Assessing Drought Stress of Sugarcane Cultivars Using Unmanned Vehicle System (UAS)-Based Vegetation Indices and Physiological Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
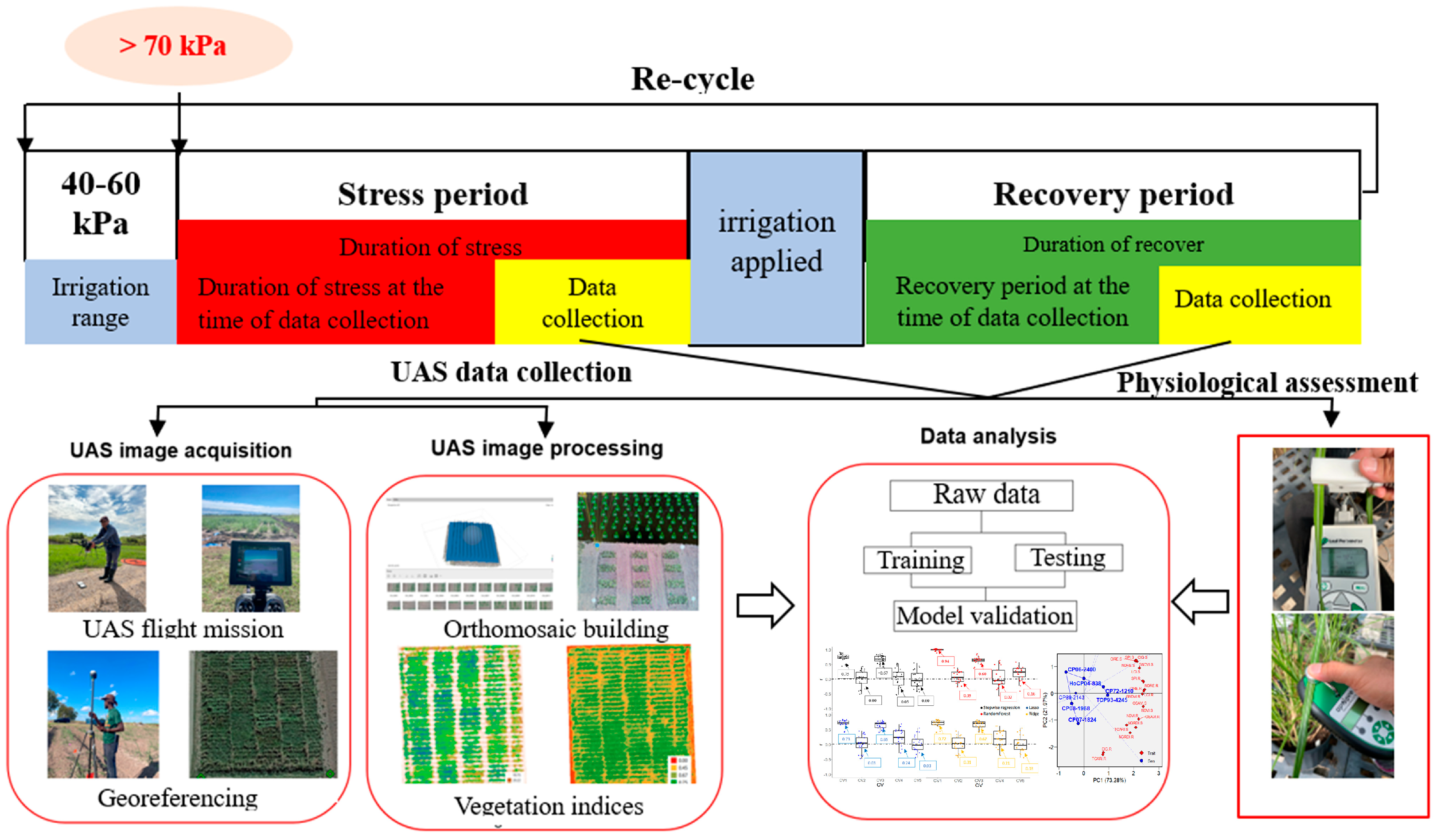
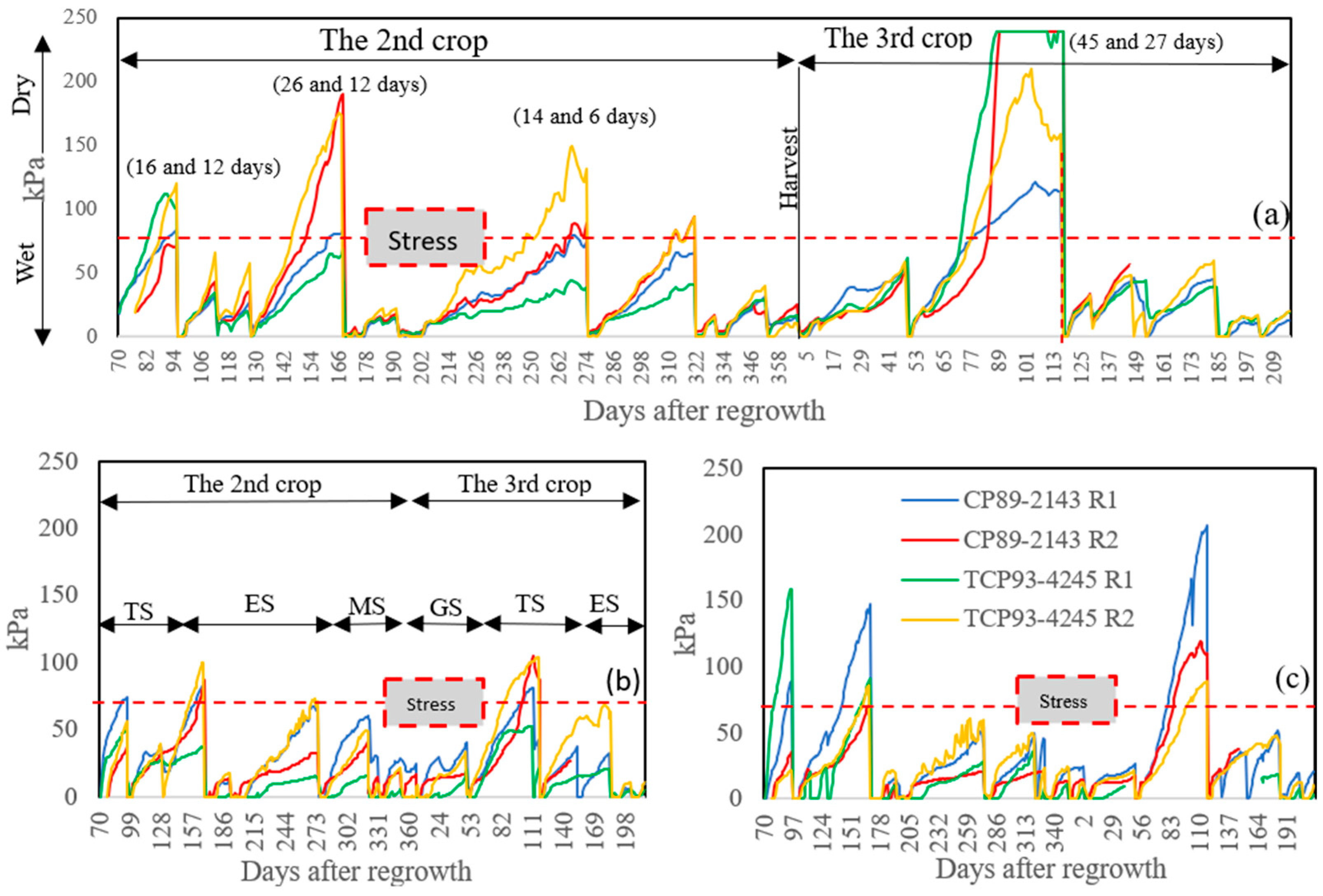
2.2. Drought Stress and Recovery Determination
2.3. UAS Data Collection and Processing
2.3.1. UAS Image Acquisition
2.3.2. Image Processing
2.4. Physiological Assessments
2.4.1. Stomatal Conductance (Gs)
2.4.2. Chlorophyll Content Meter (Chl)
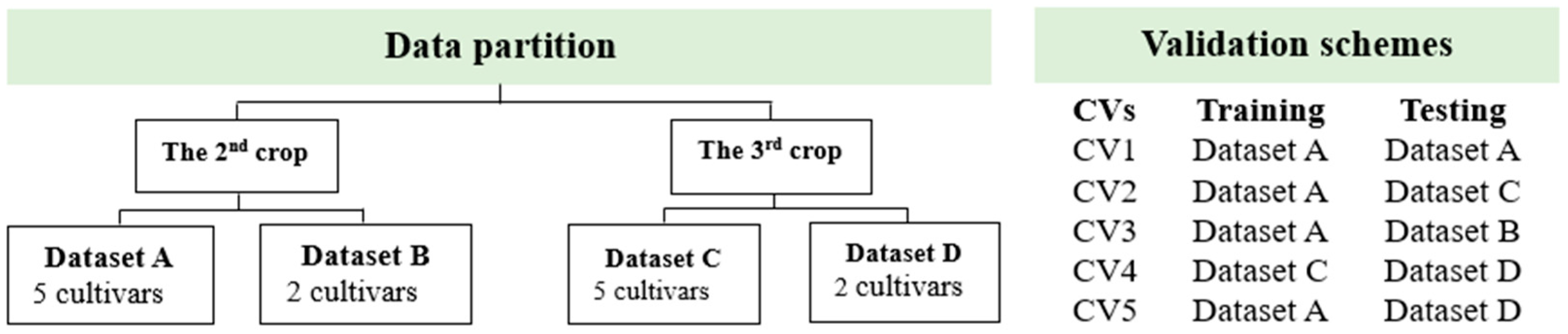
2.5. Data Analysis
2.5.1. Identification of Drought-Tolerant Cultivars of Sugarcane
2.5.2. Vegetation Indices (VIs)-Based Gs and Chl Prediction Model
3. Results
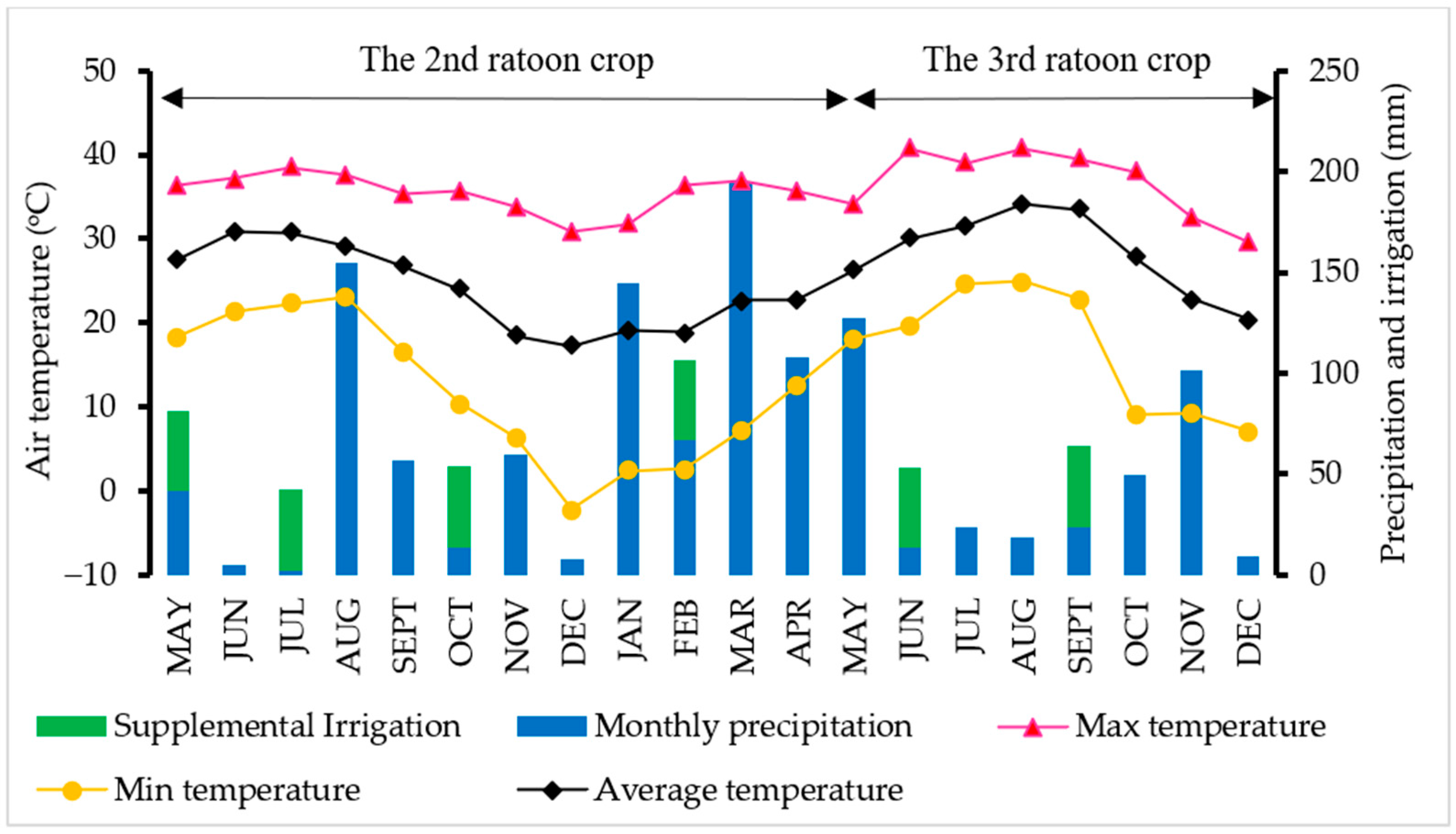
3.1. Soil Moisture and Weather Data across the Growing Season
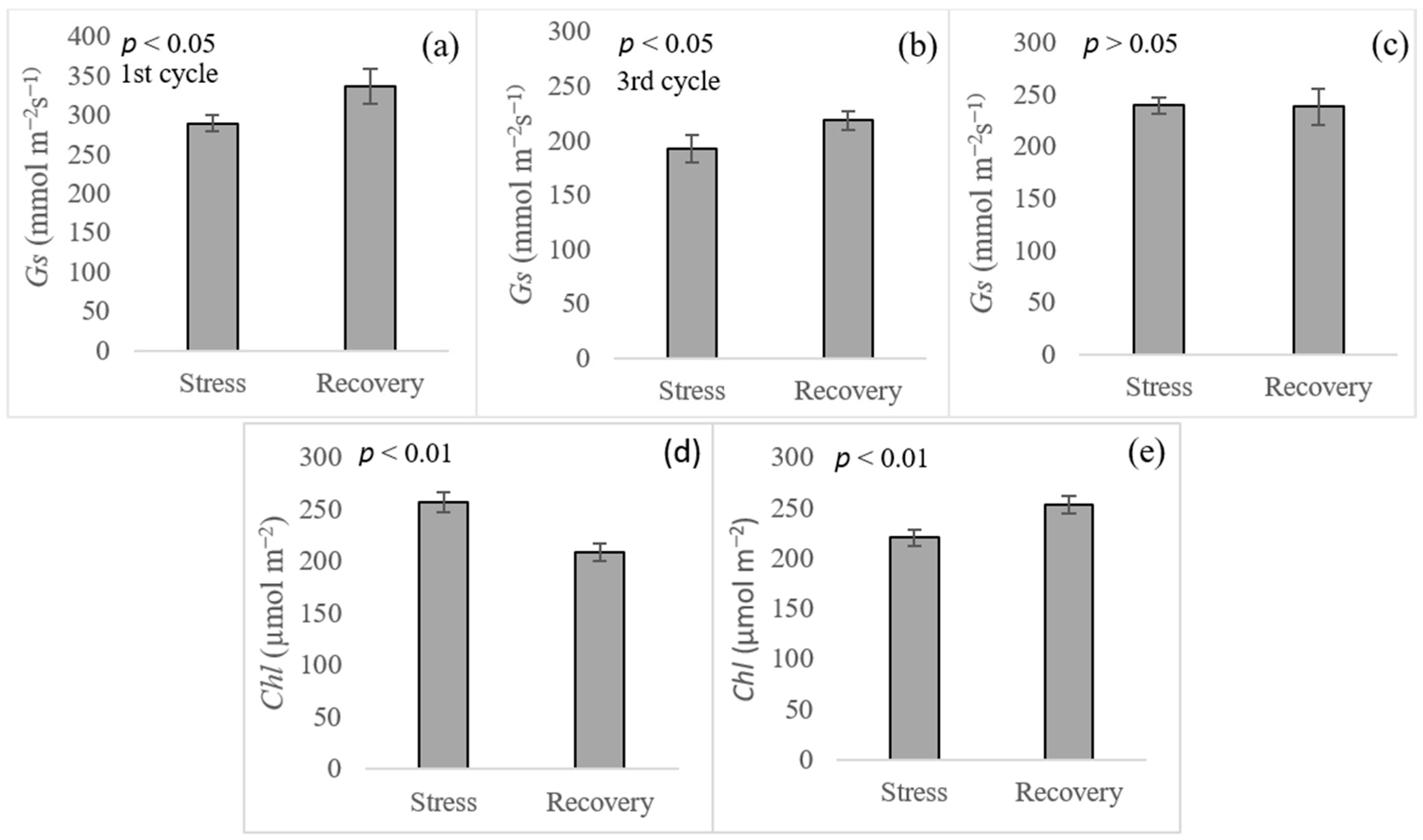
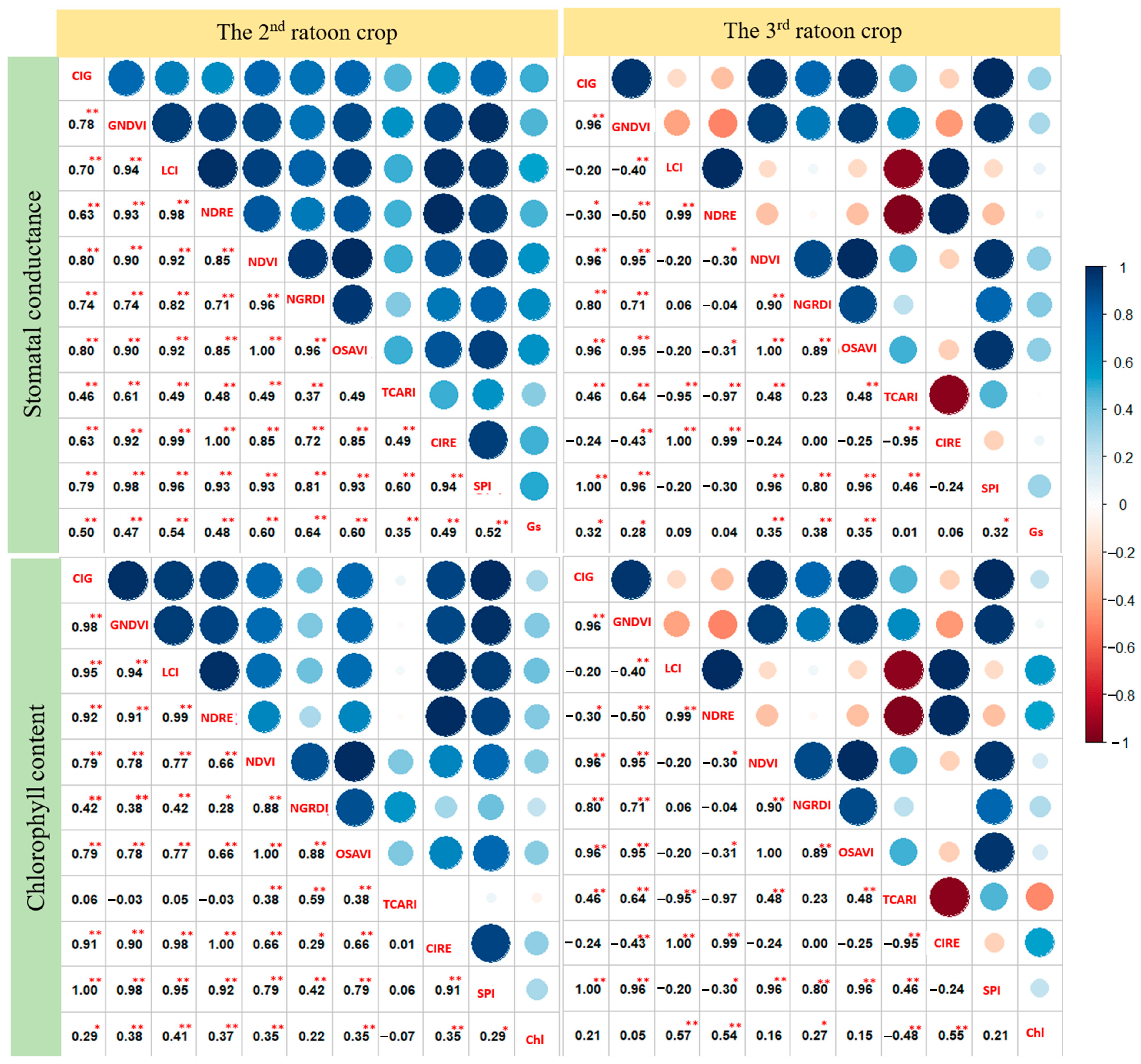
3.2. Analysis of Variance and Correlation among the Studied Traits
3.3. Identification of Drought-Tolerant Cultivar
3.3.1. Cultivar Ranking Based on the Performance of Vegetation Indices (VIs)
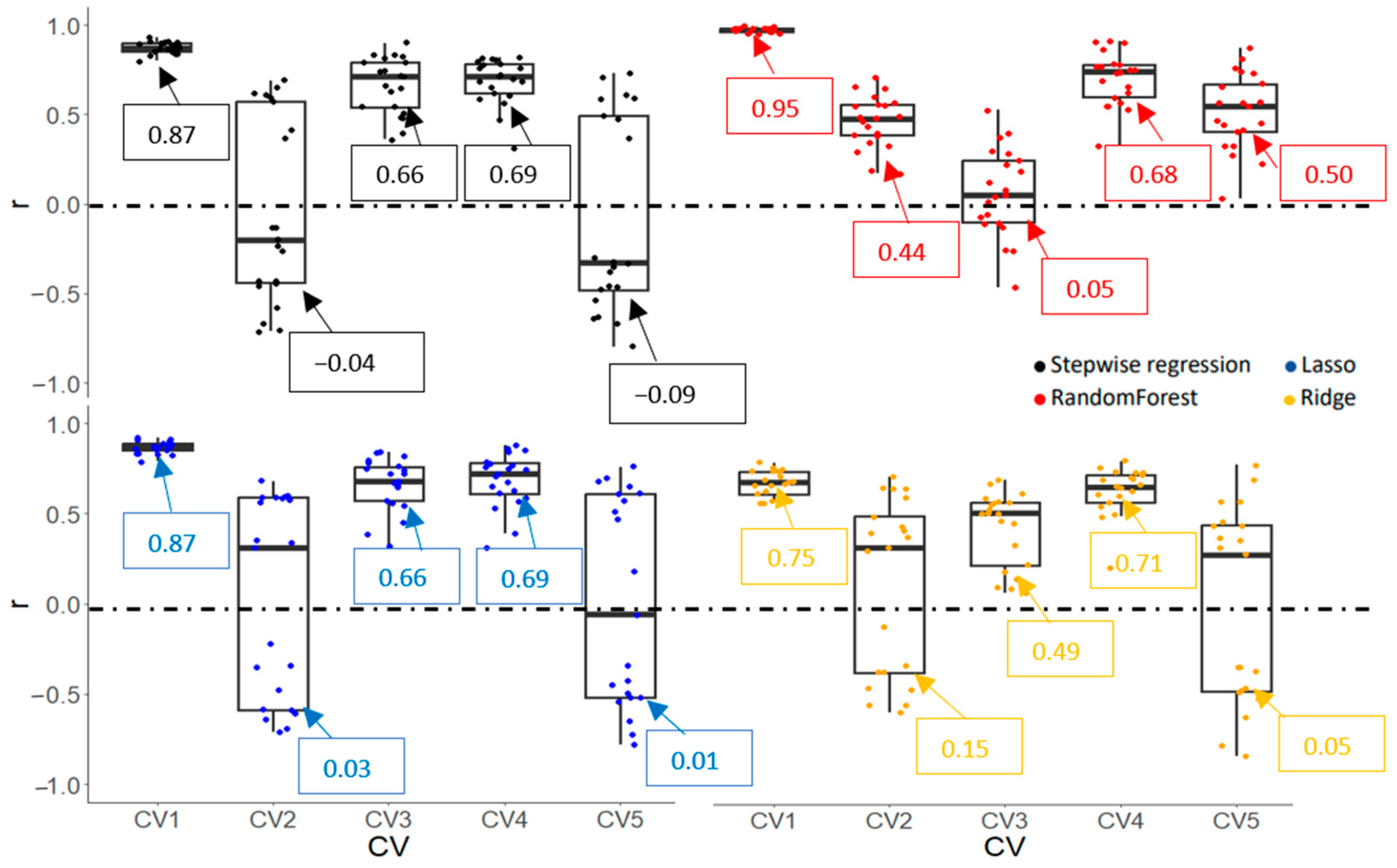
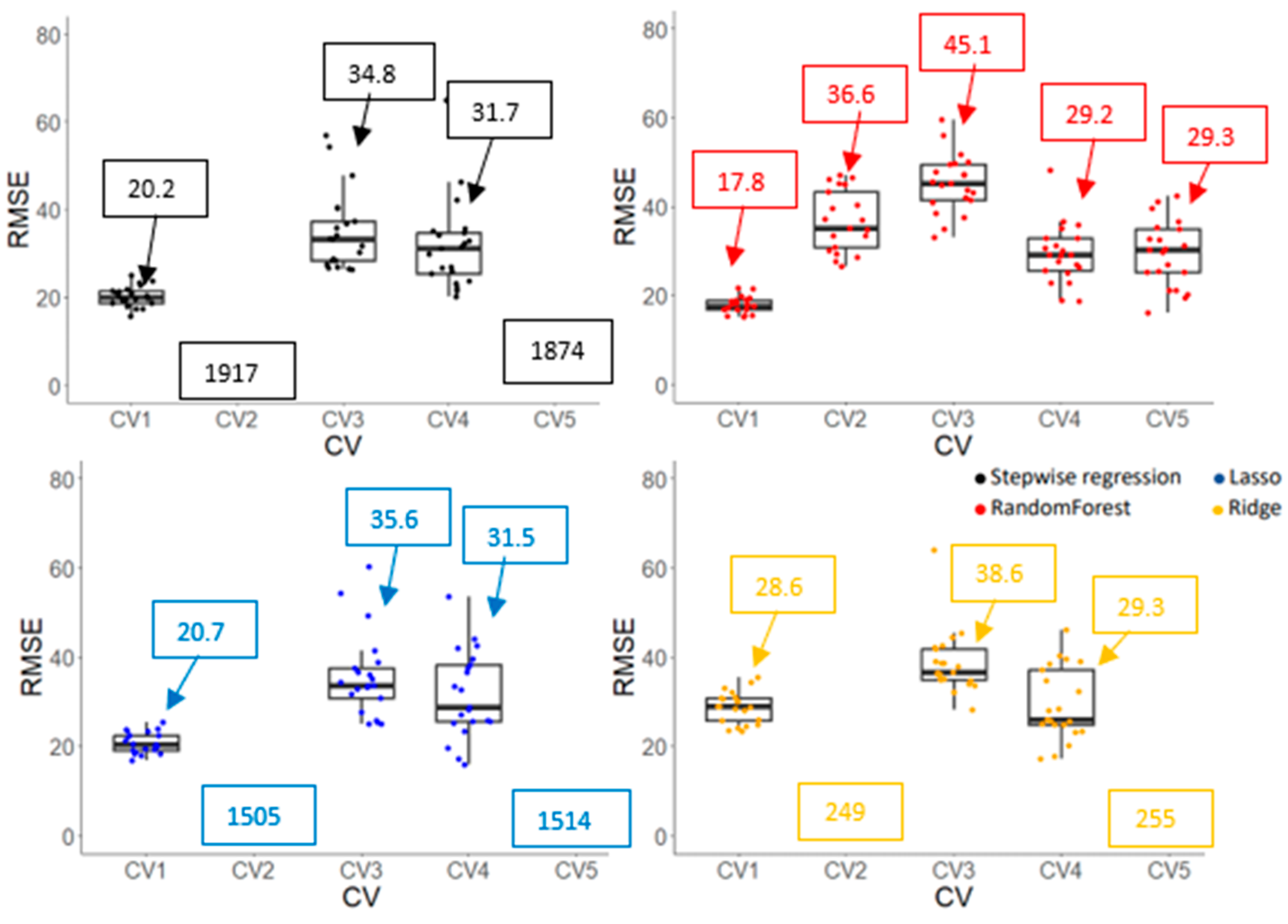
3.3.2. Model Accuracy and Cultivar Ranking Based on Predicted Values Derived from Prediction Models
4. Discussion
4.1. Soil Moisture and Weather Data across the Growing Season
4.2. Analysis of Variance and Correlation among the Studied Traits
4.3. Identification of Drought-Tolerant Cultivar
4.4. Limitations and Future Investigations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Piao, S.; Huntingford, C.; Peñuelas, J.; Yang, H.; Xu, H.; Chen, A.; Friedlingstein, P.; Keenan, T.F.; Sitch, S.; et al. Global Variations in Critical Drought Thresholds that Impact Vegetation. Natl. Sci. Rev. 2023, 10, nwad049. [Google Scholar] [CrossRef]
- Lamba, K.; Kumar, M.; Singh, V.; Chaudhary, L.; Sharma, R.; Yashveer, S.; Dalal, M.S. Heat Stress Tolerance Indices for Identification of the Heat Tolerant Wheat Genotypes. Sci. Rep. 2023, 13, 10842. [Google Scholar] [CrossRef] [PubMed]
- Cheavegatti-Gianotto, A.; de Abreu, H.M.C.; Arruda, P.; Bespalhok Filho, J.C.; Burnquist, W.L.; Creste, S.; di Ciero, L.; Ferro, J.A.; de Oliveira Figueira, A.V.; de Sousa Filgueiras, T.; et al. Sugarcane (Saccharum X officinarum): A Reference Study for the Regulation of Genetically Modified Cultivars in Brazil. Trop. Plant Biol. 2011, 4, 62–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, X.; Dai, M. Improving Crop Drought Resistance with Plant Growth Regulators and Rhizobacteria: Mechanisms, Applications, and Perspectives. Plant Commun. 2022, 3, 100228. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Saifullah; Ashraf, M.Y.; Ehsanullah. Role of Mineral Nutrition in Alleviation of Drought Stress in Plants. Aust. J. Crop Sci. 2011, 5, 764–777. [Google Scholar]
- Dlamini, P.J. Drought Stress Tolerance Mechanisms and Breeding Effort in Sugarcane: A Review of Progress and Constraints in South Africa. Plant Stress 2021, 2, 100027. [Google Scholar] [CrossRef]
- Chapae, C.; Songsri, P.; Gonkhamdee, S.; Jongrungklang, N. Understanding Drought Responses of Sugarcane Cultivars Controlled under Low Water Potential Conditions. Chil. J. Agric. Res. 2020, 80, 370–380. [Google Scholar] [CrossRef]
- Yahaya, M.A.; Shimelis, H. Drought Stress in Sorghum: Mitigation Strategies, Breeding Methods and Technologies—A Review. J. Agron. Crop Sci. 2022, 208, 127–142. [Google Scholar] [CrossRef]
- Devi, K.; Gomathi, R.; Arun Kumar, R.; Manimekalai, R.; Selvi, A. Field Tolerance and Recovery Potential of Sugarcane Varieties Subjected to Drought. Indian. J. Plant Physiol. 2018, 23, 271–282. [Google Scholar] [CrossRef]
- Basnayake, J.; Jackson, P.A.; Inman-Bamber, N.G.; Lakshmanan, P. Sugarcane for Water-Limited Environments. Genetic Variation in Cane Yield and Sugar Content in Response to Water Stress. J. Exp. Bot. 2012, 63, 6023–6033. [Google Scholar] [CrossRef]
- Wagih, M.E.; Kaiulo, J.V. Screening Sugarcane Varieties for Drought Tolerance. Sci. New Guinea 2001, 26, 38–45. [Google Scholar]
- De Silva, M.A.; Jifon, J.L.; da Silva, J.A.G.; Sharma, V. Use of Physiological Parameters as Fast Tools to Screen for Drought Tolerance in Sugarcane. Braz. J. Plant Physiol. 2007, 19, 193–201. [Google Scholar] [CrossRef]
- Gomathi, R.; Krishnapriya, V.; Arunkumar, R.; Govindaraj, P.; Ram, B. Physiological Traits Imparting Drought Stress Tolerance to Promising Sugarcane (Saccharum spp.) Clones. Plant Physiol. Rep. 2020, 25, 509–515. [Google Scholar] [CrossRef]
- Sajid, M.; Amjid, M.; Munir, H.; Ahmad, M.; Zulfiqar, U.; Ali, M.F.; Abul Farah, M.; Ahmed, M.A.A.; Artyszak, A. Comparative Analysis of Growth and Physiological Responses of Sugarcane Elite Genotypes to Water Stress and Sandy Loam Soils. Plants 2023, 12, 2759. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, M.; Baker, S.; Rudd, J.C.; Ibrahim, A.M.H.; Chang, A.; Xue, Q.; Jung, J.; Landivar, J.; Auvermann, B. Assessing the Effect of Drought on Winter Wheat Growth Using Unmanned Aerial System (UAS)-Based Phenotyping. Remote Sens. 2021, 13, 1144. [Google Scholar] [CrossRef]
- Han, X.; Wei, Z.; Chen, H.; Zhang, B.; Li, Y.; Du, T. Inversion of Winter Wheat Growth Parameters and Yield Under Different Water Treatments Based on UAV Multispectral Remote Sensing. Front. Plant Sci. 2021, 12, 609876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, W.; Niu, Y.; Chávez, J.L.; Shao, G.; Zhang, H. Evaluating the Sensitivity of Water Stressed Maize Chlorophyll and Structure Based on UAV Derived Vegetation Indices. Comput. Electron. Agric. 2021, 185, 106174. [Google Scholar] [CrossRef]
- Hoffman, N.; Singels, A.; Joshi, S. Aerial Phenotyping for Sugarcane Yield and Drought Tolerance. Field Crop. Res. 2024, 308, 109275. [Google Scholar] [CrossRef]
- Thapa, S.; Rudd, J.C.; Xue, Q.; Bhandari, M.; Reddy, S.K.; Jessup, K.E.; Liu, S.; Devkota, R.N.; Baker, J.; Baker, S. Use of NDVI for Characterizing Winter Wheat Response to Water Stress in a Semi-Arid Environment. J. Crop Improv. 2019, 33, 633–648. [Google Scholar] [CrossRef]
- Wen, P.; Meng, Y.; Gao, C.; Guan, X.; Wang, T.; Feng, W. Field Identification of Drought Tolerant Wheat Genotypes Using Canopy Vegetation Indices Instead of Plant Physiological and Biochemical Traits. Ecol. Indic. 2023, 154, 110781. [Google Scholar] [CrossRef]
- Narmilan, A.; Gonzalez, F.; Salgadoe, A.S.A.; Kumarasiri, U.W.L.M.; Weerasinghe, H.A.S.; Kulasekara, B.R. Predicting Canopy Chlorophyll Content in Sugarcane Crops Using Machine Learning Algorithms and Spectral Vegetation Indices Derived from UAV Multispectral Imagery. Remote Sens. 2022, 14, 1140. [Google Scholar] [CrossRef]
- Khuimphukhieo, I.; Marconi, T.; Enciso, J.; da Silva, J.A. The Use of UAS-Based High Throughput Phenotyping (HTP) to Assess Sugarcane Yield. J. Agric. Food Res. 2023, 11, 100501. [Google Scholar] [CrossRef]
- Cholula, U.; da Silva, J.A.; Marconi, T.; Thomasson, J.A.; Solorzano, J.; Enciso, J. Forecasting Yield and Lignocellulosic Composition of Energy Cane Using Unmanned Aerial Systems. Agronomy 2020, 10, 718. [Google Scholar] [CrossRef]
- Park, J.-W.; Benatti, T.R.; Marconi, T.; Yu, Q.; Solis-Gracia, N.; Mora, V.; da Silva, J.A. Cold Responsive Gene Expression Profiling of Sugarcane and Saccharum Spontaneum with Functional Analysis of a Cold Inducible Saccharum Homolog of NOD26-like Intrinsic Protein to Salt and Water Stress. PLoS ONE 2015, 10, e0125810. [Google Scholar] [CrossRef]
- Shabbir, R.; Zhaoli, L.; Yueyu, X.; Zihao, S.; Pinghua, C. Transcriptome Analysis of Sugarcane Response to Sugarcane Yellow Leaf Virus Infection Transmitted by the Vector Melanaphis Sacchari. Front. Plant Sci. 2022, 13, 921674. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.R.; White, W.H.; Dufrene, E.O.; Tew, T.L.; Pan, Y.-B.; Duet, M.J.; Verdun, D.L.; Hale, A.L.; Dalley, C.D.; Grisham, M.P.; et al. Registration of ‘HoCP 04-838’ Sugarcane. J. Plant Regist. 2018, 12, 324–332. [Google Scholar] [CrossRef]
- Zhao, D.; Comstock, J.C.; Sandhu, H.S.; Glaz, B.; Edmé, S.J.; Davidson, R.W.; Sood, S.; Gilbert, R.A.; McCorkle, K.; Glynn, N.C. Registration of ‘CP 06-2400’ Sugarcane. J. Plant Regist. 2015, 9, 71–77. [Google Scholar] [CrossRef]
- Richard, J.; Yong-Bao, P.; Hannah, P.; Alice, W.; Paul, W. New Crop Production and Protection Practices to Increase Sugarcane Ratoon Longevity and Maximize Economic Sustainability; USDA: Washington, DC, USA, 2022. [Google Scholar]
- Sandhu, H.; Gilbert, A.R. Performance of CP Sugarcane Cultivars Grown in Different Locations in Florida; IFAS: Gainesville, FL, USA, 2017. [Google Scholar]
- Davidson, R.W.; Scott, A.W.; Hernandez, E.; Gordon, V.S.; McCord, P.; Sandhu, H.S.; Zhao, D.; Comstock, J.C.; Sood, S.; Singh, M.P.; et al. Registration of ‘CP 08-1968’ Sugarcane. J. Plant Regist. 2019, 13, 178–186. [Google Scholar] [CrossRef]
- Irvine, J.E.; Rozeff, N.; Hernandez, E.; Miller, J.D. Registration of ‘TCP 93-4245’ Sugarcane. Crop Sci. 2003, 43, 1132. [Google Scholar] [CrossRef]
- Ferreira, T.H.S.; Tsunada, M.S.; Bassi, D.; Araújo, P.; Mattiello, L.; Guidelli, G.V.; Righetto, G.L.; Gonçalves, V.R.; Lakshmanan, P.; Menossi, M. Sugarcane Water Stress Tolerance Mechanisms and Its Implications on Developing Biotechnology Solutions. Front. Plant Sci. 2017, 8, 1077. [Google Scholar] [CrossRef]
- Awika, H.O.; Solorzano, J.; Cholula, U.; Shi, A.; Enciso, J.; Avila, C.A. Prediction Modeling for Yield and Water-Use Efficiency in Spinach Using Remote Sensing via an Unmanned Aerial System. Smart Agric. Technol. 2021, 1, 100006. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; Gitelson, A.A. Remote Estimation of Crop and Grass Chlorophyll and Nitrogen Content Using Red-Edge Bands on Sentinel-2 and -3. Int. J. Appl. Earth Obs. Geoinf. 2013, 23, 344–351. [Google Scholar] [CrossRef]
- Qi, H.; Wu, Z.; Zhang, L.; Li, J.; Zhou, J.; Jun, Z.; Zhu, B. Monitoring of Peanut Leaves Chlorophyll Content Based on Drone-Based Multispectral Image Feature Extraction. Comput. Electron. Agric. 2021, 187, 106292. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote Estimation of Canopy Chlorophyll Content in Crops. Geophys. Res. Lett. 2005, 32, L08403. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote Sensing of Chlorophyll Concentration in Higher Plant Leaves. Adv. Space Res. 1998, 22, 689–692. [Google Scholar] [CrossRef]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T.; et al. Coincident Detection of Crop Water Stress, Nitrogen Status and Canopy Density Using Ground-Based Multispectral Data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2019; ASA-CSSA-SSSA: Madison, WI, USA, 2000. [Google Scholar]
- Rouse, J.W.; Haas, R.H.; Shell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Monitoring Vegetation Systems in the Great Plains with ERTS; NASA Goddard Space Flight Center: Greenbelt, MD, USA, 1974; pp. 309–317. [Google Scholar]
- Gitelson, A.A.; Viña, A.; Arkebauer, T.J.; Rundquist, D.C.; Keydan, G.; Leavitt, B. Remote Estimation of Leaf Area Index and Green Leaf Biomass in Maize Canopies. Geophys. Res. Lett. 2003, 30, 52-1–52-4. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of Soil-Adjusted Vegetation Indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated Narrow-Band Vegetation Indices for Prediction of Crop Chlorophyll Content for Application to Precision Agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of Leaf-Area Index from Quality of Light on the Forest Floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Basnayake, J.; Jackson, P.A.; Inman-Bamber, N.G.; Lakshmanan, P. Sugarcane for Water-Limited Environments. Variation in Stomatal Conductance and Its Genetic Correlation with Crop Productivity. J. Exp. Bot. 2015, 66, 3945–3958. [Google Scholar] [CrossRef]
- Adak, A.; Murray, S.C.; Anderson, S.L. Temporal Phenomic Predictions from Unoccupied Aerial Systems Can Outperform Genomic Predictions. G3 2023, 13, jkac294. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.A.; de Miranda, J.H.; Cooke, R.A.C. Water Management for Sugarcane and Corn under Future Climate Scenarios in Brazil. Agric. Water Manag. 2018, 201, 199–206. [Google Scholar] [CrossRef]
- De Almeida Silva, M.; Jifon, J.L.; Sharma, V.; da Silva, J.A.G.; Caputo, M.M.; Damaj, M.B.; Guimarães, E.R.; Ferro, M.I.T. Use of Physiological Parameters in Screening Drought Tolerance in Sugarcane Genotypes. Sugar Tech. 2011, 13, 191–197. [Google Scholar] [CrossRef]
- Dien, D.C.; Yamakawa, T.; Mochizuki, T.; Htwe, A.Z. Dry Weight Accumulation, Root Plasticity, and Stomatal Conductance in Rice (Oryza sativa L.) Varieties under Drought Stress and Re-Watering Conditions. Am. J. Plant Sci. 2017, 08, 3189–3206. [Google Scholar] [CrossRef]
- Takai, T.; Yano, M.; Yamamoto, T. Canopy Temperature on Clear and Cloudy Days Can Be Used to Estimate Varietal Differences in Stomatal Conductance in Rice. Field Crops Res. 2010, 115, 165–170. [Google Scholar] [CrossRef]
- Lu, Z.; Percy, R.G.; Qualset, C.O.; Zeiger, E. Stomatal Conductance Predicts Yields in Irrigated Pima Cotton and Bread Wheat Grown at High Temperatures. J. Exp. Bot. 1998, 49, 453–460. [Google Scholar] [CrossRef]
- Jiang, G.M.; Sun, J.Z.; Liu, H.Q.; Qu, C.M.; Wang, K.J.; Guo, R.J.; Bai, K.Z.; Gao, L.M.; Kuang, T.Y. Changes in the Rate of Photosynthesis Accompanying the Yield Increase in Wheat Cultivars Released in the Past 50 years. J. Plant Res. 2003, 116, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Garkar, R.M.; Bharud, R.W.; Mate, S.N. Effect of Water Stress on Chlorophyll, Nitrate Reductase Activity and Cane Yield in Sugarcane (Saccharum officinarum L.). J. Sugarcane Res. 2011, 1, 43–49. [Google Scholar]
- Olivares-Villegas, J.J.; Reynolds, M.P.; McDonald, G.K. Drought-Adaptive Attributes in the Seri/Babax Hexaploid Wheat Population. Funct. Plant Biol. 2007, 34, 189. [Google Scholar] [CrossRef]
- Chen, W.; Sheng, Z.; Cai, Y.; Li, Q.; Wei, X.; Xie, L.; Jiao, G.; Shao, G.; Tang, S.; Wang, J.; et al. Rice Morphogenesis and Chlorophyll Accumulation Is Regulated by the Protein Encoded by NRL3 and Its Interaction with NAL9. Front. Plant Sci. 2019, 10, 175. [Google Scholar] [CrossRef]
- Benešová, M.; Holá, D.; Fischer, L.; Jedelský, P.L.; Hnilička, F.; Wilhelmová, N.; Rothová, O.; Kočová, M.; Procházková, D.; Honnerová, J.; et al. The Physiology and Proteomics of Drought Tolerance in Maize: Early Stomatal Closure as a Cause of Lower Tolerance to Short-Term Dehydration? PLoS ONE 2012, 7, e38017. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.S.A.; Vialet-Chabrand, S.; Lawson, T. Role of Blue and Red Light in Stomatal Dynamic Behaviour. J. Exp. Bot. 2020, 71, 2253–2269. [Google Scholar] [CrossRef] [PubMed]
- Clevers, J.G.P.W.; de Jong, S.M.; Epema, G.F.; van der Meer, F.; Bakker, W.H.; Skidmore, A.K.; Addink, E.A. MERIS and the Red-Edge Position. Int. J. Appl. Earth Obs. Geoinf. 2001, 3, 313–320. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. The MERIS Terrestrial Chlorophyll Index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Stansluos, A.A.L.; Öztürk, A.; Niedbała, G.; Türkoğlu, A.; Haliloğlu, K.; Szulc, P.; Omrani, A.; Wojciechowski, T.; Piekutowska, M. Genotype–Trait (GT) Biplot Analysis for Yield and Quality Stability in Some Sweet Corn (Zea mays L. Saccharata Sturt.) Genotypes. Agronomy 2023, 13, 1538. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Identification of Drought Tolerant Genotypes Using Physiological Traits in Soybean. Physiol. Mol. Biol. Plants 2019, 25, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Elfanah, A.M.S.; Darwish, M.A.; Selim, A.I.; Elmoselhy, O.M.A.; Ali, A.M.; El-Maghraby, M.A.; Abdelhamid, M.T. Hyperspectral Reflectance and Agro-Physiological Traits for Field Identification of Salt-Tolerant Wheat Genotypes Using the Genotype by Yield*trait Biplot Technique. Front. Plant Sci. 2023, 14, 1165113. [Google Scholar] [CrossRef]
- Khuimphukhieo, I.; Bhandari, M.; Enciso, J.; da Silva, J. Estimating Sugarcane Yield and Its Components Using Unmanned Aerial Systems (Uas)-Based High Throughput Phenotyping (Htp). SSRN, 2024; preprint. [Google Scholar] [CrossRef]
- Adak, A.; Anderson, S.L.; Murray, S.C. Pedigree-management-flight Interaction for Temporal Phenotype Analysis and Temporal Phenomic Prediction. Plant Phenome J. 2023, 6, e20057. [Google Scholar] [CrossRef]




| Cultivars | Characteristic Description | References |
|---|---|---|
| CP72-1210 | Cold susceptible and yellow leaf disease | [24,25] |
| HoCP04-838 | Resistant to smut, mosaic caused by Sorghum mosaic virus, brown rust, sugarcane borer, ratoon stunt but susceptible to yellow leaf | [26] |
| CP06-2400 | High cane, sucrose yields, acceptable levels of resistance to brown rust, orange rust, leaf scald, ratoon stunt, smut, and freeze tolerance | [27] |
| CP07-1824 | Resistant to sugarcane borer in South Texas | [28] |
| CP89-2143 | High, stable sucrose concentration and sucrose yield | [29] |
| CP08-1968 | High cane and sucrose yield on sand soils. Acceptable levels of brown rust, smut, mosaic, yellow leaf, ratoon stunt, moderate to good freeze tolerance but susceptible to orange rust | [30] |
| TCP93-4245 | High sugar yield, acceptable ratoon ability, resistance to ratoon stunting disease, Mexican rice borer, and sugarcane borer | [31] |
| Ratoons | Cycles | Stress Triggered | UAS Data Collection | Physiological Data Collection | Irrigation Applied | UAS Data Collection | Physiological Data Collection |
|---|---|---|---|---|---|---|---|
| 2nd crop | 1st | 30 July 2022 | 10 August 2022 | 11 August 2022 | 14 August 2022 † | 18 August 2022 | 17 August 2022 |
| 2nd | 1 October 2022 | 12 October 2022 | - | 26 October 2022 | 2 November 2022 | - | |
| 3rd | 22 January 2023 | 27 January 2023 | 25 January 2023 | 9 February 2023 | 14 February 2023 | 15 February 2023 | |
| 3rd crop | 21 July 2023 | 16 August 2023 | 17 August 2023 | 4 September 2023 | 22 September 2023 | 19 September 2023 |
| Vegetation Indices (VIs) | Equation † | References |
|---|---|---|
| Green chlorophyll index | GIG = (NIR/G) − 1 | [36] |
| Green normalized difference vegetation index | GNDVI = (NIR − G)/(NIR + G) | [37] |
| Leaf chlorophyll index | LCI = (NIR − RE)/(NIR + R) | [21] |
| Normalized difference red edge index | NDRE = (NIR − RE)/(NIR + RE) | [38] |
| Normalized difference vegetation index | NDVI = (NIR − R)/(NIR + R) | [39] |
| Normalized green, red difference index | NGRDI = (G − R)/(G + R) | [40] |
| Optimized soil-adjusted vegetation index | OSAVI = (1 + 0.16) × (NIR − R)/(NIR + R +0.16) | [41] |
| Transformed chlorophyll absorption in refection index | TCARI = 3 × [(RE − R) − 0.2 × (RE − G) × (RE/R)] | [42] |
| Red-edge chlorophyll index | CIRE = (NIR/RE) − 1 | [36] |
| Simple ration index | SPI = NIR/G | [43] |
| C2 | C3 | C4 | C5 | C6 | C7 | |
|---|---|---|---|---|---|---|
| C1 † | C1, C2 (I1 ‡) (I1) | C1, C3 (I2) | C1, C4 (I3) | C1, C5 (I4) | C1, C6 (I5) | C1, C7 (I6) |
| C2 | C2, C3 (I7) | C2, C4 (I8) | C2, C5 (I9) | C2, C6 (I10) | C2, C7 (I11) | |
| C3 | C3, C4 (I12) | C3, C5 (I13) | C3, C6 (I14) | C3, C7 (I15) | ||
| C4 | C4, C5 (I16) | C4, C6 (I17) | C4, C7 (I18) | |||
| C5 | C5, C6 (I19) | C5, C7 (I20) | ||||
| C6 | C6, C7 (I21) |
| Cultivars | The 1st Cycle | The 3rd Cycle | ||||
|---|---|---|---|---|---|---|
| Gs.S † | Gs.R | Gs.S | Gs.R | Chl.S | Chl.R | |
| CP06-2400 | 240.6 ± 120.9 | 280.4 ± 37.0 c | 164.9 ± 36.6 bc | 211.1 ± 32.9 | 258.0 ± 10.5 abc | 204.1 ± 17.5 ab |
| CP07-1824 | 292.0 ± 88.8 | 440.0 ± 96.4 a | 206.2 ± 12.2 ab | 221.9 ± 50.1 | 244.4 ± 35.6 bc | 209.2 ± 25.7 a |
| CP08-1968 | 282.7 ± 71.7 | 329.7 ± 98.8 bc | 209.5 ± 61.1 ab | 246.0 ± 18.3 | 278.9 ± 31.7 ab | 228.6 ± 43.7 a |
| CP72-1210 | 308.6 ± 131.8 | 394.3 ± 95.8 ab | 207.3 ± 8.1 ab | 231.4 ± 28.9 | 278.3 ± 2.7 ab | 235.1 ± 38.6 a |
| CP89-2143 | 289.2 ± 106.9 | 286.8 ± 83.1 c | 139.4 ± 22.7 c | 198.8 ± 18.0 | 227.2 ± 21.8 c | 198.7 ± 19.6 ab |
| HoCP04-838 | 280.5 ± 78.0 | 301.9 ± 83.9 bc | 182.6 ± 59.6 bc | 182.5 ± 63.3 | 226.3 ± 31.1 c | 169.5 ± 31.8 b |
| TCP93-4245 | 336.8 ± 83.9 | 326.7 ± 38.4 bc | 239.9 ± 45.1 a | 240.1 ± 33.5 | 288.9 ± 30.3 a | 216.7 ± 10.2 a |
| F-test | ns ‡ | * | * | ns | ** | * |
| CV (%) | 28.57 | 19.05 | 19.78 | 16.09 | 9.87 | 12.78 |
| Cultivars | The 3rd Ratoon Crop | |||
|---|---|---|---|---|
| Gs.S † | Gs.R | Chl.S | Chl.R | |
| CP06-2400 | 217.9 ± 38.1 | 195.6 ± 62.5 b | 203.4 ± 19.3 bc | 230.1 ± 14.2 |
| CP07-1824 | 275.5 ± 22.7 | 300.6 ± 60.4 a | 201.0 ± 13.7 c | 262.1 ± 18.0 |
| CP08-1968 | 233.0 ± 28.9 | 223.3 ± 48.3 b | 224.2 ± 33.6 abc | 249.2 ± 51.2 |
| CP72-1210 | 259.2 ± 46.9 | 235.5 ± 29.6 ab | 240.7 ± 39.0 ab | 260.2 ± 19.7 |
| CP89-2143 | 228.3 ± 25.6 | 179.2 ± 37.2 b | 194.4 ± 17.1 c | 232.3 ± 6.2 |
| HoCP04-838 | 218.4 ± 18.3 | 294.4 ± 36.0 a | 253.9 ± 30.0 a | 295.9 ± 39.1 |
| TCP93-4245 | 244.5 ± 52.7 | 241.4 ± 29.8 ab | 226.4 ± 13.5 abc | 241.4 ± 17.5 |
| F-test | ns ‡ | * | * | ns |
| CV (%) | 14.02 | 19.46 | 11.95 | 11.18 |
| Crops | Traits | Model | CVs † | Cultivars | Ranking Based on MVs | MVs | PVs | Ranking Based on PVs |
|---|---|---|---|---|---|---|---|---|
| The 2nd ratoon | Gs (1st) | Ridge | CV3 | TCP93-4245 | 1 | 336.8 | 313.3 | 1 |
| CP72-1210 | 2 | 308.6 | 292.8 | 2 | ||||
| CP07-1824 | 3 | 292.0 | 274.9 | 4 | ||||
| Gs (3rd) | Ridge | CV3 | TCP93-4245 | 1 | 239.9 | 211.6 | 4 | |
| CP08-1968 | 2 | 209.5 | 215.1 | 2 | ||||
| CP72-1210 | 3 | 207.3 | 214.0 | 3 | ||||
| Chl (3rd) | Lasso | CV3 | TCP93-4245 | 1 | 288.9 | 275.4 | 2 | |
| CP08-1968 | 2 | 278.9 | 242.2 | 4 | ||||
| CP72-1210 | 3 | 278.3 | 275.8 | 1 | ||||
| The 3rd ratoon | Gs | Random forest | CV5 | CP07-1824 | 1 | 275.5 | 210.2 | 3 |
| CP72-1210 | 2 | 259.2 | 263.5 | 1 | ||||
| TCP93-4245 | 3 | 244.5 | 246.9 | 2 | ||||
| Chl | Random forest | CV5 | HoCP04-838 | 1 | 253.9 | 235.9 | 3 | |
| CP72-1210 | 2 | 240.7 | 240.1 | 2 | ||||
| TCP93-4245 | 3 | 226.4 | 245.4 | 1 |
| Crops | Traits | Model | CVs † | Cultivars | Ranking Based on MVs | MVs | PVs | Ranking Based on PVs |
|---|---|---|---|---|---|---|---|---|
| The 2nd ratoon | Gs (1st) | Ridge | CV3 | CP07-1824 | 1 | 440.0 | 319.0 | 5 |
| CP72-1210 | 2 | 394.3 | 339.6 | 3 | ||||
| TCP93-4245 | 3 | 326.7 | 376.7 | 1 | ||||
| Gs (3rd) | Ridge | CV3 | CP08-1968 | 1 | 246.0 | 237.8 | 2 | |
| TCP93-4245 | 2 | 240.1 | 215.3 | 5 | ||||
| CP72-1210 | 3 | 231.4 | 228.5 | 3 | ||||
| Chl (3rd) | Lasso | CV3 | CP72-1210 | 1 | 235.1 | 243.5 | 1 | |
| CP08-1968 | 2 | 228.6 | 212.2 | 4 | ||||
| TCP93-4245 | 3 | 216.7 | 230.0 | 3 | ||||
| The 3rd ratoon | Gs | Random forest | CV5 | CP07-1824 | 1 | 300.6 | 276.2 | 2 |
| HoCP04-838 | 2 | 294.4 | 270.3 | 4 | ||||
| TCP93-4245 | 3 | 241.4 | 277.5 | 1 | ||||
| Chl | Random forest | CV5 | HoCP04-838 | 1 | 295.9 | 260.1 | 1 | |
| CP07-1824 | 2 | 262.1 | 236.2 | 4 | ||||
| CP72-1210 | 3 | 260.2 | 239.8 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuimphukhieo, I.; Bhandari, M.; Enciso, J.; da Silva, J.A. Assessing Drought Stress of Sugarcane Cultivars Using Unmanned Vehicle System (UAS)-Based Vegetation Indices and Physiological Parameters. Remote Sens. 2024, 16, 1433. https://doi.org/10.3390/rs16081433
Khuimphukhieo I, Bhandari M, Enciso J, da Silva JA. Assessing Drought Stress of Sugarcane Cultivars Using Unmanned Vehicle System (UAS)-Based Vegetation Indices and Physiological Parameters. Remote Sensing. 2024; 16(8):1433. https://doi.org/10.3390/rs16081433
Chicago/Turabian StyleKhuimphukhieo, Ittipon, Mahendra Bhandari, Juan Enciso, and Jorge A. da Silva. 2024. "Assessing Drought Stress of Sugarcane Cultivars Using Unmanned Vehicle System (UAS)-Based Vegetation Indices and Physiological Parameters" Remote Sensing 16, no. 8: 1433. https://doi.org/10.3390/rs16081433






