Analyst: An ArcView extension for modeling solar radiation at landscape scales. In Proceedings of the 9th Annual ESRI User Conference, San Diego, CA, USA, 26–30 July 1999; pp. 1–31. [Google Scholar]
- Allen, R.G.; Trezza, R.; Tasumi, M. Analytical integrated functions for daily solar radiation on slopes. Agric. For. Meteorol. 2006, 139, 55–73. [Google Scholar] [CrossRef]
- Huang, S.; Rich, P.M.; Crabtree, R.L.; Potter, C.S.; Fu, P. Modeling monthly near-surface air temperature from solar radiation and lapse rate: Application over complex terrain in Yellowstone National Park. Phys. Geogr. 2008, 29, 158–178. [Google Scholar] [CrossRef]
- Ashcroft, M.B. A method for improving landscape scale temperature predictions and the implications for vegetation modelling. Ecol. Model. 2006, 197, 394–404. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Ime4: Linear Mixed-Effects Models Using Eigen and S4. R package Version 1.1-27.1. 2021. Available online: https://cran.r-project.org/web/packages/lme4/ (accessed on 4 October 2021).
- Hijmans, R.J.; Van Etten, J. raster: Geographic Data Analysis and Modeling. R Package Version 2.5–8. 2016. Available online: https://cran.r-project.org/web/packages/raster/ (accessed on 4 October 2021).
- Leutner, B.; Horning, N.; Schwalb-Willmann, J.R. Tools for Remote Sensing Data Analysis. R Package Version 0.2. 2019. Available online: https://rdrr.io/cran/RStoolbox/ (accessed on 4 October 2021).
- Urbanek, S. jpeg: Read and Write JPEG Images. R Package Version 0.1–8. 2014. Available online: http://www.rforge.net/jpeg/ (accessed on 4 October 2021).
- Grolemund, G.; Wickham, H. Dates and times made easy with lubridate. J. Stat. Softw. 2011, 40, 1–25. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wilke, C. cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. R Package Version 0.9. 2. 2020. Available online: https://cran.r-project.org/web/packages/cowplot/ (accessed on 4 October 2021).
- Deutscher Wetterdienst. Klimastatusbericht Deutschland Jahr 2020; Deutscher Wetterdienst: Offenbach, Germany, 2021. [Google Scholar]
- Pfeifroth, U.; Sanchez-Lorenzo, A.; Manara, V.; Trentmann, J.; Hollmann, R. Trends and variability of surface solar radiation in Europe based on surface-and satellite-based data records. J. Geophys. Res. Atmos. 2018, 123, 1735–1754. [Google Scholar] [CrossRef]
- Leuchner, M.; Hertel, C.; Menzel, A. Spatial variability of photosynthetically active radiation in European beech and Norway spruce. Agric. For. Meteorol. 2011, 151, 1226–1232. [Google Scholar] [CrossRef]
- Deutscher Wetterdienst. Klimastatusbericht Deutschland Jahr 2019; Deutscher Wetterdienst: Offenbach, Germany, 2020. [Google Scholar]
- Rautiainen, M. Retrieval of leaf area index for a coniferous forest by inverting a forest reflectance model. Remote Sens. Environ. 2005, 99, 295–303. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and model inversion methods with narrowband optical indices for chlorophyll content estimation in closed forest canopies with hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef] [Green Version]
- Friedl, M.; Henebry, G.; Reed, B.; Huete, A. Land surface phenology NASA white paper. NASA Doc. 2006, 15, 2011. [Google Scholar]
- Cheng, Y.; Vrieling, A.; Fava, F.; Meroni, M.; Marshall, M.; Gachoki, S. Phenology of short vegetation cycles in a Kenyan rangeland from PlanetScope and Sentinel-2. Remote Sens. Environ. 2020, 248, 112004. [Google Scholar] [CrossRef]
- Tian, F.; Cai, Z.; Jin, H.; Hufkens, K.; Scheifinger, H.; Tagesson, T.; Smets, B.; Van Hoolst, R.; Bonte, K.; Ivits, E. Calibrating vegetation phenology from Sentinel-2 using eddy covariance, PhenoCam, and PEP725 networks across Europe. Remote Sens. Environ. 2021, 260, 112456. [Google Scholar] [CrossRef]
- Li, N.; Zhan, P.; Pan, Y.; Zhu, X.; Li, M.; Zhang, D. Comparison of remote sensing time-series smoothing methods for grassland spring phenology extraction on the Qinghai–Tibetan Plateau. Remote Sens. 2020, 12, 3383. [Google Scholar] [CrossRef]
- Xin, Q.; Li, J.; Li, Z.; Li, Y.; Zhou, X. Evaluations and comparisons of rule-based and machine-learning-based methods to retrieve satellite-based vegetation phenology using MODIS and USA National Phenology Network data. Int. J. Appl. Earth Obs. Geoinf. 2020, 93, 102189. [Google Scholar] [CrossRef]
- Teuling, A.J.; Taylor, C.M.; Meirink, J.F.; Melsen, L.A.; Miralles, D.G.; Van Heerwaarden, C.C.; Vautard, R.; Stegehuis, A.I.; Nabuurs, G.-J.; de Arellano, J.V.-G. Observational evidence for cloud cover enhancement over western European forests. Nat. Commun. 2017, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]



| Quadrant | Center Coordinates (°E, °N) | Elevation (m) | Mean Annual Temp. (°C) | Mean Annual Precip. (mm) | Camera Sites (Section 2.1) | Groundover. Indivs. (Section 2.2) | Ground Under. Indivs. (Section 2.2) | Sentinel-2 Indivs. (Section 2.5.2 Dataset 1) | Sentinel-2 Pixels (Section 2.5.2 Dataset 2) |
|---|---|---|---|---|---|---|---|---|---|
| Q1 | 9.77, 49.76 | 294 | 9.2 | 635 | 3 | 3 | 1 | 4 | 12,356 |
| Q2 | 9.94, 49.89 | 309 | 9.1 | 678 | 2 | 3 | 1 | 20 | 15,923 |
| Q3 | 10.44, 50.07 | 328 | 8.8 | 701 | - | 3 | 2 | 8 | - |
| Q4 | 11.75, 48.92 | 449 | 8.6 | 675 | 3 | 4 | 3 | 11 | 15,384 |
| Q5 | 11.85, 48.58 | 490 | 8.4 | 650 | - | 3 | 3 | 13 | 132 |
| Q6 | 10.53, 49.92 | 412 | 8.2 | 809 | - | 3 | 3 | 12 | 17,029 |
| Q7 | 11.70, 49.13 | 543 | 7.9 | 785 | 2 | 4 | 3 | 8 | 3614 |
| Q8 | 13.36, 48.78 | 491 | 7.8 | 1215 | 1 | 3 | 3 | 10 | 3910 |
| Q9 | 13.54, 48.88 | 785 | 6.8 | 1367 | 2 | 3 | 3 | 6 | - |
| Total | 13 | 29 | 22 | 92 | 68,348 |
| Dependent Variable | Method | Fixed Effect | Coefficient | Centered Intercept | n | ||
|---|---|---|---|---|---|---|---|
| Models Camera and Visual Ground Observations | |||||||
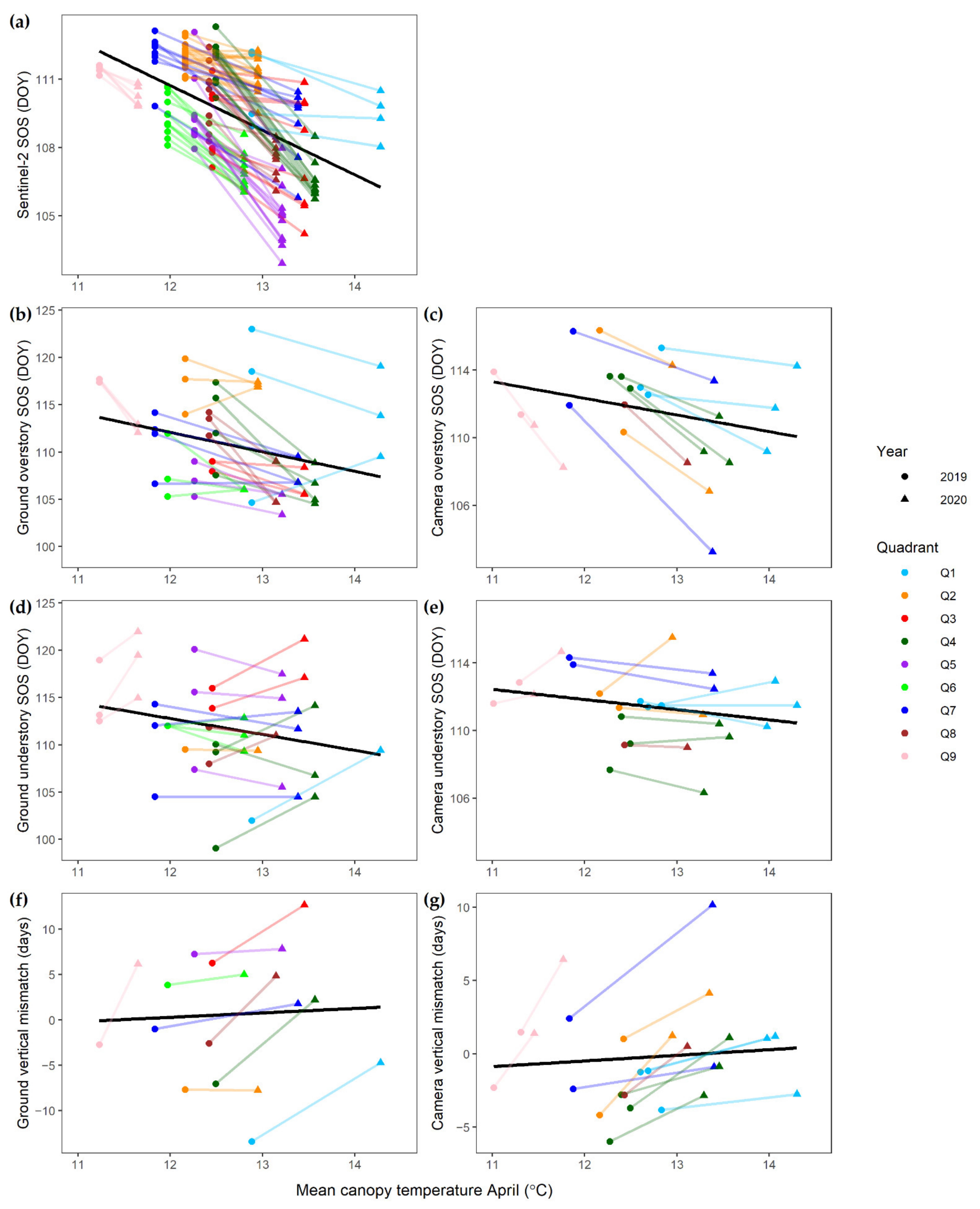
| SOS overstory (DOY) | Camera | CT | −2.574 *** | 111.494 *** | 26 | ||
| SOS overstory (DOY) | Ground | CT | −2.860 *** | 110.705 *** | 58 | ||
| SOS understory (DOY) | Camera | CT | −0.175 | 111.450 *** | 26 | ||
| SOS understory (DOY) | Ground | CT | +0.644 | 111.632 *** | 44 | ||
| Vertical mismatch (days) | Camera | CT | +2.522 *** | 0.072 | 26 | ||
| Vertical mismatch (days) | Ground | CT | +3.901 ** | 0.085 | 18 | ||
| Models Sentinel-2 Observations | |||||||
| CT coefficient | Height coefficient | Interaction coefficient | |||||
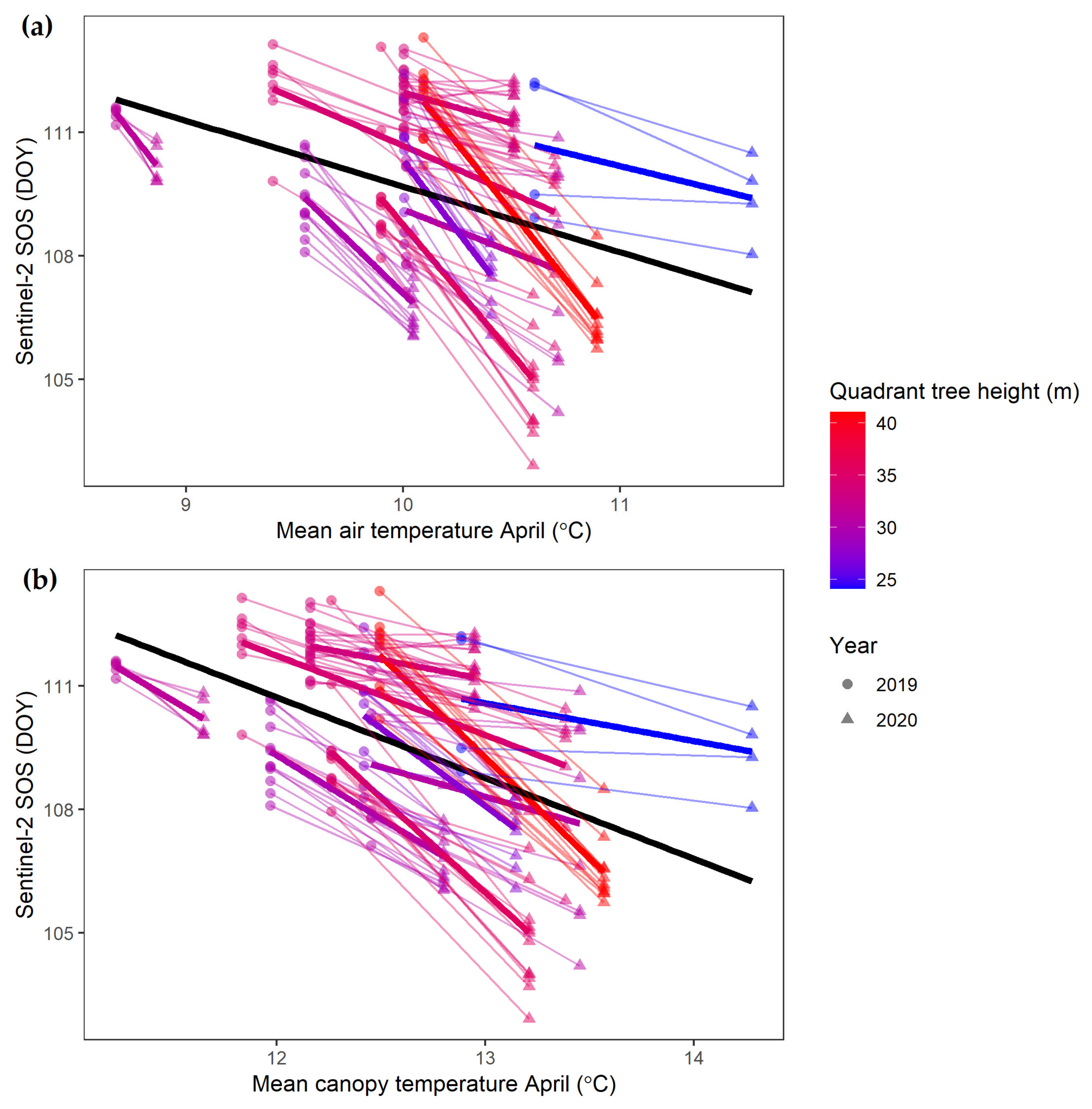
| SOS (DOY) | Sentinel-2 | CT * height | −2.619 *** | −0.025 | −0.082 *** | 109.416 *** | 184 |
| 2019 | 2020 | |||||||
|---|---|---|---|---|---|---|---|---|
| Quadrant | Intercept (DOY) | Height Effect | SOS at 1 m (DOY) | SOS at 30 m (DOY) | Intercept (DOY) | Height Effect | SOS at 1 m (DOY) | SOS at 30 m (DOY) |
| Q1 | 110.8 | −0.003 | 110.8 | 110.7 | 109.6 | −0.02 | 109.6 | 108.9 |
| Q2 | 110.2 | −0.008 | 110.2 | 109.9 | 110.0 | −0.05 | 110.0 | 108.4 |
| Q4 | 112.2 | −0.062 | 112.2 | 110.4 | 111.6 | −0.19 | 111.4 | 105.8 |
| Q5 | 110.5 | +0.038 | 110.6 | 111.6 | 108.9 | −0.14 | 108.7 | 104.7 |
| Q6 | 109.9 | −0.028 | 109.8 | 109.0 | 109.0 | −0.09 | 108.9 | 106.4 |
| Q7 | 112.2 | −0.012 | 112.2 | 111.9 | 112.0 | −0.16 | 111.8 | 107.1 |
| Q8 | 110.4 | −0.046 | 110.4 | 109.1 | 112.0 | −0.27 | 111.8 | 103.9 |
| Mean | 110.9 | −0.017 | 110.9 | 110.4 | 110.5 | −0.13 | 110.3 | 106.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uphus, L.; Lüpke, M.; Yuan, Y.; Benjamin, C.; Englmeier, J.; Fricke, U.; Ganuza, C.; Schwindl, M.; Uhler, J.; Menzel, A. Climate Effects on Vertical Forest Phenology of Fagus sylvatica L., Sensed by Sentinel-2, Time Lapse Camera, and Visual Ground Observations. Remote Sens. 2021, 13, 3982. https://doi.org/10.3390/rs13193982
Uphus L, Lüpke M, Yuan Y, Benjamin C, Englmeier J, Fricke U, Ganuza C, Schwindl M, Uhler J, Menzel A. Climate Effects on Vertical Forest Phenology of Fagus sylvatica L., Sensed by Sentinel-2, Time Lapse Camera, and Visual Ground Observations. Remote Sensing. 2021; 13(19):3982. https://doi.org/10.3390/rs13193982
Chicago/Turabian StyleUphus, Lars, Marvin Lüpke, Ye Yuan, Caryl Benjamin, Jana Englmeier, Ute Fricke, Cristina Ganuza, Michael Schwindl, Johannes Uhler, and Annette Menzel. 2021. "Climate Effects on Vertical Forest Phenology of Fagus sylvatica L., Sensed by Sentinel-2, Time Lapse Camera, and Visual Ground Observations" Remote Sensing 13, no. 19: 3982. https://doi.org/10.3390/rs13193982






