VIS-NIR-SWIR Hyperspectroscopy Combined with Data Mining and Machine Learning for Classification of Predicted Chemometrics of Green Lettuce
Abstract
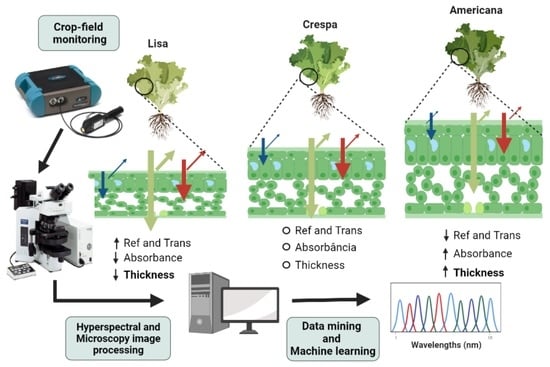
:1. Introduction
2. Material and Methods
2.1. Plant Material, Growth Conditions, and Experimental Design
2.2. Extraction of Leaf Pigments
2.3. Optical Microscopy Analysis
2.4. Optical Reflectance, Transmittance, and Absorbance Properties of Leaves
2.5. Statistical Analyses
2.5.1. Descriptive and Univariate Statistical Analyses
2.5.2. Analysis of Leaf Spectral Fingerprints
2.5.3. Principal Component Analysis (PCA)
2.5.4. Multivariate Curve Resolution (MCR)
2.5.5. Linear Discriminant Analysis (LDA)
2.5.6. Support Vector Machine (SVM)
2.5.7. K-Nearest Neighbour (KNN)
2.5.8. Partial Least Squares Regression (PLSR) by Analysis of Spectroscopy Data
3. Results
3.1. Descriptive Analysis-Based Biochemical and Biophysical Attributes of Lettuce
3.2. Hyperspectral Analysis of Leaves
3.3. Principal Component Analysis (PCA)
3.4. Multivariate Curve Resolution (MCR)
3.5. Regression Coefficient (RC) and Variable Importance in Projection (VIP)
3.6. Model Evaluation of Chemometric Parameters
3.7. Estimation of Thickness-Based Data Mining and Machine Learning by PLSR Method
4. Discussion
4.1. Descriptive Analysis
4.2. Analysis of Hyperspectral Curves
4.3. Machine Learning-Based PCA Classification
4.4. Partial Least Squares Regression (PLSR) of Predicted Chemometrics
4.5. Data Mining and Machine Learning-Based Modelling by Hyperspectroscopy Data
4.6. Regression Coefficient (RC) and Variable Importance in Projection (VIP)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buturi, C.V.; Sabatino, L.; Mauro, R.P.; Navarro-León, E.; Blasco, B.; Leonardi, C.; Giuffrida, F. Iron Biofortification of Greenhouse Soilless Lettuce: An Effective Agronomic Tool to Improve the Dietary Mineral Intake. Agronomy 2022, 12, 1793. [Google Scholar] [CrossRef]
- Hong, J.; Xu, F.; Chen, G.; Huang, X.; Wang, S.; Du, L.; Ding, G. Evaluation of the Effects of Nitrogen, Phosphorus, and Potassium Applications on the Growth, Yield, and Quality of Lettuce (Lactuca Sativa L.). Agronomy 2022, 12, 2477. [Google Scholar] [CrossRef]
- Muneer, S.; Kim, E.; Park, J.; Lee, J. Influence of Green, Red and Blue Light Emitting Diodes on Multiprotein Complex Proteins and Photosynthetic Activity under Different Light Intensities in Lettuce Leaves (Lactuca Sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horf, M.; Vogel, S.; Drücker, H.; Gebbers, R.; Olfs, H.-W. Optical Spectrometry to Determine Nutrient Concentrations and Other Physicochemical Parameters in Liquid Organic Manures: A Review. Agronomy 2022, 12, 514. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Carillo, P.; Colla, G.; Graziani, G.; Di Mola, I.; Mori, M.; Kyriacou, M.C.; Rouphael, Y.; Soteriou, G.A.; et al. Plant-Derived Biostimulants Differentially Modulate Primary and Secondary Metabolites and Improve the Yield Potential of Red and Green Lettuce Cultivars. Agronomy 2022, 12, 1361. [Google Scholar] [CrossRef]
- Matysiak, B.; Ropelewska, E.; Wrzodak, A.; Kowalski, A.; Kaniszewski, S. Yield and Quality of Romaine Lettuce at Different Daily Light Integral in an Indoor Controlled Environment. Agronomy 2022, 12, 1026. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Pattaro, M.; Herrig Furlanetto, R.; Nanni, M.R.; Camargos Antunes, W. High Resolution Leaf Spectral Signature as a Tool for Foliar Pigment Estimation Displaying Potential for Species Differentiation. J. Plant Physiol. 2020, 249, 153161. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Antunes, W.C.; Nanni, M.R. Rapid Quantification Method for Yield, Calorimetric Energy and Chlorophyll a Fluorescence Parameters in Nicotiana Tabacum L. Using Vis-NIR-SWIR Hyperspectroscopy. Plants 2022, 11, 2406. [Google Scholar] [CrossRef]
- Moriwaki, T.; Falcioni, R.; Tanaka, F.A.O.; Cardoso, K.A.K.; Souza, L.A.; Benedito, E.; Nanni, M.R.; Bonato, C.M.; Antunes, W.C. Nitrogen-Improved Photosynthesis Quantum Yield Is Driven by Increased Thylakoid Density, Enhancing Green Light Absorption. Plant Sci. 2019, 278, 1–11. [Google Scholar] [CrossRef]
- Rodrigues, M.; Berti de Oliveira, R.; Leboso Alemparte Abrantes dos Santos, G.; Mayara de Oliveira, K.; Silveira Reis, A.; Herrig Furlanetto, R.; Antônio Yanes Bernardo Júnior, L.; Silva Coelho, F.; Rafael Nanni, M. Rapid Quantification of Alkaloids, Sugar and Yield of Tobacco (Nicotiana Tabacum L.) Varieties by Using Vis–NIR–SWIR Spectroradiometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 274, 121082. [Google Scholar] [CrossRef]
- Sobejano-Paz, V.; Mikkelsen, T.N.; Baum, A.; Mo, X.; Liu, S.; Köppl, C.J.; Johnson, M.S.; Gulyas, L.; García, M. Hyperspectral and Thermal Sensing of Stomatal Conductance, Transpiration, and Photosynthesis for Soybean and Maize under Drought. Remote Sens. 2020, 12, 3182. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Zhihua, L.; Jiyong, S.; Zhai, X.; Wang, S.; Mariod, A.A. Rapid Prediction of Phenolic Compounds and Antioxidant Activity of Sudanese Honey Using Raman and Fourier Transform Infrared (FT-IR) Spectroscopy. Food Chem. 2017, 226, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Chicati, M.S.; Nanni, M.R.; Chicati, M.L.; Furlanetto, R.H.; Cezar, E.; De Oliveira, R.B. Hyperspectral Remote Detection as an Alternative to Correlate Data of Soil Constituents. Remote Sens. Appl. Soc. Environ. 2019, 16, 100270. [Google Scholar] [CrossRef]
- Furlanetto, R.H.; Moriwaki, T.; Falcioni, R.; Pattaro, M.; Vollmann, A.; Sturion Junior, A.C.; Antunes, W.C.; Nanni, M.R. Hyperspectral Reflectance Imaging to Classify Lettuce Varieties by Optimum Selected Wavelengths and Linear Discriminant Analysis. Remote Sens. Appl. Soc. Environ. 2020, 20, 100400. [Google Scholar] [CrossRef]
- da Silva Junior, C.A.; Nanni, M.R.; Shakir, M.; Teodoro, P.E.; de Oliveira-Júnior, J.F.; Cezar, E.; de Gois, G.; Lima, M.; Wojciechowski, J.C.; Shiratsuchi, L.S. Soybean Varieties Discrimination Using Non-Imaging Hyperspectral Sensor. Infrared Phys. Technol. 2018, 89, 338–350. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Bonato, C.M.; de Souza, L.A.; Nanni, M.R.; Antunes, W.C. Distinct Growth Light and Gibberellin Regimes Alter Leaf Anatomy and Reveal Their Influence on Leaf Optical Properties. Environ. Exp. Bot. 2017, 140, 86–95. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Lorenzini, G.; Nali, C.; Pellegrini, E.; Saponaro, V.; Hoshika, Y.; Arab, L.; Rennenberg, H.; Paoletti, E. Hyperspectral Reflectance of Light-Adapted Leaves Can Predict Both Dark- and Light-Adapted Chl Fluorescence Parameters, and the Effects of Chronic Ozone Exposure on Date Palm (Phoenix Dactylifera). Int. J. Mol. Sci. 2020, 21, 6441. [Google Scholar] [CrossRef]
- El-Hendawy, S.; Al-Suhaibani, N.; Mubushar, M.; Tahir, M.U.; Marey, S.; Refay, Y.; Tola, E. Combining Hyperspectral Reflectance and Multivariate Regression Models to Estimate Plant Biomass of Advanced Spring Wheat Lines in Diverse Phenological Stages under Salinity Conditions. Appl. Sci. 2022, 12, 1983. [Google Scholar] [CrossRef]
- Braga, P.; Crusiol, L.G.T.; Nanni, M.R.; Caranhato, A.L.H.; Fuhrmann, M.B.; Nepomuceno, A.L.; Neumaier, N.; Farias, J.R.B.; Koltun, A.; Gonçalves, L.S.A.; et al. Vegetation Indices and NIR-SWIR Spectral Bands as a Phenotyping Tool for Water Status Determination in Soybean. Precis. Agric. 2021, 22, 249–266. [Google Scholar] [CrossRef]
- Ralbovsky, N.M.; Smith, J.P. Multivariate Curve Resolution for Analysis of Raman Hyperspectral Imaging Data Sets for Enzyme Immobilization. Chem. Data Collect. 2022, 38, 100835. [Google Scholar] [CrossRef]
- Féret, J.-B.; le Maire, G.; Jay, S.; Berveiller, D.; Bendoula, R.; Hmimina, G.; Cheraiet, A.; Oliveira, J.C.; Ponzoni, F.J.; Solanki, T.; et al. Estimating Leaf Mass per Area and Equivalent Water Thickness Based on Leaf Optical Properties: Potential and Limitations of Physical Modeling and Machine Learning. Remote Sens. Environ. 2019, 231, 110959. [Google Scholar] [CrossRef]
- Jin, J.; Arief Pratama, B.; Wang, Q. Tracing Leaf Photosynthetic Parameters Using Hyperspectral Indices in an Alpine Deciduous Forest. Remote Sens. 2020, 12, 1124. [Google Scholar] [CrossRef] [Green Version]
- Sexton, T.; Sankaran, S.; Cousins, A.B. Predicting Photosynthetic Capacity in Tobacco Using Shortwave Infrared Spectral Reflectance. J. Exp. Bot. 2021, 72, 4373–4383. [Google Scholar] [CrossRef] [PubMed]
- Shorten, P.R.; Leath, S.R.; Schmidt, J.; Ghamkhar, K. Predicting the Quality of Ryegrass Using Hyperspectral Imaging. Plant Methods 2019, 15, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmer, G.F.; Santos, R.O.; Teixeira, I.D.; de Cassia de Souza Schneider, R.; Helfer, G.A.; Costa, A. Ben Rapid Quantification of Constituents in Tobacco by NIR Fiber-optic Probe. J. Chemom. 2020, 34, e3303. [Google Scholar] [CrossRef]
- Falcioni, R.; Moriwaki, T.; Furlanetto, R.H.; Nanni, M.R.; Antunes, W.C. Simple, Fast and Efficient Methods for Analysing the Structural, Ultrastructural and Cellular Components of the Cell Wall. Plants 2022, 11, 995. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, P.J. Image Processing with ImageJ. Biophotonics Int. 2012, 11, 36–42. [Google Scholar]
- R-Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2020. Available online: https://www.R-project.org (accessed on 5 October 2022).
- Pimentel-Gomes, F. Statistics Course Experimental, 1st ed.; FEALQ: Piracicaba, Brazil, 2009. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Education: Upper Saddle River, NJ, USA, 2010; ISBN 0-13-100846-3. [Google Scholar]
- Franca, T.; Goncalves, D.; Cena, C. ATR-FTIR Spectroscopy Combined with Machine Learning for Classification of PVA/PVP Blends in Low Concentration. Vib. Spectrosc. 2022, 120, 103378. [Google Scholar] [CrossRef]
- Nanni, M.R.; Cezar, E.; da Silva Junior, C.A.; Silva, G.F.C.; da Silva Gualberto, A.A. Partial Least Squares Regression (PLSR) Associated with Spectral Response to Predict Soil Attributes in Transitional Lithologies. Arch. Agron. Soil Sci. 2018, 64, 682–695. [Google Scholar] [CrossRef]
- Cozzolino, D.; Vadell, A.; Ballesteros, F.; Galietta, G.; Barlocco, N. Combining Visible and Near-Infrared Spectroscopy with Chemometrics to Trace Muscles from an Autochthonous Breed of Pig Produced in Uruguay: A Feasibility Study. Anal. Bioanal. Chem. 2006, 385, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Pierna, J.A.F.; Baeten, V.; Renier, A.M.; Cogdill, R.P.; Dardenne, P. Combination of Support Vector Machines (SVM) and near-Infrared (NIR) Imaging Spectroscopy for the Detection of Meat and Bone Meal (MBM) in Compound Feeds. J. Chemom. 2004, 18, 341–349. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, Z.; Zhang, G.; Chen, S.; Zhao, Y.; Lu, J. Analysis and Classification of Kidney Stones Based on Raman Spectroscopy. Biomed. Opt. Express 2018, 9, 4175. [Google Scholar] [CrossRef] [PubMed]
- Gholamy, A.; Kreinovich, V.; Kosheleva, O. Why 70/30 or 80/20 Relation between Training and Testing Sets: A Pedagogical Explanation. El Paso, TX, USA. 2018. Available online: https://www.cs.utep.edu/vladik/2018/tr18-09.pdf (accessed on 5 October 2022).
- Izenman, A.J. Modern Multivariate Statistical Techniques, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Jolliffe, I.T. (Ed.) Principal Component Analysis, 2nd ed.; Springer USA: New York, NY, USA, 2002; Volume 30. [Google Scholar]
- Sheskin, D.J. Handbook of Parametric and Nonparametric Statistical Procedures, 1st ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Minasny, B.; McBratney, A.B.; Malone, B.P.; Wheeler, I. Digital Mapping of Soil Carbon. Adv. Agron. 2013, 3, 1–47. [Google Scholar]
- de Oliveira Moura, L.; de Carvalho Lopes, D.; Steidle Neto, A.J.; de Castro Louback Ferraz, L.; de Almeida Carlos, L.; Martins, L.M. Evaluation of Techniques for Automatic Classification of Lettuce Based on Spectral Reflectance. Food Anal. Methods 2016, 9, 1799–1806. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, X.; Li, Y.; Li, S.; Zhang, Y. Hyperspectral Identification of Chlorophyll Fluorescence Parameters of Suaeda Salsa in Coastal Wetlands. Remote Sens. 2021, 13, 2066. [Google Scholar] [CrossRef]
- Calviño-Cancela, M.; Martín-Herrero, J. Spectral Discrimination of Vegetation Classes in Ice-Free Areas of Antarctica. Remote Sens. 2016, 8, 856. [Google Scholar] [CrossRef] [Green Version]
- Falcioni, R.; Moriwaki, T.; Perez-Llorca, M.; Munné-Bosch, S.; Gibin, M.S.; Sato, F.; Pelozo, A.; Pattaro, M.C.; Giacomelli, M.E.; Rüggeberg, M.; et al. Cell Wall Structure and Composition Is Affected by Light Quality in Tomato Seedlings. J. Photochem. Photobiol. B Biol. 2020, 203, 111745. [Google Scholar] [CrossRef]
- Silva, C.A.; Nanni, M.R.; Teodoro, P.E.; Silva, G.F.C. Vegetation Indices for Discrimination of Soybean Areas: A New Approach. Agron. J. 2017, 109, 1331–1343. [Google Scholar] [CrossRef]
- Baranović, G.; Šegota, S. Infrared Spectroscopy of Flavones and Flavonols. Reexamination of the Hydroxyl and Carbonyl Vibrations in Relation to the Interactions of Flavonoids with Membrane Lipids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 473–486. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of Polyphenols and Antioxidant Properties of Five Lettuce Varieties and Escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Kitazaki, K.; Fukushima, A.; Nakabayashi, R.; Okazaki, Y.; Kobayashi, M.; Mori, T.; Nishizawa, T.; Reyes-Chin-Wo, S.; Michelmore, R.W.; Saito, K.; et al. Metabolic Reprogramming in Leaf Lettuce Grown Under Different Light Quality and Intensity Conditions Using Narrow-Band LEDs. Sci. Rep. 2018, 8, 7914. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Marino, G.; LLusia, J.; Morfopoulos, C.; Farré-Armengol, G.; Filella, I. Photochemical Reflectance Index as an Indirect Estimator of Foliar Isoprenoid Emissions at the Ecosystem Level. Nat. Commun. 2013, 4, 2604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvão, L.S.; Formaggio, A.R.; Tisot, D.A. Discrimination of Sugarcane Varieties in Southeastern Brazil with EO-1 Hyperion Data. Remote Sens. Environ. 2005, 94, 523–534. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Fortini, E.A.; de Müller, L.A.C.; Batista, D.S.; Vieira, L.M.; Silva, P.O.; do Amaral, C.H.; Poethig, R.S.; Otoni, W.C. Leaf Development Stages and Ontogenetic Changes in Passionfruit (Passiflora Edulis Sims.) Are Detected by Narrowband Spectral Signal. J. Photochem. Photobiol. B Biol. 2020, 209, 111931. [Google Scholar] [CrossRef]
- Lysenko, V.; Guo, Y.; Kosolapov, A.; Usova, E.; Varduny, T.; Krasnov, V. Polychromatic Fourier-PAM Fluorometry and Hyperspectral Analysis of Chlorophyll Fluorescence from Phaseolus Vulgaris Leaves: Effects of Green Light. Inf. Process. Agric. 2020, 7, 204–211. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Q. Selection of Informative Spectral Bands for PLS Models to Estimate Foliar Chlorophyll Content Using Hyperspectral Reflectance. IEEE Trans. Geosci. Remote Sens. 2019, 57, 3064–3072. [Google Scholar] [CrossRef]
- Ge, Y.; Atefi, A.; Zhang, H.; Miao, C.; Ramamurthy, R.K.; Sigmon, B.; Yang, J.; Schnable, J.C. High-Throughput Analysis of Leaf Physiological and Chemical Traits with VIS–NIR–SWIR Spectroscopy: A Case Study with a Maize Diversity Panel. Plant Methods 2019, 15, 66. [Google Scholar] [CrossRef] [Green Version]
- Straková, P.; Larmola, T.; Andrés, J.; Ilola, N.; Launiainen, P.; Edwards, K.; Minkkinen, K.; Laiho, R. Quantification of Plant Root Species Composition in Peatlands Using FTIR Spectroscopy. Front. Plant Sci. 2020, 11, 597. [Google Scholar] [CrossRef]
- Juan, A. Multivariate Curve Resolution for Hyperspectral Image Analysis. In Hyperspectral Imaging; Amigo, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 115–150. [Google Scholar]
- Olmos, V.; Marro, M.; Loza-Alvarez, P.; Raldúa, D.; Prats, E.; Padrós, F.; Piña, B.; Tauler, R.; de Juan, A. Combining Hyperspectral Imaging and Chemometrics to Assess and Interpret the Effects of Environmental Stressors on Zebrafish Eye Images at Tissue Level. J. Biophotonics 2018, 11, e201700089. [Google Scholar] [CrossRef] [Green Version]
- Boshkovski, B.; Doupis, G.; Zapolska, A.; Kalaitzidis, C.; Koubouris, G. Hyperspectral Imagery Detects Water Deficit and Salinity Effects on Photosynthesis and Antioxidant Enzyme Activity of Three Greek Olive Varieties. Sustainability 2022, 14, 1432. [Google Scholar] [CrossRef]
- Gitelson, A.; Solovchenko, A. Non-Invasive Quantification of Foliar Pigments: Possibilities and Limitations of Reflectance- and Absorbance-Based Approaches. J. Photochem. Photobiol. B Biol. 2018, 178, 537–544. [Google Scholar] [CrossRef] [PubMed]








| Parameter | Lisa | Crespa | Americana | Minimum | Maximum | CV(%) |
|---|---|---|---|---|---|---|
| Chl a (mg m−2) | 339.2 ± 0.70 c | 442.8 ± 1.38 b | 630.0 ± 1.36 a | 283.8 | 735.4 | 25.8 |
| Chl b (mg m−2) | 181.0 ± 0.37 c | 213.5 ± 0.61 b | 307.2 ± 0.61 a | 153.5 | 353.4 | 23.1 |
| Chl a+b (mg m−2) | 520.2 ± 1.00 c | 656.3 ± 1.98 b | 937.2 ± 1.97 a | 439.2 | 1088.8 | 24.9 |
| Car (mg m−2) | 121.0 ± 0.28 c | 157.4 ± 0.45 b | 211.5 ± 0.45 a | 93.2 | 244.6 | 23.1 |
| Chl a (mg g−1) | 17.2 ± 0.06 c | 27.1 ± 0.13 a | 20.4 ± 0.05 b | 13.7 | 38.9 | 19.9 |
| Chl b (mg g−1) | 9.2 ± 0.03 b | 13.0 ± 0.06 a | 9.9 ± 0.02 b | 6.7 | 18.7 | 16.5 |
| Chl a+b (mg g−1) | 26.4 ± 0.08 c | 40.1 ± 0.19 a | 30.3 ± 0.07 b | 20.4 | 57.7 | 18.7 |
| Car (mg g−1) | 6.1 ± 0.02 c | 9.6 ± 0.04 a | 6.8 ± 0.02 b | 4.9 | 13.5 | 20.7 |
| Chl a/Chl b | 1.9 ± 0.01 b | 2.1 ± 0.01 a | 2.1 ± 0.01 a | 1.6 | 2.2 | 4.7 |
| Car/Chl a+b | 0.2 ± 0.01 a | 0.2 ± 0.01 a | 0.2 ± 0.01 a | 0.2 | 0.3 | 2.6 |
| Thickness (µm) | 261.1 ± 0.03 c | 303.7 ± 0.29 b | 368.7 ± 0.07 a | 258.7 | 375.7 | 14.3 |
| Spectroscopy | Multivariate | Selection | Most Responsive VIP by Wavelength (nm) |
|---|---|---|---|
| Reflectance | PC1 | 12 | 485, 552, 680, 710, 1079, 1185, 1392, 1440, 1546, 1683, 1923, 2199 |
| PC2 | 7 | 552, 710, 1368, 1450, 1831, 2030, 2228 | |
| PC3 | 11 | 470, 555, 680, 705, 920, 968, 1070, 1180, 1929 | |
| MCR1 | 11 | 552, 709, 975, 1104, 1183, 1376, 1440, 1588, 1739, 1831, 2230 | |
| MCR2 | 8 | 550, 745, 1078, 1364, 1447, 1595, 1935, 2200 | |
| MCR3 | 9 | 494, 565, 661, 770, 964, 1085, 1174, 1226, 1664 | |
| Transmittance | PC1 | 12 | 550, 679, 704, 942, 1170, 1399, 1433, 1523, 1671, 1852, 1926, 2227 |
| PC2 | 6 | 548, 676, 704, 1375, 1835, 2020 | |
| PC3 | 9 | 970, 1059, 1071, 1423, 1667, 1879, 1920, 2072, 2193 | |
| MCR1 | 9 | 552, 703, 968, 1168, 1382, 1446, 1553, 1842, 2250 | |
| MCR2 | 11 | 550, 663, 706, 995, 1166, 1397, 1512, 1680, 1861, 1933, 2142 | |
| MCR3 | 9 | 551, 755, 923, 1056, 1172, 1277, 1423, 1670, 2215 | |
| Absorbance | PC1 | 12 | 550, 674, 710, 969, 1174 1394, 1437, 1543, 1679, 1841, 1926, 2210 |
| PC2 | 7 | 457, 550, 676, 705, 1373, 1450, 2020 | |
| PC3 | 12 | 435, 498, 550, 674, 718, 1160, 1257, 1367, 1461, 1832, 2003, 2230 | |
| MCR1 | 7 | 445, 555, 678, 704, 1375, 1833, 2265 | |
| MCR2 | 9 | 555, 708, 972, 1174, 1395, 1520, 1852, 1920, 2186 | |
| MCR3 | 6 | 445, 550, 680, 1442, 1927, 2220 |
| PLSR Models | Measurements | PLSR Parameters | ||||
|---|---|---|---|---|---|---|
| R2 | Offset | RMSE | RPD | Bias | ||
| Calibration | Reflectance | 0.988 | 3.88 | 4.95 | 9.13 | - |
| Transmittance | 0.990 | 3.12 | 4.43 | 10.04 | - | |
| Absorbance | 0.996 | 2.35 | 3.86 | 15.81 | - | |
| Cross-Validation | Reflectance | 0.980 | 5.46 | 5.94 | 7.07 | - |
| Transmittance | 0.987 | 3.63 | 5.07 | 8.77 | - | |
| Absorbance | 0.993 | 2.19 | 4.06 | 11.95 | - | |
| Predicted | Reflectance | 0.986 | 3.55 | 5.60 | 8.45 | 0.206 |
| Transmittance | 0.973 | 4.62 | 7.75 | 6.09 | 0.126 | |
| Absorbance | 0.991 | 2.98 | 6.21 | 10.54 | 0.102 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcioni, R.; Gonçalves, J.V.F.; Oliveira, K.M.d.; Antunes, W.C.; Nanni, M.R. VIS-NIR-SWIR Hyperspectroscopy Combined with Data Mining and Machine Learning for Classification of Predicted Chemometrics of Green Lettuce. Remote Sens. 2022, 14, 6330. https://doi.org/10.3390/rs14246330
Falcioni R, Gonçalves JVF, Oliveira KMd, Antunes WC, Nanni MR. VIS-NIR-SWIR Hyperspectroscopy Combined with Data Mining and Machine Learning for Classification of Predicted Chemometrics of Green Lettuce. Remote Sensing. 2022; 14(24):6330. https://doi.org/10.3390/rs14246330
Chicago/Turabian StyleFalcioni, Renan, João Vitor Ferreira Gonçalves, Karym Mayara de Oliveira, Werner Camargos Antunes, and Marcos Rafael Nanni. 2022. "VIS-NIR-SWIR Hyperspectroscopy Combined with Data Mining and Machine Learning for Classification of Predicted Chemometrics of Green Lettuce" Remote Sensing 14, no. 24: 6330. https://doi.org/10.3390/rs14246330