Development of a Spectral Index for the Detection of Yellow-Flowering Vegetation
Abstract
:1. Introduction
2. Data
2.1. Surface Reflectance Data
2.2. Crop Products
2.2.1. EUCROPMAP 2018
2.2.2. RapeseedMap10
2.3. Ground Phenological Observations
3. Development of Yellowness Index
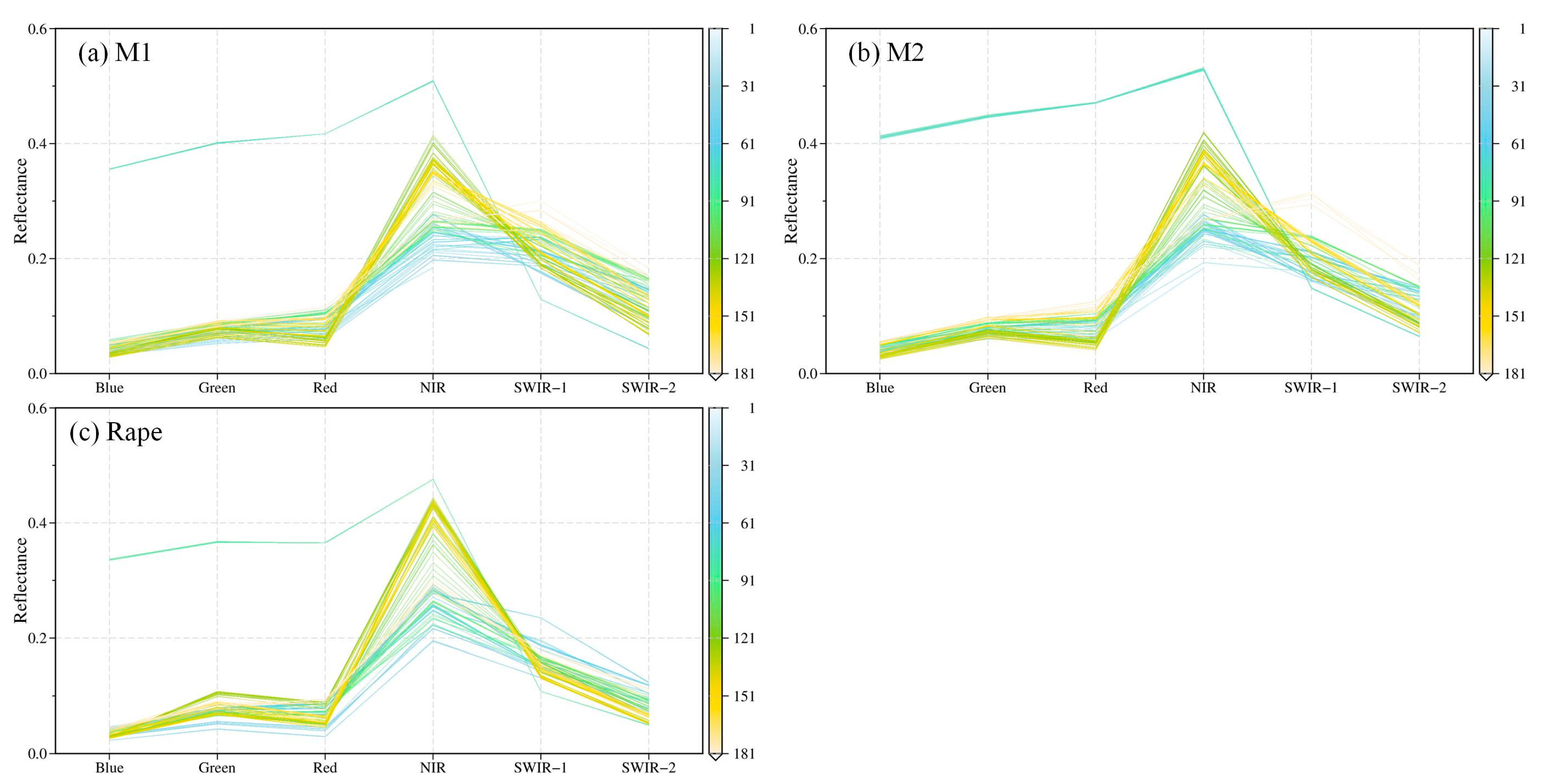
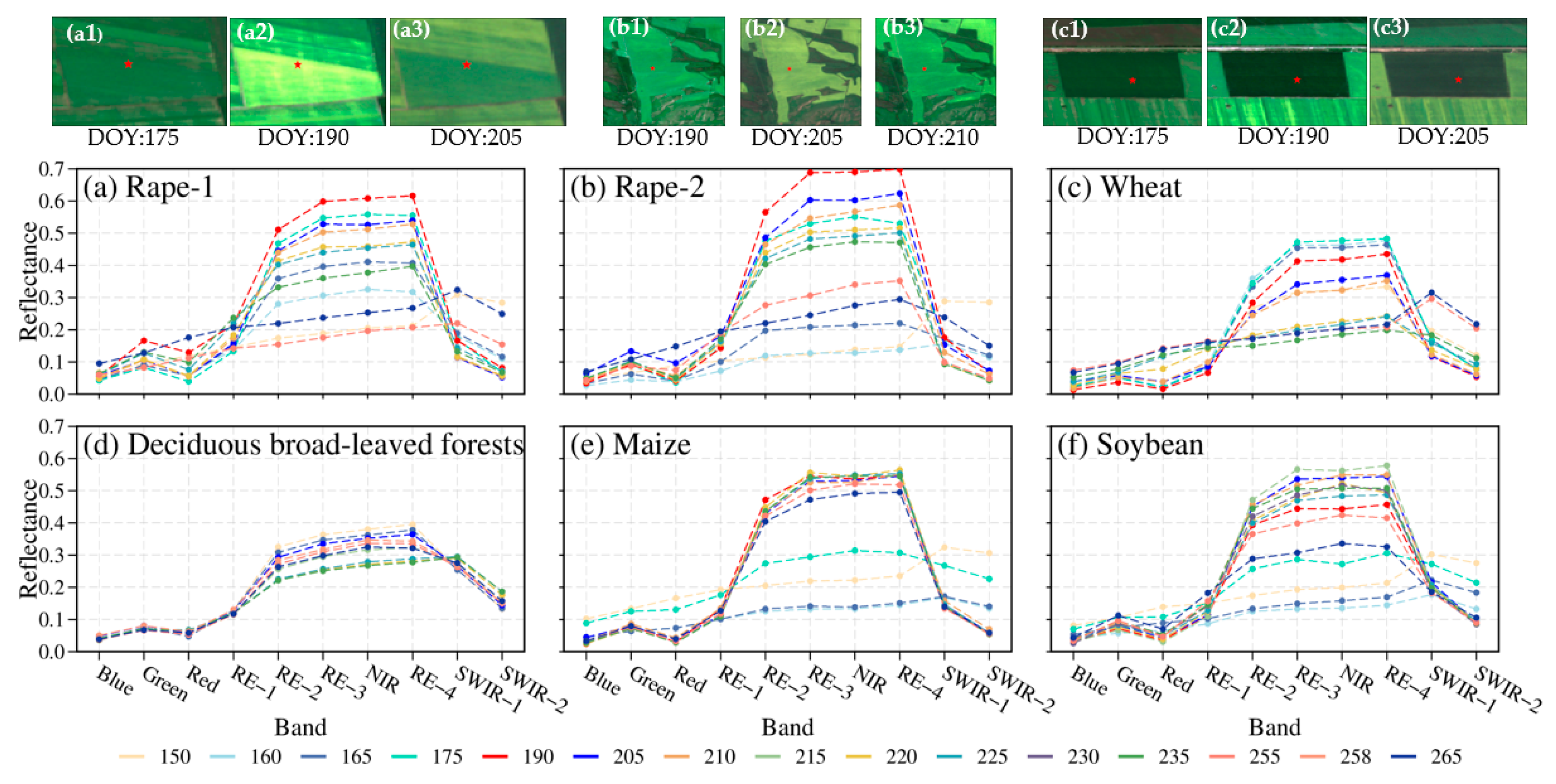
3.1. Investigation of the Time-Series Spectral Characteristics for Rape and Other Crops
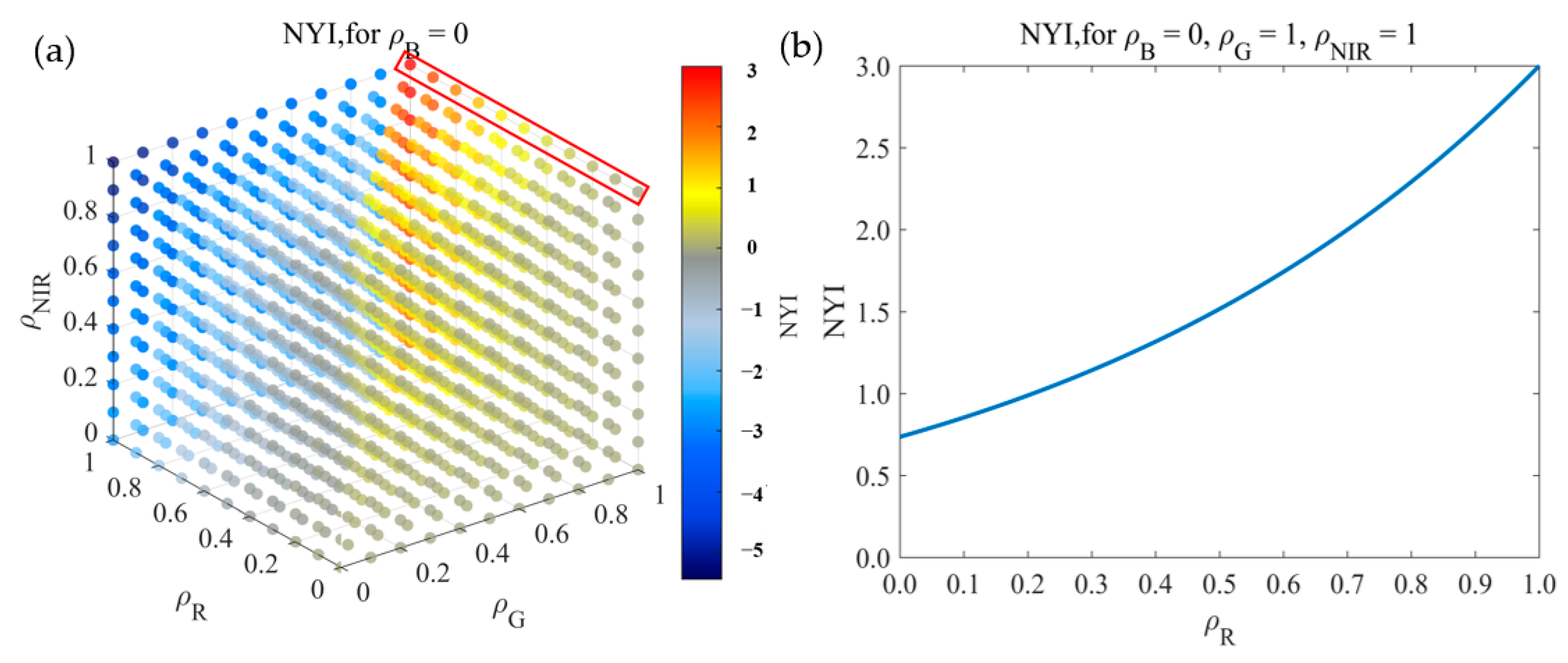
3.2. Construction of Yellowness Index
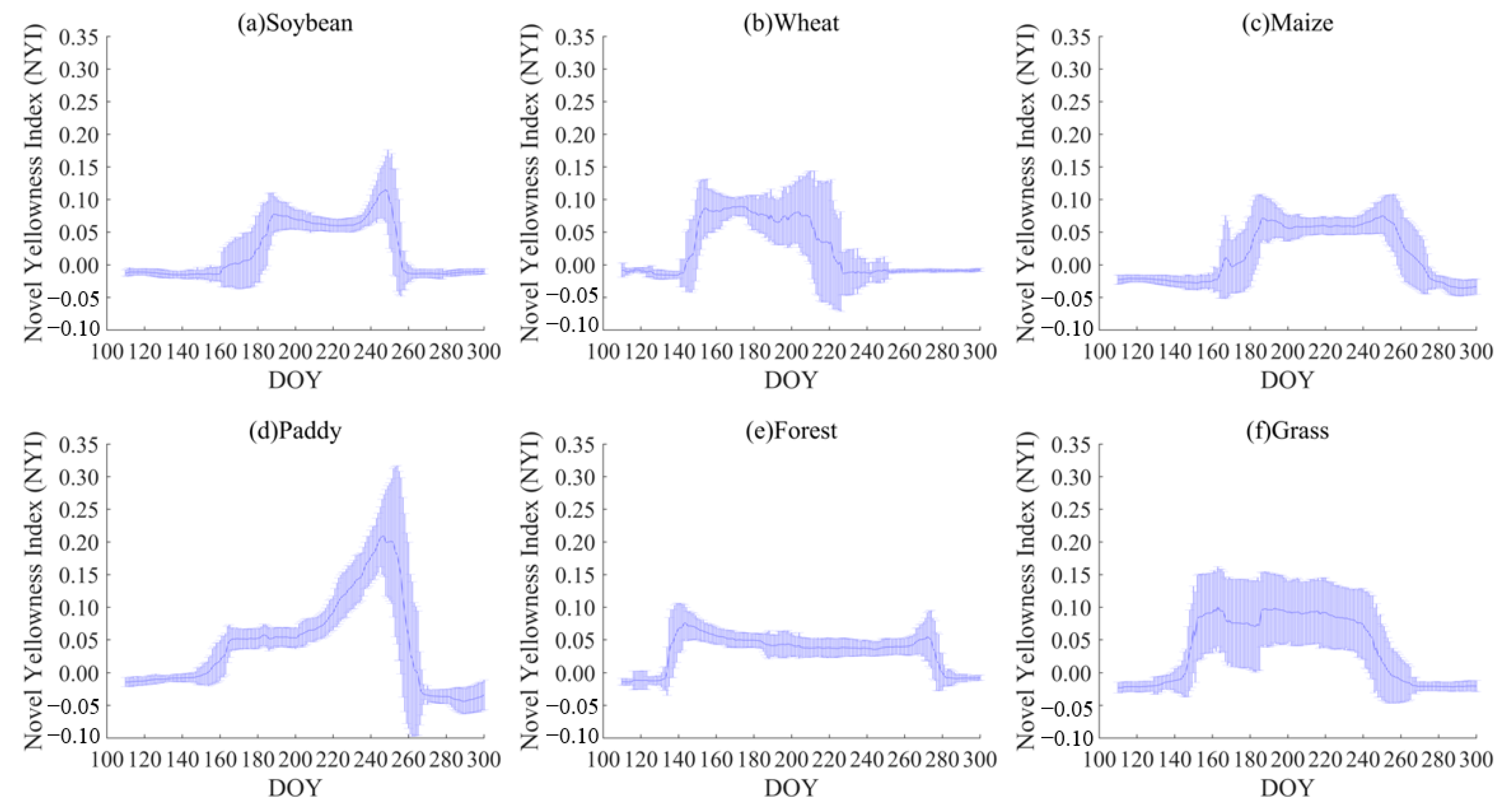
3.3. Yellowness Index of Typical Ground Objects
4. Results and Discussion
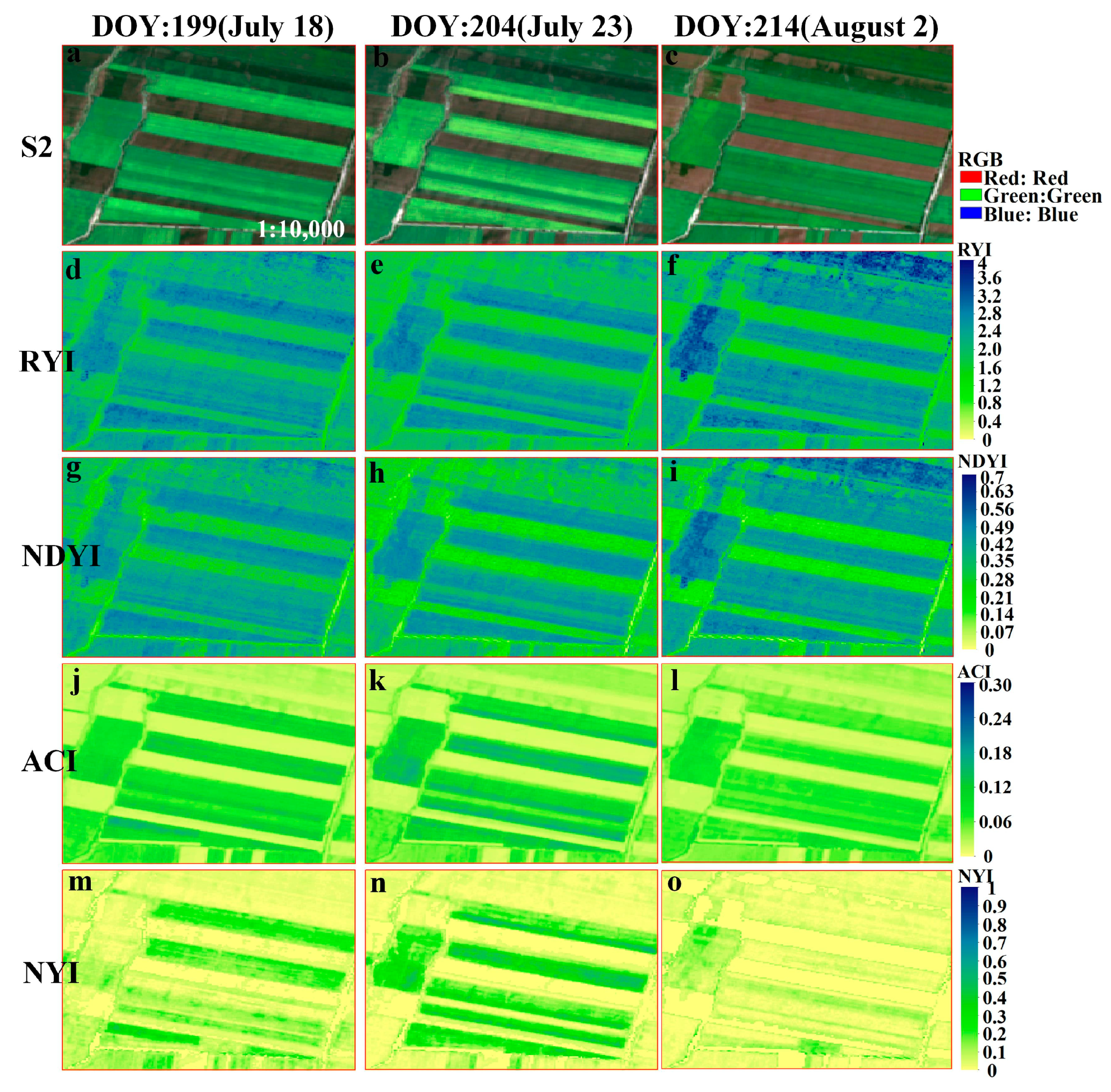
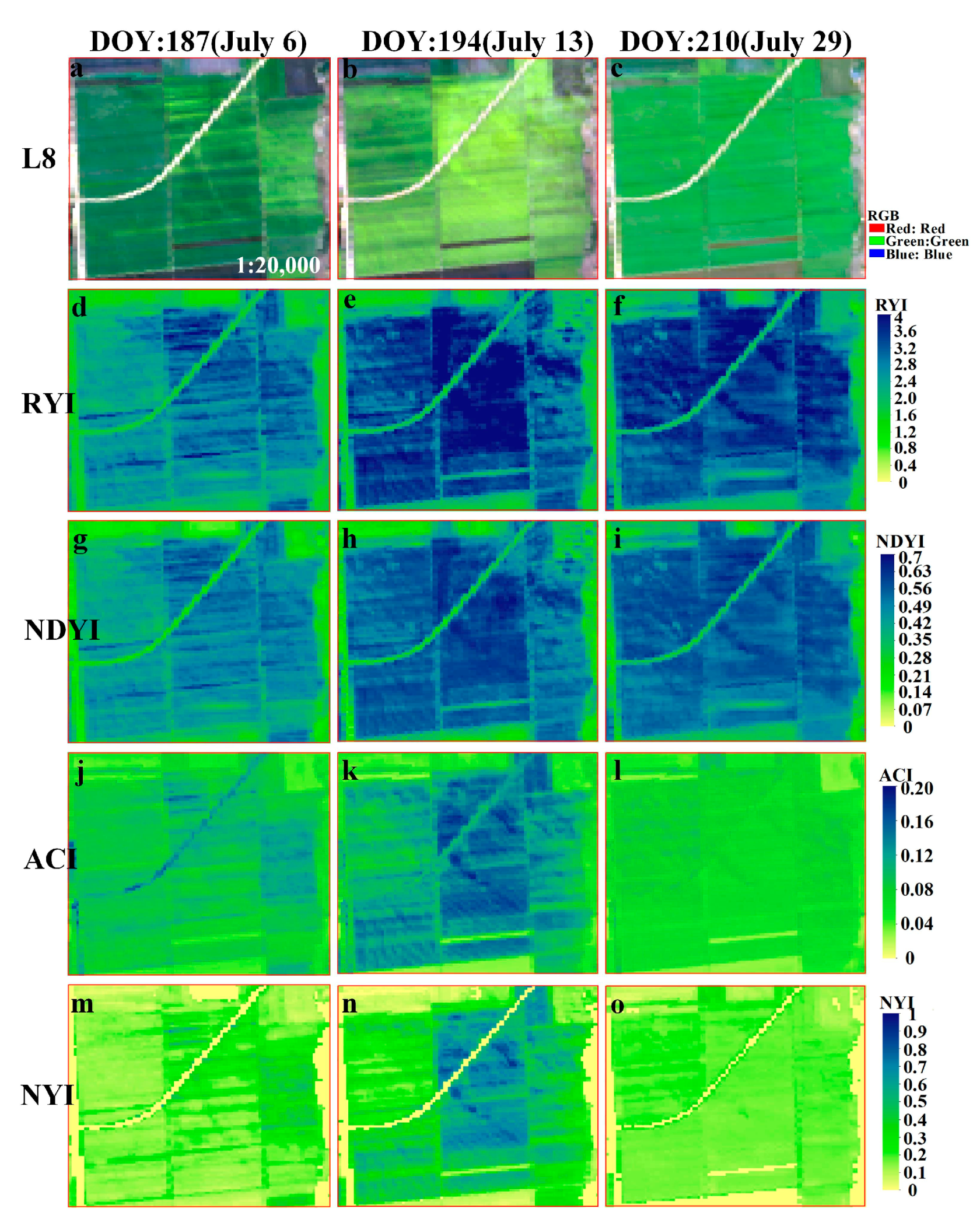
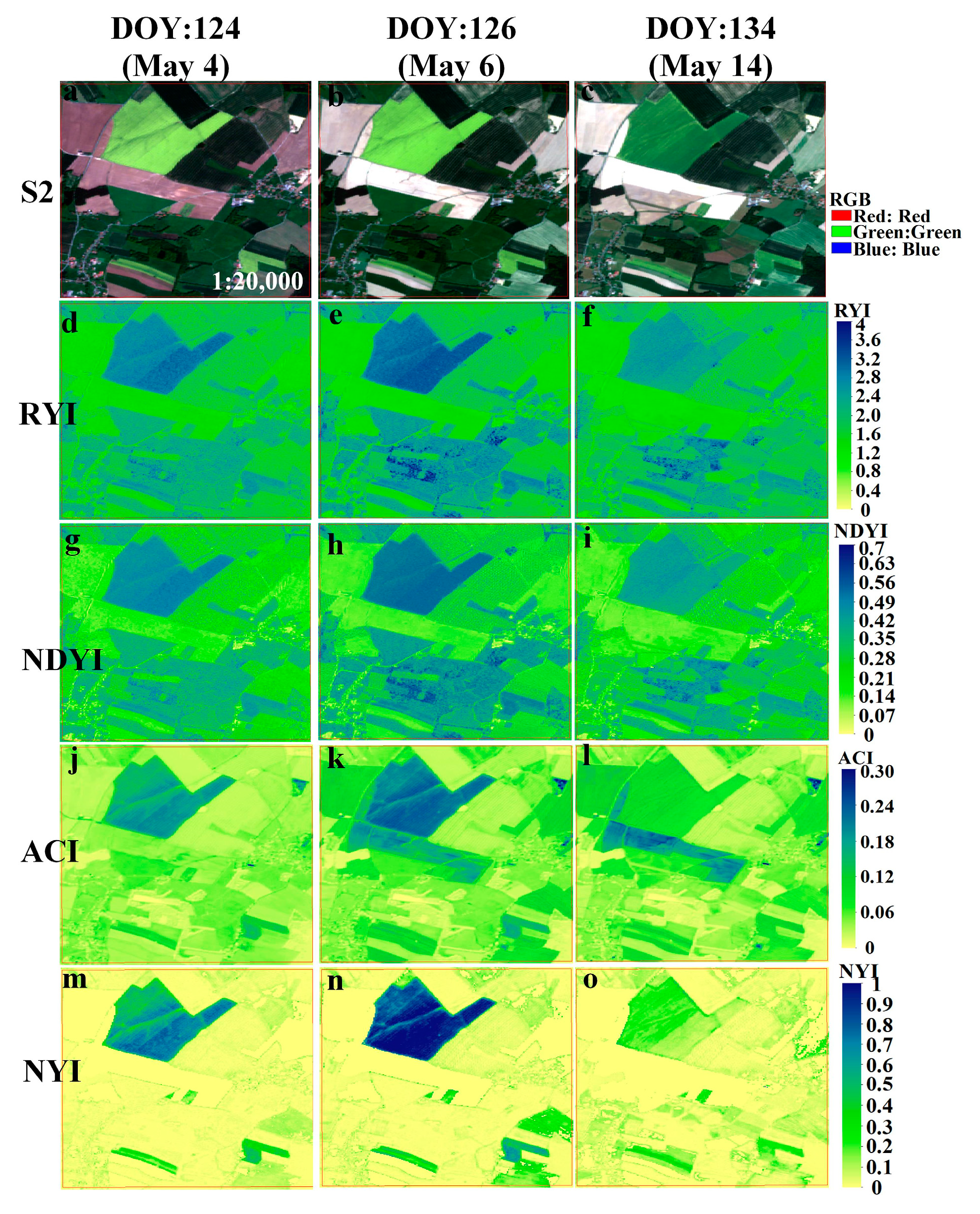
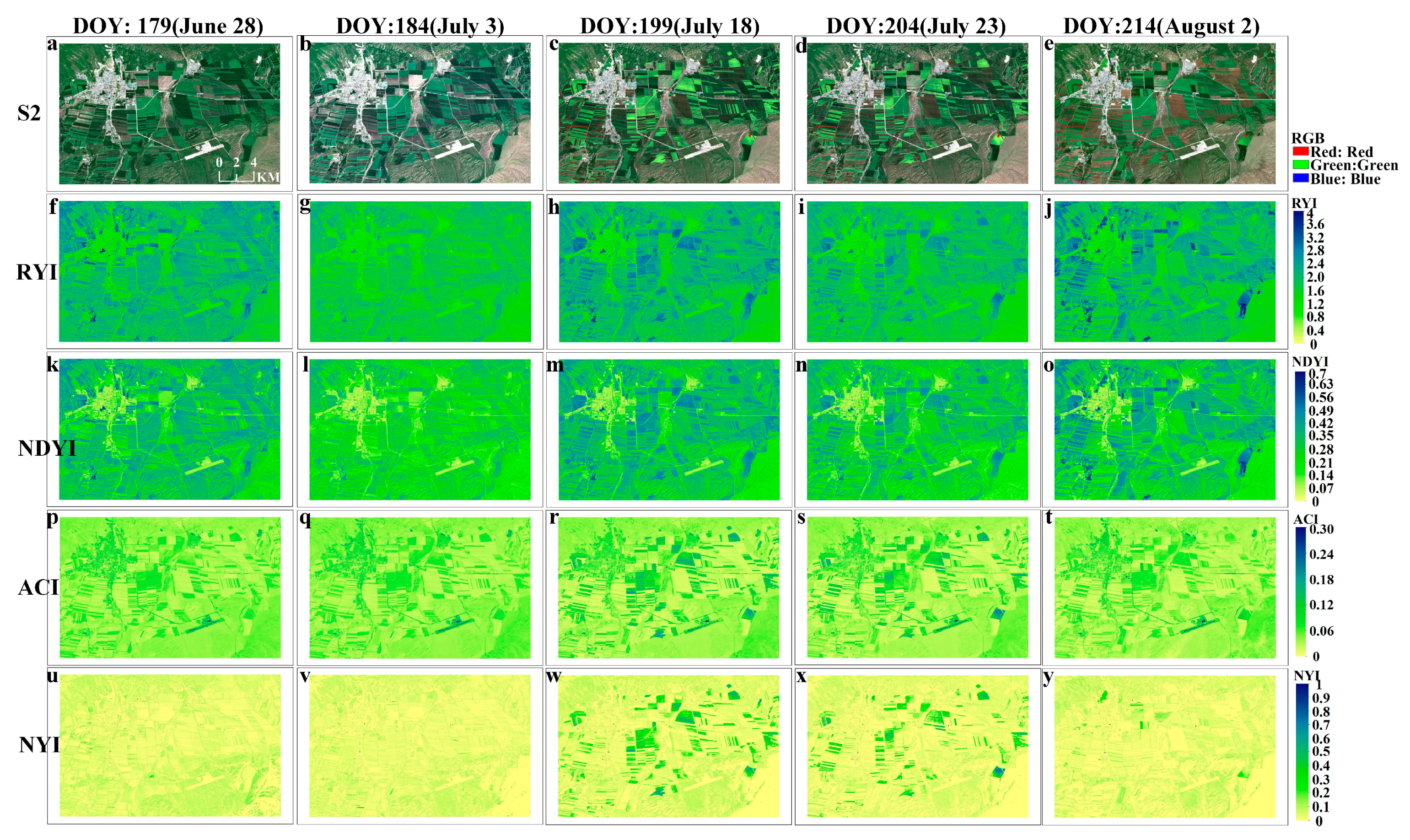
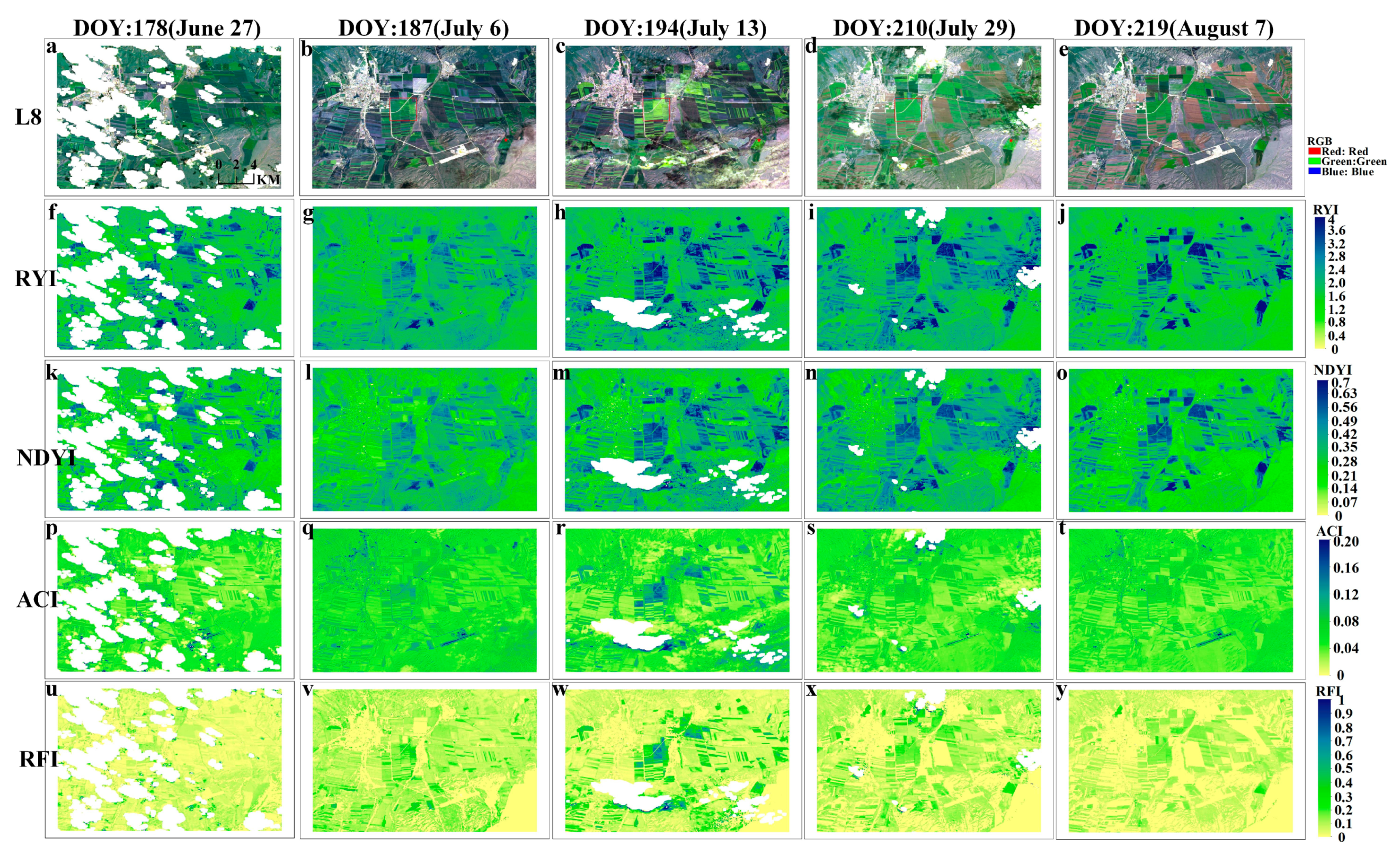
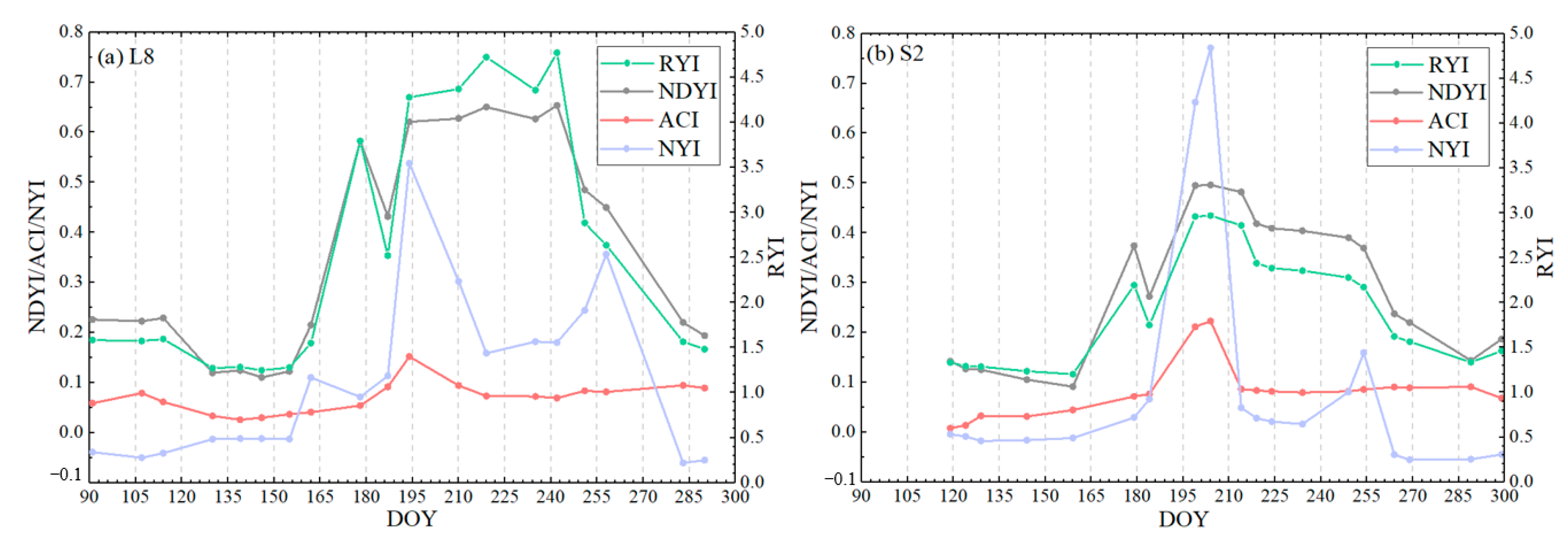
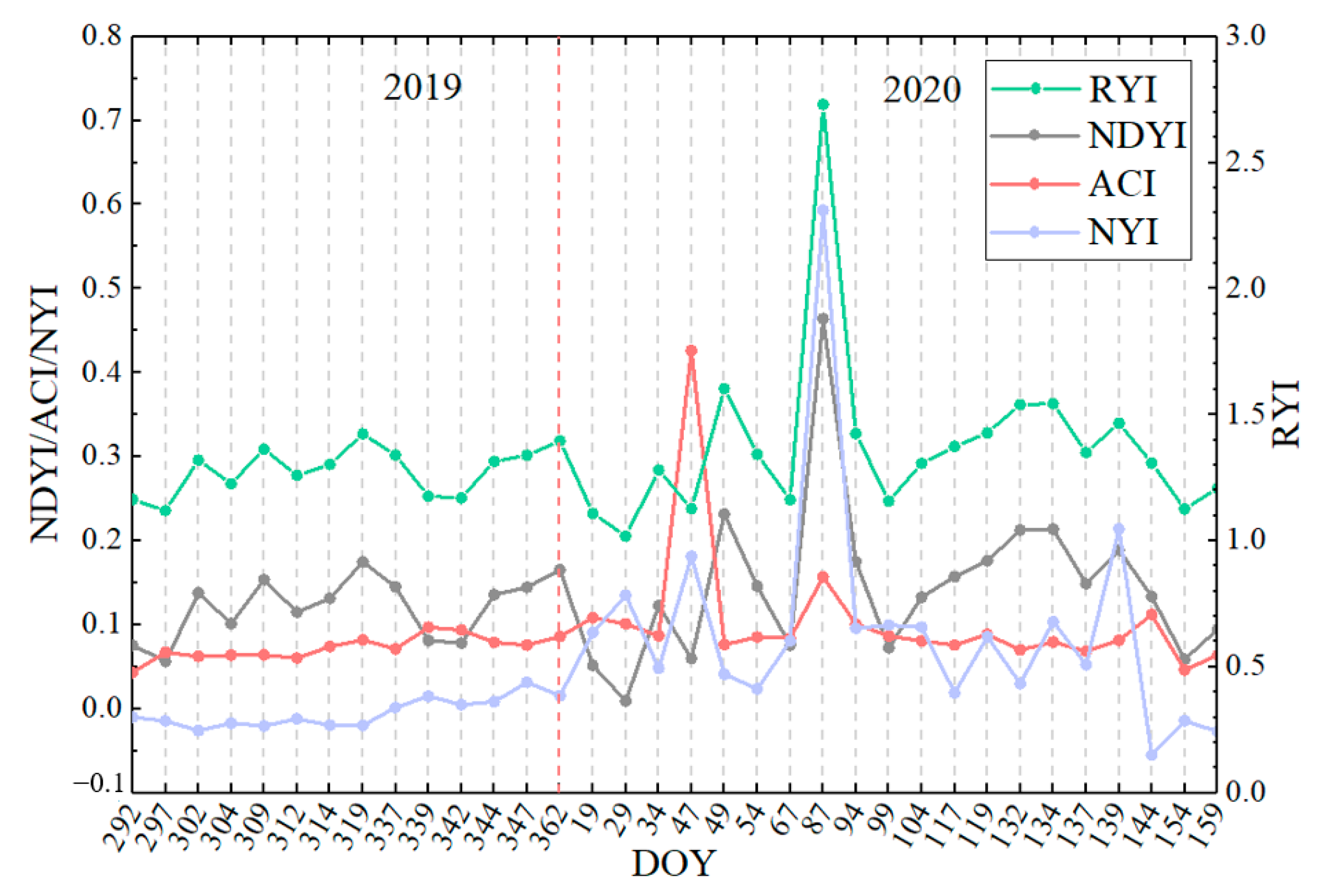
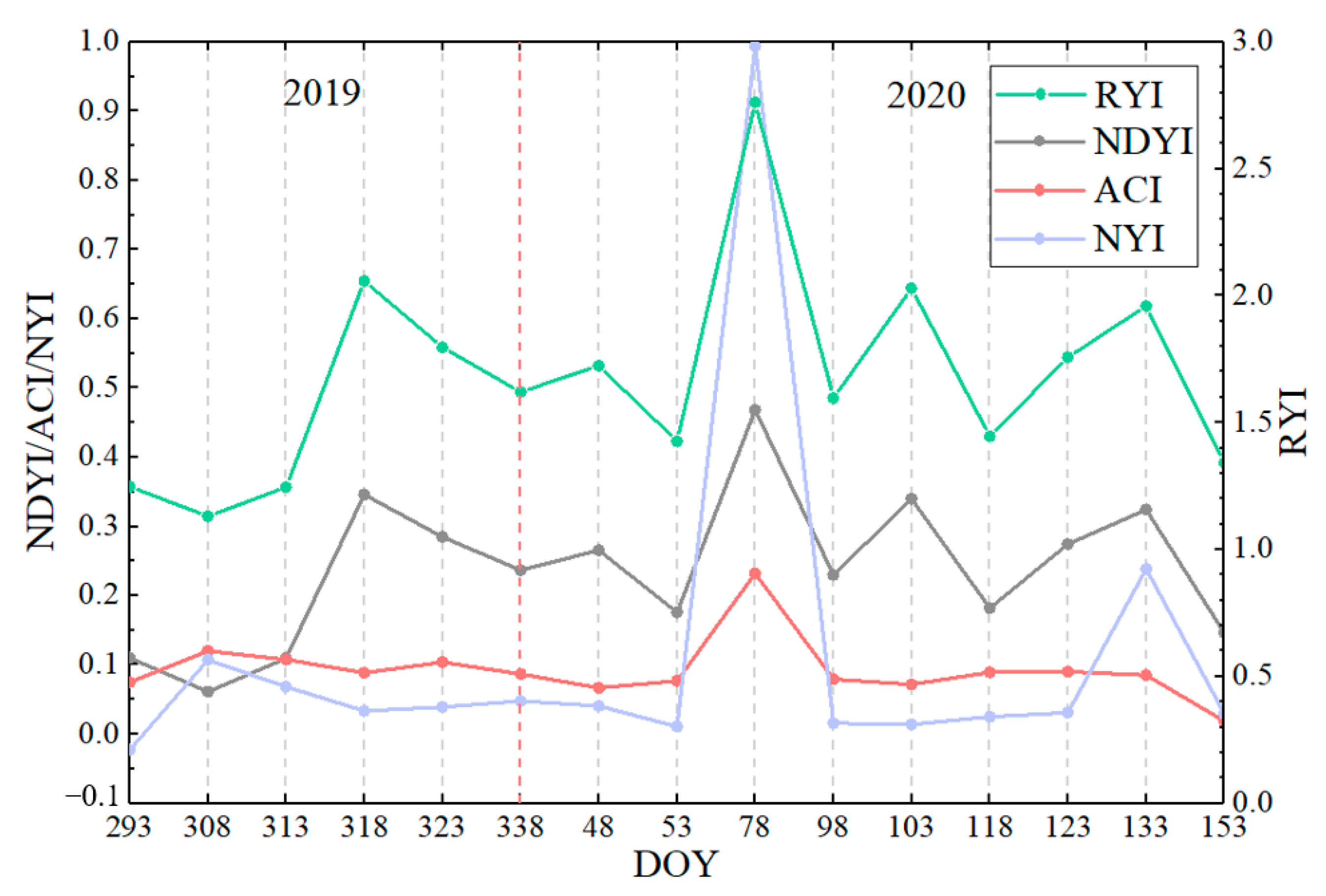
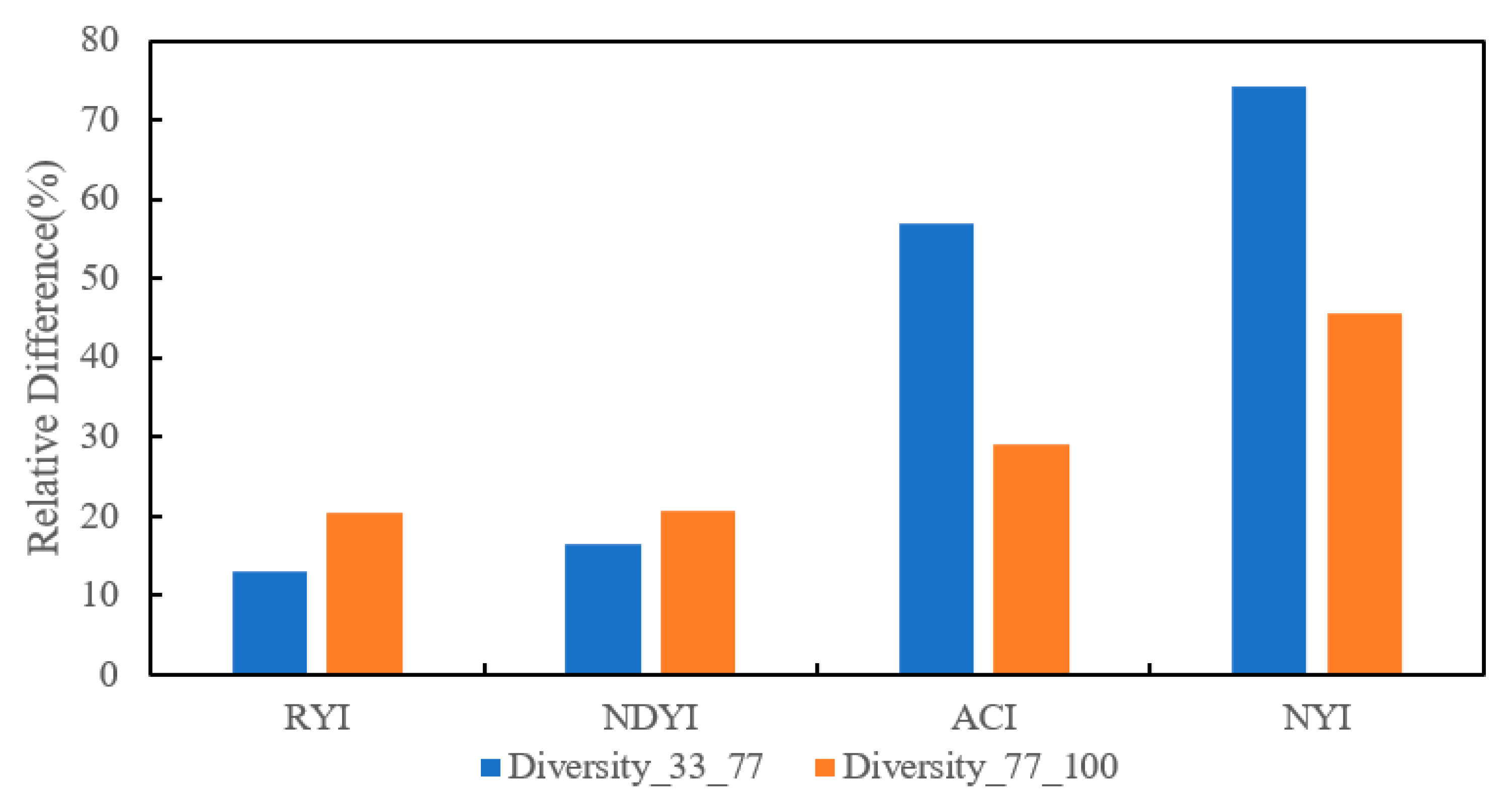
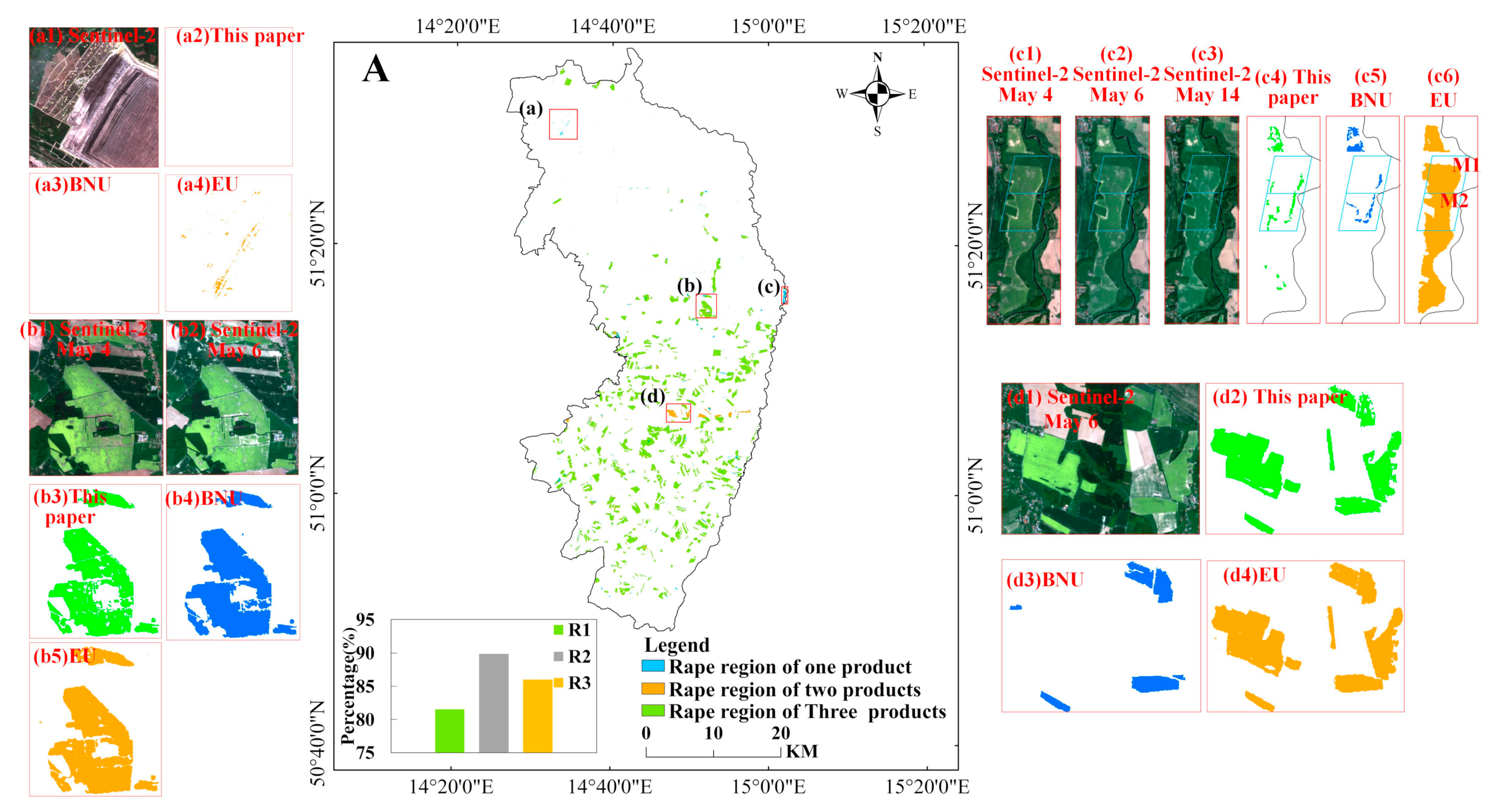
4.1. Comparison of Different Observation Scales
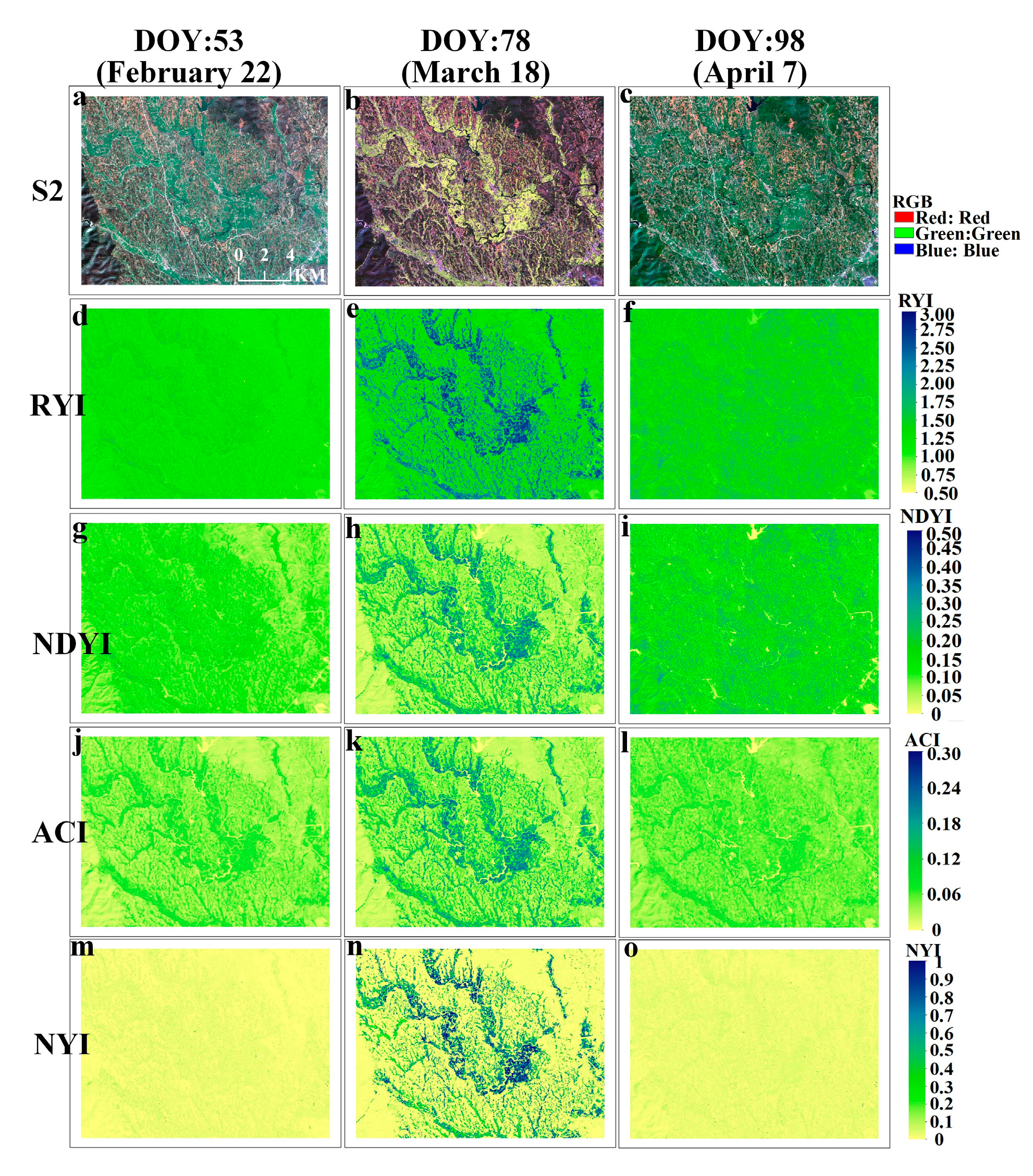
4.2. Comparison of Different Species of Yellow-Flowering Vegetation
4.3. Comparison of Different Flower Densities
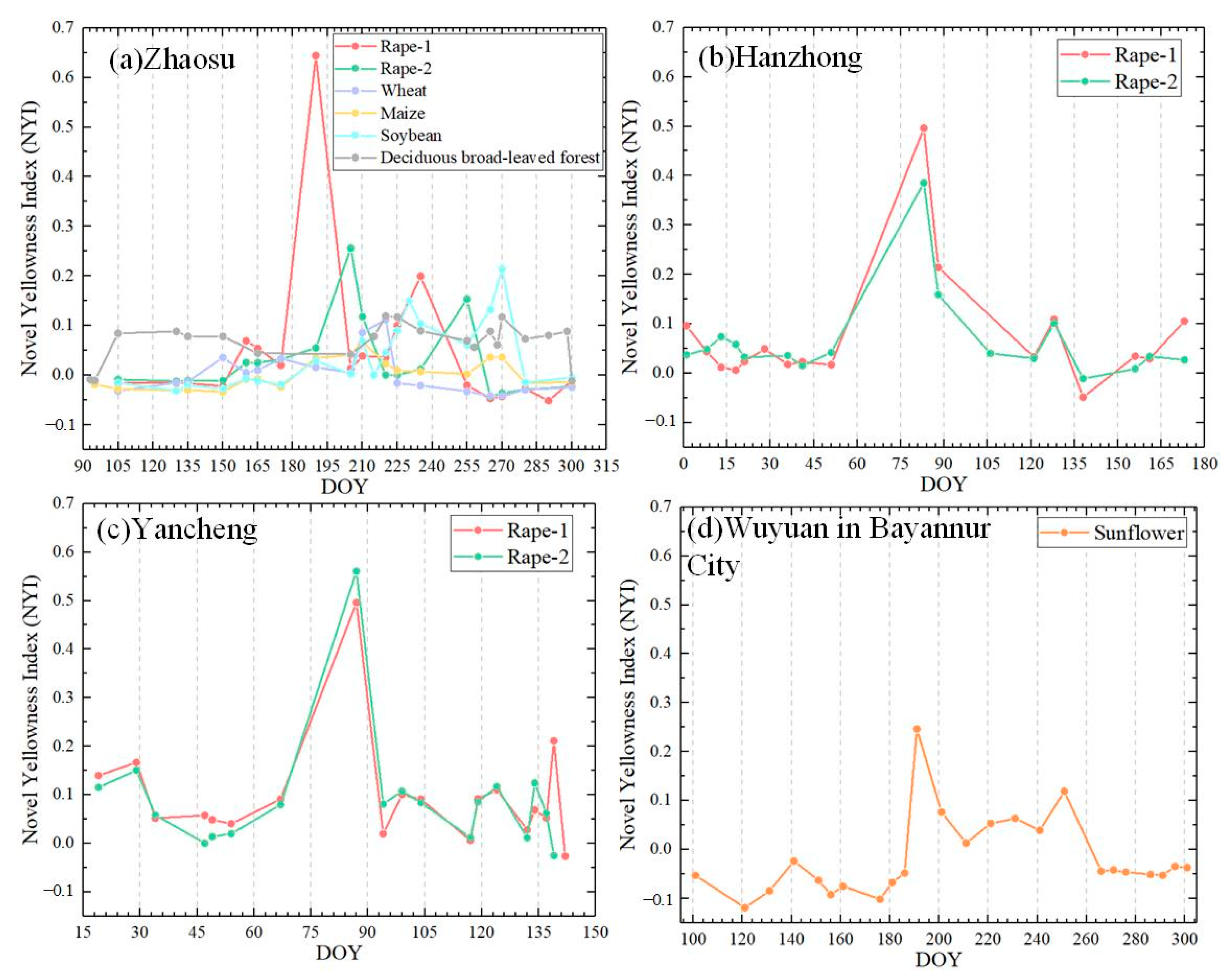
4.4. Comparison with Ground Phenological Observations
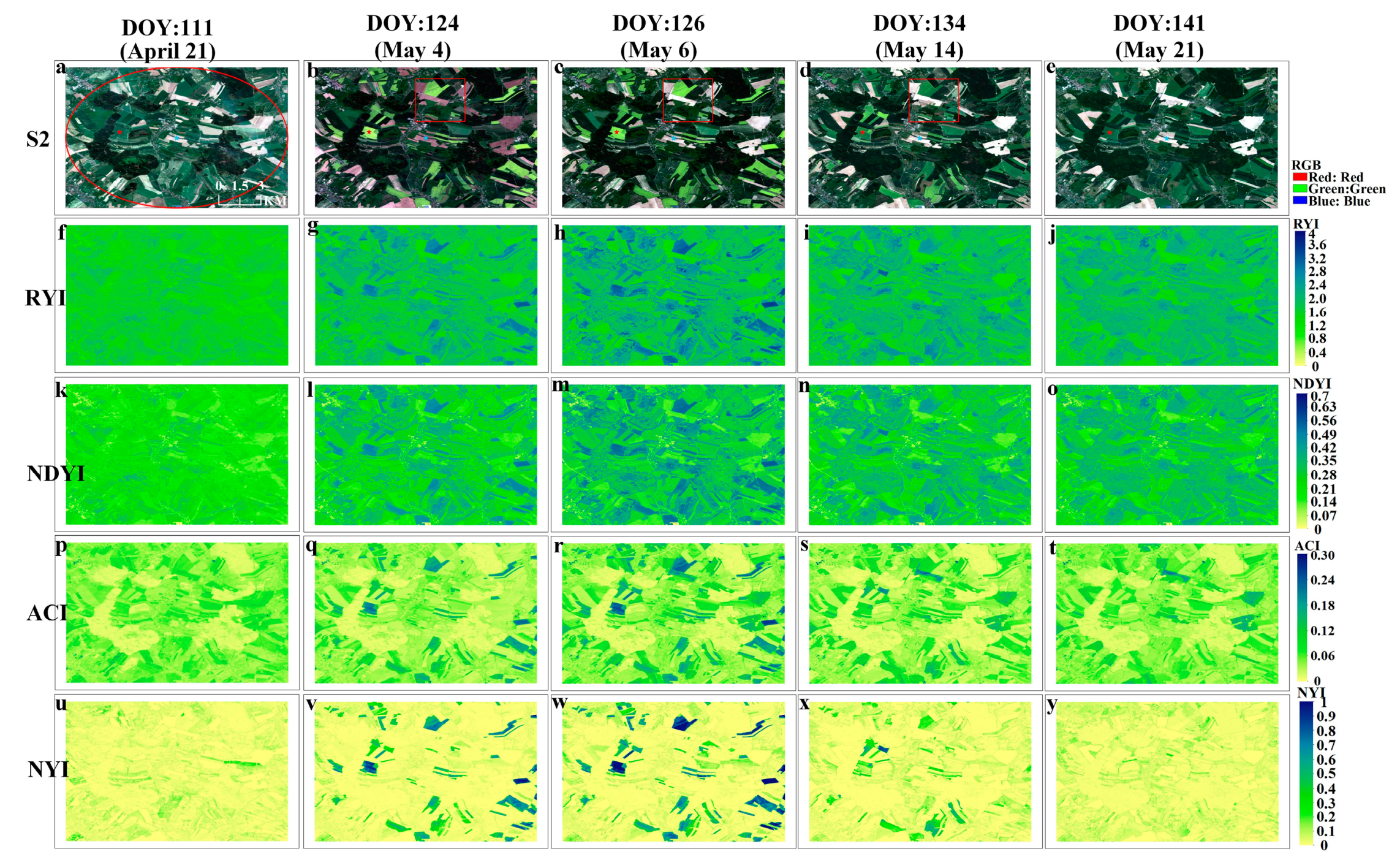
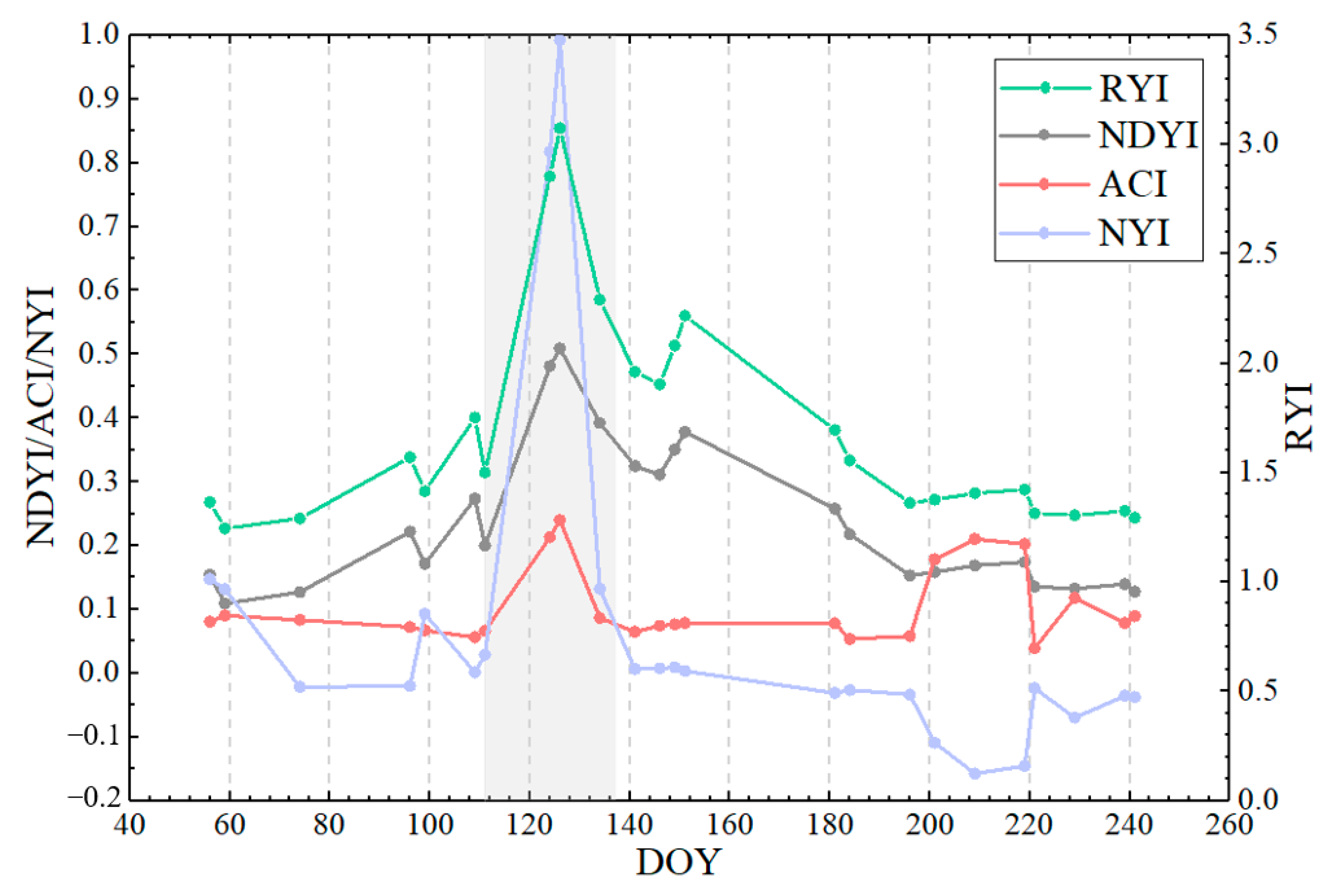
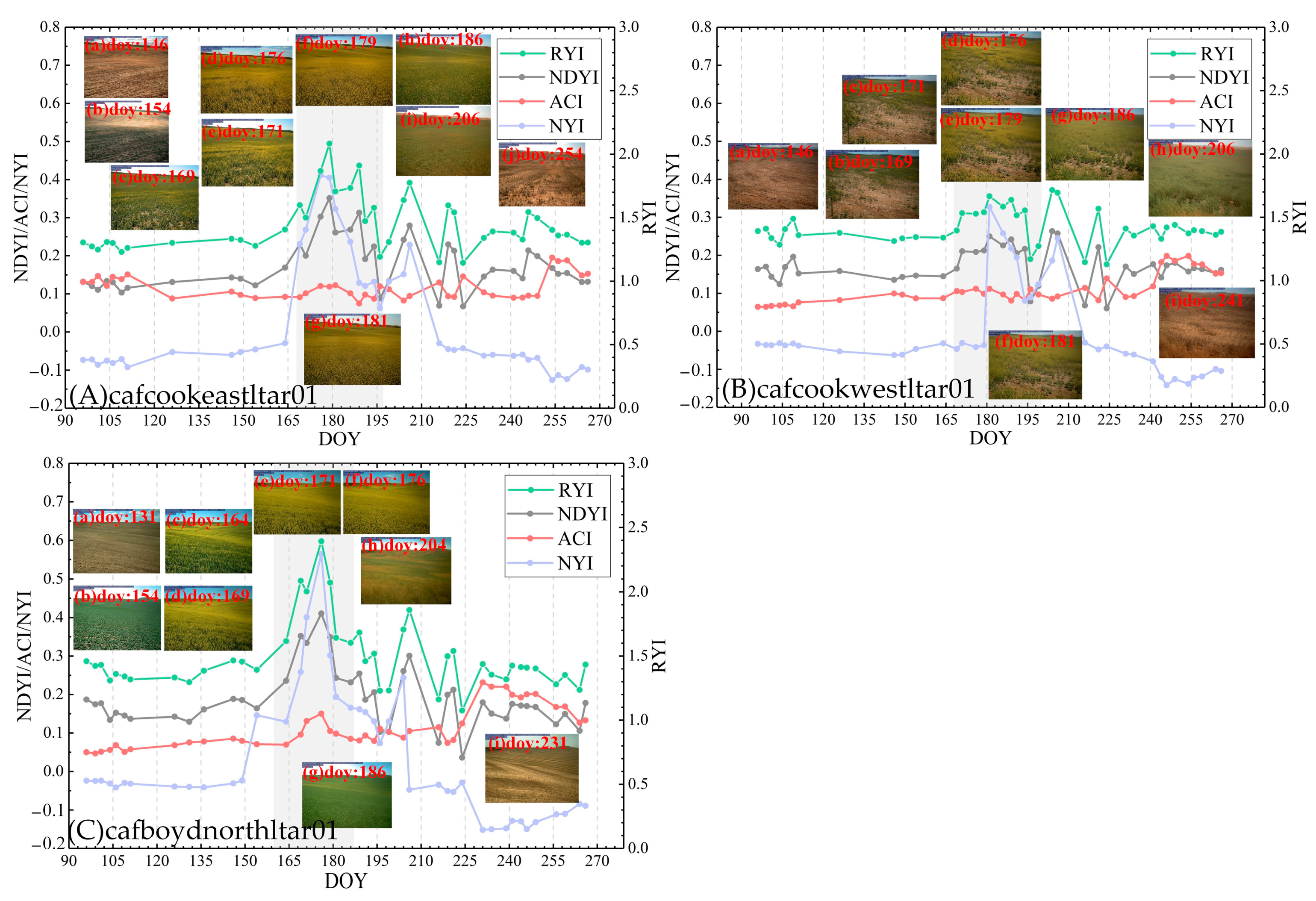
4.5. Application of Yellowness Index
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Fox, M.; Steltzer, H.; Trlica, M.J.; McMaster, G.S.; Andales, A.A.; LeCain, D.R.; Morgan, J.A. Elevated CO2 further lengthens growing season under warming conditions. Nature 2014, 510, 259–262. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Zhang, Y.; Dong, J.; Xiao, X. Green-up dates in the Tibetan Plateau have continuously advanced from 1982 to 2011. Proc. Natl. Acad. Sci. USA 2013, 110, 4309–4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, Q. Monitoring interannual variation in global crop yield using long-term AVHRR and MODIS observations. ISPRS J. Photogramm. Remote Sens. 2016, 114, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, N.E.; Ives, A.R. Effects of experimental shifts in flowering phenology on plant-pollinator interactions. Ecol. Lett. 2011, 14, 69–74. [Google Scholar] [CrossRef]
- Gezon, Z.J.; Inouye, D.W.; Irwin, R.E. Phenological change in a spring ephemeral: Implications for pollination and plant reproduction. Glob. Chang. Biol. 2016, 22, 1779–1793. [Google Scholar] [CrossRef]
- Forrest, J.R.K. Plant-pollinator interactions and phenological change: What can we learn about climate impacts from experiments and observations? Oikos 2015, 124, 4–13. [Google Scholar] [CrossRef]
- Burkle, L.A.; Marlin, J.C.; Knight, T.M. Plant-pollinator interactions over 120 years: Loss of species, co-occurrence, and function. Science 2013, 339, 1611–1615. [Google Scholar] [CrossRef] [Green Version]
- Garibaldi, L.A.; Carvalheiro, L.G.; Leonhardt, S.D.; Aizen, M.A.; Blaauw, B.R.; Isaacs, R.; Kuhlmann, M.; Kleijn, D.; Klein, A.M.; Kremen, C.; et al. From research to action: Enhancing crop yield through wild pollinators. Front. Ecol. Environ. 2014, 12, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Schiessl, S.; Iniguez-Luy, F.; Qian, W.; Snowdon, R.J. Diverse regulatory factors associate with flowering time and yield responses in winter-type Brassica napus. BMC Genom. 2015, 16, 737. [Google Scholar] [CrossRef] [Green Version]
- Stratonovitch, P.; Semenov, M.A. Heat tolerance around flowering in wheat identified as a key trait for increased yield potential in Europe under climate change. J. Exp. Bot. 2015, 66, 3599–3609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craufurd, P.Q.; Wheeler, T.R. Climate change and the flowering time of annual crops. J. Exp. Bot. 2009, 60, 2529–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, D.J.; Callow, J.N.; Duncan, J.M.A.; Setterfield, S.A.; Pauli, N. Satellite prediction of forest flowering phenology. Remote Sens. Environ. 2021, 255, 112197. [Google Scholar] [CrossRef]
- Shuai, Y.; Schaaf, C.; Zhang, X.; Strahler, A.; Roy, D.; Morisette, J.; Wang, Z.; Nightingale, J.; Nickeson, J.; Richardson, A.D.; et al. Daily MODIS 500 m reflectance anisotropy direct broadcast (DB) products for monitoring vegetation phenology dynamics. Int. J. Remote Sens. 2013, 34, 5997–6016. [Google Scholar] [CrossRef]
- Misra, G.; Cawkwell, F.; Wingler, A. Status of Phenological Research Using Sentinel-2 Data: A Review. Remote Sens. 2020, 12, 2760. [Google Scholar] [CrossRef]
- Chen, B.; Jin, Y.F.; Brown, P. An enhanced bloom index for quantifying floral phenology using multi-scale remote sensing observations. ISPRS J. Photogramm. Remote Sens. 2019, 156, 108–120. [Google Scholar] [CrossRef]
- Sulik, J.J.; Long, D.S. Spectral indices for yellow canola flowers. Int. J. Remote Sens. 2015, 36, 2751–2765. [Google Scholar] [CrossRef]
- Sulik, J.J.; Long, D.S. Spectral considerations for modeling yield of canola. Remote Sens. Environ. 2016, 184, 161–174. [Google Scholar] [CrossRef] [Green Version]
- D’Andrimont, R.; Taymans, M.; Lemoine, G.; Ceglar, A.; Yordanov, M.; van der Velde, M. Detecting flowering phenology in oil seed rape parcels with Sentinel-1 and -2 time series. Remote Sens. Environ. 2020, 239, 111660. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Z.; Cao, J. Developing a New Method to Identify Flowering Dynamics of Rapeseed Using Landsat 8 and Sentinel-1/2. Remote Sens. 2021, 13, 105. [Google Scholar] [CrossRef]
- Ashourloo, D.; Shahrabi, H.S.; Azadbakht, M.; Aghighi, H.; Nematollahi, H.; Alimohammadi, A.; Matkan, A.A. Automatic canola mapping using time series of sentinel 2 images. ISPRS J. Photogramm. Remote Sens. 2019, 156, 63–76. [Google Scholar] [CrossRef]
- Tian, H.; Chen, T.; Li, Q.; Mei, Q.; Wang, S.; Yang, M.; Wang, Y.; Qin, Y. A Novel Spectral Index for Automatic Canola Mapping by Using Sentinel-2 Imagery. Remote Sens. 2022, 14, 1113. [Google Scholar] [CrossRef]
- Rengarajan, R.; Choate, M.; Storey, J.; Franks, S.; Micijevic, E. Landsat Collection-2 geometric calibration updates. In Proceedings of the Earth Observing Systems XXV, Online, 24 August–4 September 2020; pp. 85–95. [Google Scholar]
- Languille, F.; Déchoz, C.; Gaudel, A.; Greslou, D.; De Lussy, F.; Trémas, T.; Poulain, V. Sentinel-2 geometric image quality commissioning: First results. In Proceedings of the Image and Signal Processing for Remote Sensing XXI, Toulouse, France, 21–23 September 2015; pp. 61–73. [Google Scholar]
- D’Andrimont, R.; Verhegghen, A.; Lemoine, G.; Kempeneers, P.; Meroni, M.; van der Velde, M. From parcel to continental scale—A first European crop type map based on Sentinel-1 and LUCAS Copernicus in-situ observations. Remote Sens. Environ. 2021, 266, 112708. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Z.; Luo, Y.; Cao, J.; Zhang, L.; Zhang, J.; Li, Z. Developing a phenology-and pixel-based algorithm for mapping rapeseed at 10m spatial resolution using multi-source data. Earth Syst. Sci. Data 2021, 2021, 1–31. [Google Scholar] [CrossRef]
- Kaspar, F.; Zimmermann, K.; Polte-Rudolf, C. An overview of the phenological observation network and the phenological database of Germany’s national meteorological service (Deutscher Wetterdienst). Adv. Sci. Res. 2015, 11, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Böttcher, U.; Rampin, E.; Hartmann, K.; Zanetti, F.; Flenet, F.; Morison, M.; Kage, H. A phenological model of winter oilseed rape according to the BBCH scale. Crop Pasture Sci. 2016, 67, 345–358. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hufkens, K.; Milliman, T.; Aubrecht, D.M.; Chen, M.; Gray, J.M.; Johnston, M.R.; Keenan, T.F.; Klosterman, S.T.; Kosmala, M.; et al. Tracking vegetation phenology across diverse North American biomes using PhenoCam imagery. Sci. Data 2018, 5, 180028. [Google Scholar] [CrossRef]
- Wilson, J.; Zhang, C.; Kovacs, J. Separating Crop Species in Northeastern Ontario Using Hyperspectral Data. Remote Sens. 2014, 6, 925–945. [Google Scholar] [CrossRef] [Green Version]
- Yates, D.J.; Steven, M.D. Reflexion and absorption of solar radiation by flowering canopies of oil-seed rape (Brassica napus L.). J. Agric. Sci.-Camb. 2009, 109, 495–502. [Google Scholar] [CrossRef]
- Migdall, S.; Ohl, N.; Bach, H. Parameterisation of the Land Surface Reflectance Model SLC for Winter Rape Using Spaceborne Hyperspectral CHRIS Data. In Proceedings of the Hyperspectral 2010 Workshop, Frascati, Italy, 17–19 March 2010. [Google Scholar]
- Wójtowicz, M.; Wójtowicz, A.; Piekarczyk, J. Application of remote sensing methods in agriculture. Commun. Biometry Crop Sci. 2016, 11, 31–50. [Google Scholar]
- Shen, M.; Chen, J.; Zhu, X.; Tang, Y. Yellow flowers can decrease NDVI and EVI values: Evidence from a field experiment in an alpine meadow. Can. J. Remote Sens. 2014, 35, 99–106. [Google Scholar] [CrossRef]
- Wang, D.; Fang, S.; Yang, Z.; Wang, L.; Tang, W.; Li, Y.; Tong, C. A Regional Mapping Method for Oilseed Rape Based on HSV Transformation and Spectral Features. ISPRS Int. J. Geo-Inf. 2018, 7, 224. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Vail, S.; Duddu, H.S.N.; Parkin, I.A.P.; Guo, X.; Johnson, E.N.; Shirtliffe, S.J. Phenotyping Flowering in Canola (Brassica napus L.) and Estimating Seed Yield Using an Unmanned Aerial Vehicle-Based Imagery. Front. Plant Sci. 2021, 12, 686332. [Google Scholar] [CrossRef] [PubMed]







| Spectral Library | Feature Type—Broad Categories | Feature Type—Subcategory | Spectrometer | Spectrum Number |
|---|---|---|---|---|
| Aster | Soil | Black loam, brown fine sandy loam, grayish brown loam, light yellowish-brown clay, brown to dark brown gravelly fine sandy loam, etc. | Becknic | 41 |
| Water Body | Ice, fine snow, medium granular snow, coarse granular snow, frost, tap water | Becknic | 6 | |
| USGS | Green Vegetation | Fir, whitebark, cactus, geranium, pansy, petunia, aspen, lichen, etc. | ASD | 41 |
| AVIRIS | 39 | |||
| Veg (ENVI) | Green Vegetation | Bay laurel, red willow, coast redwood, etc. | Beckman | 16 |
| Total | 143 | |||
| Rape | Non-Rape | User Accuracy | |
|---|---|---|---|
| Rape | 67 | 3 | 95.71 |
| Non-Rape | 2 | 128 | 98.46 |
| Producer Accuracy | 97.1 | 97.71 | |
| Overall Accuracy = 97.5%, Kappa coefficient = 0.94 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, C.; Shuai, Y.; Wu, H.; Deng, X.; Zhang, X.; Xu, A. Development of a Spectral Index for the Detection of Yellow-Flowering Vegetation. Remote Sens. 2023, 15, 1725. https://doi.org/10.3390/rs15071725
Shao C, Shuai Y, Wu H, Deng X, Zhang X, Xu A. Development of a Spectral Index for the Detection of Yellow-Flowering Vegetation. Remote Sensing. 2023; 15(7):1725. https://doi.org/10.3390/rs15071725
Chicago/Turabian StyleShao, Congying, Yanmin Shuai, Hao Wu, Xiaolian Deng, Xuecong Zhang, and Aigong Xu. 2023. "Development of a Spectral Index for the Detection of Yellow-Flowering Vegetation" Remote Sensing 15, no. 7: 1725. https://doi.org/10.3390/rs15071725






