Environmental and Human Controls of Ecosystem Functional Diversity in Temperate South America
Abstract
:1. Introduction
- H1. The main drivers of EFT richness switch from climatic variables in natural environments to human influence variables in anthropogenic environments.
- H2. In natural environments of temperate South America, H2a) water availability is a stronger limiting factor for EFT richness than energy variables, and EFT richness has a positive relationship with both H2b) water availability and H2c) energy.
- H3. In natural environments, climate stability (daily and seasonal) has a positive effect on EFT richness.
- H4. Across human influence gradients, land use has a positive effect on EFT richness at low rates of human pressure, but it switches to negative effects at high rates of human intervention.
- H5. Since topographic roughness increases spatial heterogeneity, it should have a positive relationship with EFT richness.
2. Material and Methods
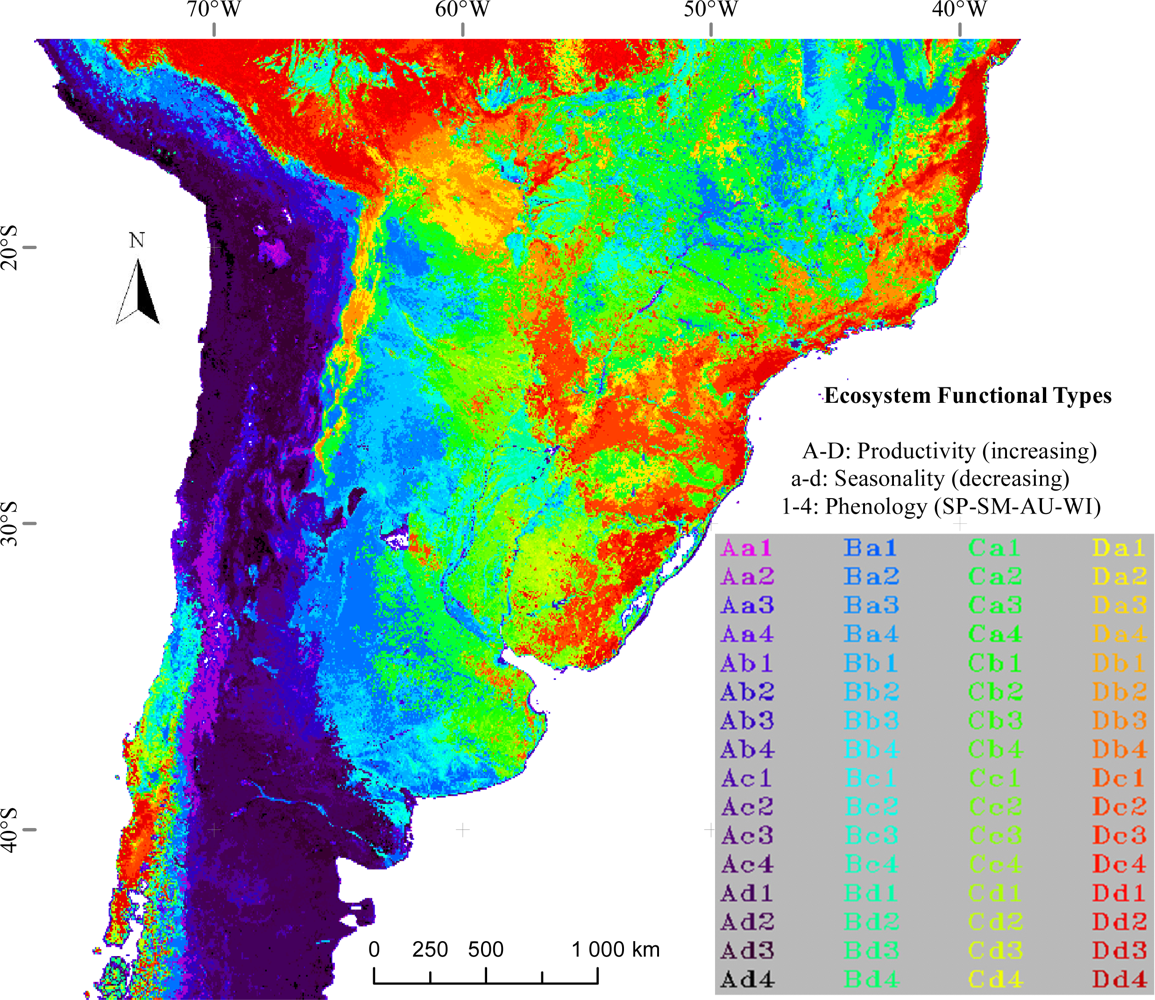
2.1. EFT Identification
2.2. Controls of Ecosystem Functional Diversity
3. Results
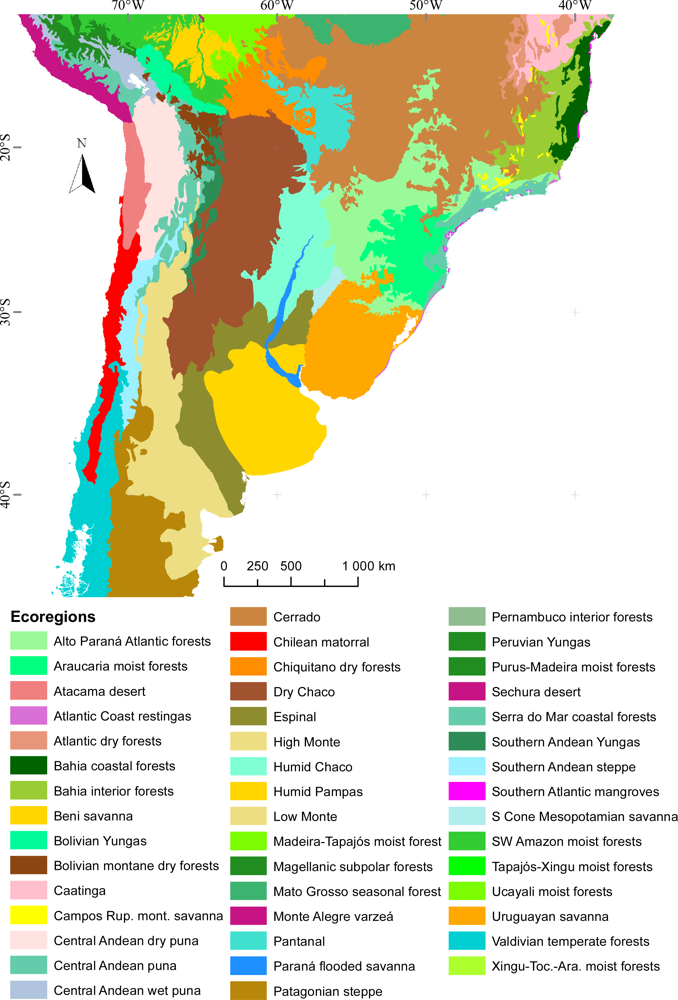
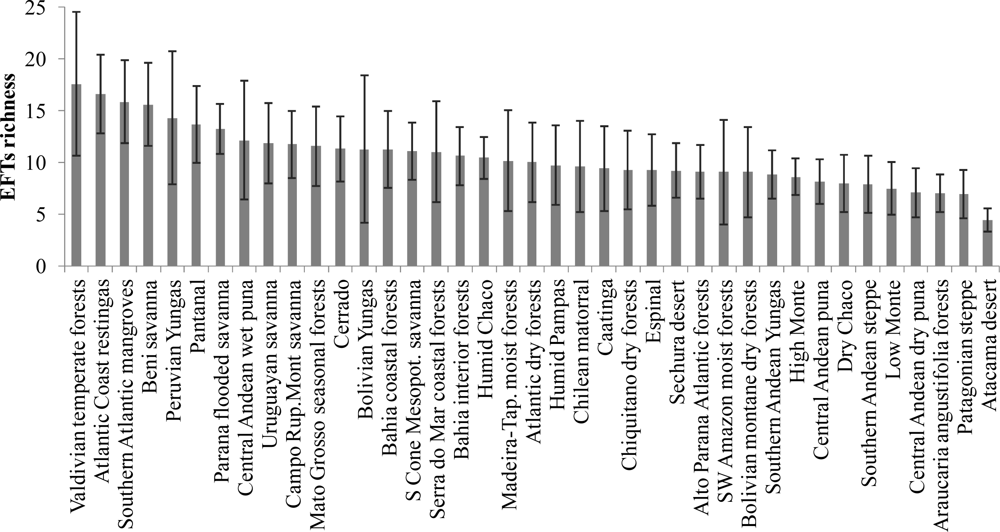
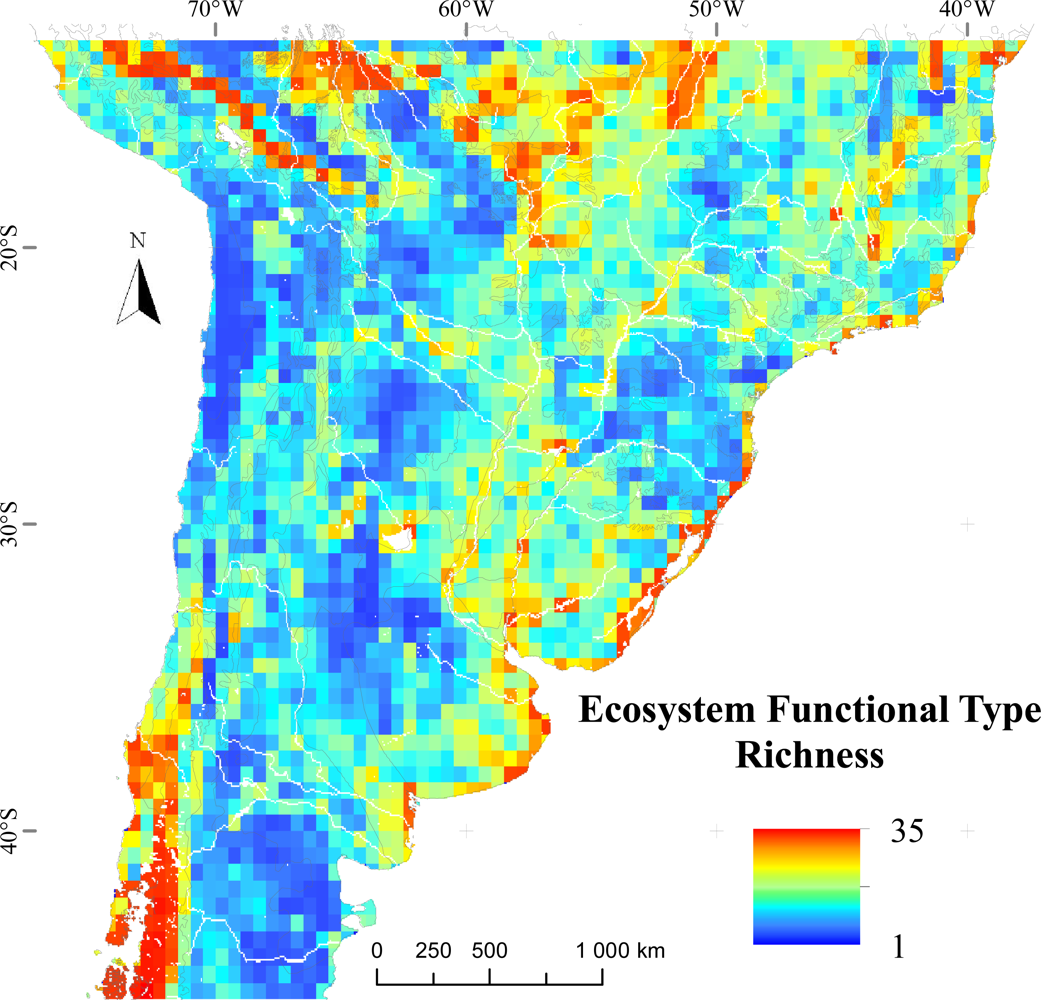
3.1. Regional Patterns of Ecosystem Functional Diversity
3.2. Environmental and Human Controls of EFT Richness
3.3. Sense of the Relationships between the EFT Richness and the Explanatory Variables
4. Discussion
5. Conclusions
Acknowledgments
References
- Noss, R.F. Indicators for monitoring biodiversity—A hierarchical approach. Conserv. Biol 1990, 4, 355–364. [Google Scholar]
- Callicott, J.B.; Crowder, L.B.; Mumford, K. Current normative concepts in conservation. Conserv. Biol 1999, 13, 22–35. [Google Scholar]
- Cabello, J.; Fernández, N.; Alcaraz-Segura, D.; Oyonarte, C.; Piñeiro, G.; Altesor, A.; Delibes, M.; Paruelo, J. The ecosystem functioning dimension in conservation: Insights from remote sensing. Biodiver. Conserv 2012, 21, 3287–3305. [Google Scholar]
- Vitousek, P.M. Beyond global warming: Ecology and global change. Ecology 1994, 75, 1861–1876. [Google Scholar]
- Milchunas, D.G.; Lauenroth, W.K. Inertia in plant community structure: State changes after cessation of nutrient enrichment stress. Ecol. Appl 1995, 5, 1195–2005. [Google Scholar]
- McNaughton, S.; Oesterheld, M.; Frank, D.; Williams, K. Ecosystem-level patterns of primary productivity and herbivory in terrestrial habitats. Nature 1989, 341, 142–144. [Google Scholar]
- Valentini, R.; Baldocchi, D.D.; Tenhunen, J.D.; Kabat, P. Ecological Controls on Land-Surface Atmospheric Interactions. In Integrating Hydrology, Ecosystem Dynamics and Biogeochemistry in Complex Landscapes; John Wiley & Sons: Berlin, Germany, 1999; pp. 105–116. [Google Scholar]
- Paruelo, J.M.; Jobbagy, E.G.; Sala, O.E. Current distribution of ecosystem functional types in temperate South America. Ecosystems 2001, 4, 683–698. [Google Scholar]
- Shugart, H.H.; Smith, T.M.; Woodward, F.I. Plant and Ecosystem Functional Types. In Plant Functional Types. Their Relevance to Ecosystem Properties and Global Change; Cambridge University Press: Cambridge, UK, 1997; pp. 20–45. [Google Scholar]
- Díaz, S.; Cabido, M. Plant functional types and ecosystem function in relation to global change. J. Veg. Sci 1997, 8, 463–474. [Google Scholar]
- Scholes, R.J.; Pickett, G.; Ellery, W.N.; Blackmore, A.C.; Smith, T.M.; Shugart, H.H.; Woodward, F.I. Plant Functional Types in African Savannas and Grasslands. In Plant Functional Types. Their Relevance to Ecosystem Properties and Global Change; Cambridge University Press: Cambridge, UK, 1997; pp. 255–268. [Google Scholar]
- Walker, B.H.; Smith, T.M.; Shugart, H.H.; Woodward, F.I. Functional Types in Non-Equilibrium Ecosystems. In Plant Functional Types. Their Relevance to Ecosystem Properties and Global Change; Cambridge University Press: Cambridge, UK, 1997; pp. 91–103. [Google Scholar]
- Aber, J.D.; Burke, I.C.; Acock, B.; Bugmann, H.K.M.; Kabat, P.; Menaut, J.C.; Noble, I.R.; Reynolds, J.F.; Steffen, W.L.; Wu, J.; Tenhunen, J.D. Group Report: Hydrological and Biogeochemical Processes in Complex Landscapes—What Is the Role of Temporal and Spatial Ecosystem Dynamics? In Integrating Hydrology, Ecosystem Dynamics and Biogeochemistry in Complex Landscapes; John Wiley & Sons: Berlin, Germany, 1999; pp. 335–356. [Google Scholar]
- Bonan, G.B.; Levis, S.; Kergoat, L.; Oleson, K.W. Landscapes as patches of plant functional types: An integrating concept for climate and ecosystem models. Glob. Biogeochem. Cy. 2002. [Google Scholar] [CrossRef]
- Duckworth, J.C.; Kent, M.; Ramsay, P.M. Plant functional types: An alternative to taxonomic plant community description in biogeography? Progr. Phys. Geogr 2000, 24, 515–542. [Google Scholar]
- Bret-Harte, M.S.; Mack, M.C.; Goldsmith, G.R.; Sloan, D.B.; DeMarco, J.; Shaver, G.R.; Ray, P.M.; Biesinger, Z.; Chapin, F.S. Plant functional types do not predict biomass responses to removal and fertilization in Alaskan tussock tundra. J. Ecol 2008, 96, 713–726. [Google Scholar]
- Lavorel, S.; Díaz, S.; Cornelissen, J.; Garnier, E.; Harrison, S.; McIntyre, S.; Pausas, J.; Pérez-Harguindeguy, N.; Roumet, C.; Urcelay, C. Plant Functional Types: Are We Getting Any Closer to the Holy Grail? In Terrestrial Ecosystems in a Changing World; Canadell, J.G., Pataki, D.E., Pitelka, L.F., Eds.; Springer: Berlin/Heidelberg, Germany/New York, NY, USA, 2007; pp. 149–164. [Google Scholar]
- Wright, J.P.; Naeem, S.; Hector, A.; Lehman, C.; Reich, P.B.; Schmid, B.; Tilman, D. Conventional functional classification schemes underestimate the relationship with ecosystem functioning. Ecol. Lett 2006, 9, 111–120. [Google Scholar]
- Soriano, A.; Paruelo, J.M. Biozones: Vegetation units defined by functional characters identifiable with the aid of satellite sensor images. Glob. Ecol. Biogeogr. Lett 1992, 2, 82–89. [Google Scholar]
- Stow, D.; Daeschner, S.; Boynton, W.; Hope, A. Arctic tundra functional types by classification of single-date and AVHRR bi-weekly NDVI composite datasets. Int. J. Remote Sens 2000, 21, 1773–1779. [Google Scholar]
- Alcaraz-Segura, D.; Paruelo, J.M.; Cabello, J. Identification of current ecosystem functional types in the Iberian Peninsula. Glob. Ecol. Biogeogr 2006, 15, 200–212. [Google Scholar]
- Piñeiro, G.; Alcaraz-Segura, D.; Paruelo, J.M.; Oyonarte, C.; Guerschman, J.P.; Escribano, P.; Cabello, J. A Functional Classification of Natural and Human-Modified Areas of “Cabo de Gata”, Spain, Based on Landsat TM Data. Proceedings of 29th International Symposium on Remote Sensing of Environment, Buenos Aires, Argentina, 8–12 April 2002.
- Fernández, N.; Paruelo, J.M.; Delibes, M. Ecosystem functioning of protected and altered Mediterranean environments: A remote sensing classification in Doñana, Spain. Remote Sens. Environ 2010, 114, 211–220. [Google Scholar]
- Pérez-Hoyos, A.; Martínez, B.; Gilabert, M.A.; García-Haro, F.J. A multi-temporal analysis of vegetation dynamics in the Iberian peninsula using MODIS-NDVI data. EARSeL eProc 2010, 9, 22–30. [Google Scholar]
- Martínez, B.; Gilabert, M.A. Vegetation dynamics from NDVI time series analysis using the wavelet transform. Remote Sens. Environ 2009, 113, 1823–1842. [Google Scholar]
- Stoyanova, J.S. Bioclimatic Concept for Assessment of Atmosphere and Forest Land-Cover Coupling at a Regional Scale. Proceedings of 29th International Conference on Alpine Meteorology, Chambéry, France, 4–8 June 2007.
- Alcaraz-Segura, D.; Berbery, E.H.; Lee, S.-J.; Paruelo, J.M. Use of ecosystem functional types to represent the interannual variability of vegetation biophysical properties in regional models. CLIVAR Exchanges 2011, 17, 23–27. [Google Scholar]
- Muller, O.; Berbery, E.H.; Alcaraz-Segura, D. The Drought Interest Group: Using Ecosystem Functional Types as Lower Boundary Conditions in Simulations of Droughts in Southern South America. Proceedings of WCRP Open Science Conference. Climate Research in Service to Society, Denver, CO, USA, 24–28 October 2011; p. C39 TH98A.
- Berbery, E.H.; Alcaraz-Segura, D.; Muller, O. Time-Varying Biophysical Properties of Terrestrial Ecosystems: Their Use in Regional Climate Modeling. Proceedings of WCRP Open Science Conference. Climate Research in Service to Society, Denver, CO, USA, 24–28 October 2011; p. C10 M36A.
- Karlsen, S.R.; Elvebakk, A.; Høgda, K.A.; Johansen, B. Satellite-based mapping of the growing season and bioclimatic zones in Fennoscandia. Glob. Ecol. Biogeogr 2006, 15, 416–430. [Google Scholar]
- Azzali, S.; Menenti, M. Mapping isogrowth zones on continental scale using temporal Fourier analysis of AVHRR-NDVI data. Int. J. Appl. Earth Obs. Geoinf 1999, 1, 9–20. [Google Scholar]
- Azzali, S.; Menenti, M. Mapping vegetation-soil-climate complexes in southern Africa using temporal Fourier analysis of NOAA-AVHRR NDVI data. Int. J. Remote Sens 2000, 21, 973–996. [Google Scholar]
- Duro, D.; Coops, N.C.; Wulder, M.A.; Han, T. Development of a large area biodiversity monitoring system driven by remote sensing. Progr. Phys. Geogr 2007, 31, 235–260. [Google Scholar]
- Mildrexler, D.J.; Zhao, M.S.; Heinsch, F.A.; Running, S.W. A new satellite-based methodology for continental-scale disturbance detection. Ecol. Appl 2007, 17, 235–250. [Google Scholar]
- Sobrino, J.A.; Julien, Y.; Morales, L. Multitemporal analysis of PAL images for the study of land cover dynamics in South America. Glob. Planet. Change 2006, 51, 172–180. [Google Scholar]
- Geerken, R.A. An algorithm to classify and monitor seasonal variations in vegetation phenologies and their inter-annual change. ISPRS J. Photogramm 2009, 64, 422–431. [Google Scholar]
- Kreft, H.; Jetz, W. Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 5925–5930. [Google Scholar]
- Hawkins, B.A.; Field, R.; Cornell, H.V.; Currie, D.J.; Guegan, J.F.; Kaufman, D.M.; Kerr, J.T.; Mittelbach, G.G.; Oberdorff, T.; O’Brien, E.M.; Porter, E.E.; Turner, J.R.G. Energy, water and broad-scale geographic patterns of species richness. Ecology 2003, 84, 3105–3117. [Google Scholar]
- Hawkins, B.A. Are we making progress toward understanding the global diversity gradient? Basic Appl. Ecol 2004, 5, 1–3. [Google Scholar]
- Hawkins, B.A.; McCain, C.M.; Davies, T.J.; Buckley, L.B.; Anacker, B.L.; Cornell, H.V.; Damschen, E.I.; Grytnes, J.-A.; Harrison, S.; Holt, R.D.; et al. Different evolutionary histories underlie congruent species richness gradients of birds and mammals. J. Biogeogr 2012, 39, 825–841. [Google Scholar]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar]
- Cadena, C.D.; Kozak, K.H.; Gómez, J.P.; Parra, J.L.; McCain, C.M.; Bowie, R.C.K.; Carnaval, A.C.; Moritz, C.; Rahbek, C.; Roberts, T.E.; et al. Latitude, elevational climatic zonation and speciation in New World vertebrates. Proc. Roy. Soc. B: Biol. Sci 2012, 279, 194–201. [Google Scholar]
- Gould, W. Remote sensing of vegetation, plant species richness and regional biodiversity hotspots. Ecol. Appl 2000, 10, 1861–1870. [Google Scholar]
- Kerr, J.T.; Ostrovsky, M. From space to species: Ecological applications for remote sensing. Trend. Ecol. Evol 2003, 18, 299–305. [Google Scholar]
- Buchanan, G.; Pearce-Higgins, J.; Grant, M.; Robertson, D.; Waterhouse, T. Characterization of moorland vegetation and the prediction of bird abundance using remote sensing. J. Biogeogr 2005, 32, 697–707. [Google Scholar]
- Innes, J.L.; Koch, B. Forest biodiversity and its assessment by remote sensing. Glob. Ecol. Biogeogr 1998, 7, 397–419. [Google Scholar]
- Turner, W.; Spector, S.; Gardiner, N.; Fladeland, M.; Sterling, E.; Steininger, M. Remote sensing for biodiversity science and conservation. Trend. Ecol. Evol 2003, 18, 306–314. [Google Scholar]
- Leyequien, E.; Verrelst, J.; Slot, M.; Schaepman-Strub, G.; Heitkonig, I.M.A.; Skidmore, A. Capturing the fugitive: Applying remote sensing to terrestrial animal distribution and diversity. Int. J. Appl. Earth Obs. Geoinf 2007, 9, 1–20. [Google Scholar]
- Wiens, J.; Sutter, R.; Anderson, M.; Blanchard, J.; Barnett, A.; Aguilar-Amuchastegui, N.; Avery, C.; Laine, S. Selecting and conserving lands for biodiversity: The role of remote sensing. Remote Sens. Environ 2009, 113, 1370–1381. [Google Scholar]
- Goetz, S.; Steinberg, D.; Dubayah, R.; Blair, B. Laser remote sensing of canopy habitat heterogeneity as a predictor of bird species richness in an eastern temperate forest, USA. Remote Sens. Environ 2007, 108, 254–263. [Google Scholar]
- Whittaker, R.J.; Nogués-Bravo, D.; Araújo, M.B. Geographical gradients of species richness: A test of the water-energy conjecture of Hawkins et al. (2003) using European data for five taxa. Glob. Ecol. Biogeogr 2007, 16, 76–89. [Google Scholar]
- Carnaval, A.C.; Hickerson, M.J.; Haddad, C.F.B.; Rodrigues, M.T.; Moritz, C. Stability predicts genetic diversity in the Brazilian Atlantic forest hotspot. Science 2009, 323, 785–789. [Google Scholar]
- Kiessling, W. Long-term relationships between ecological stability and biodiversity in Phanerozoic reefs. Nature 2005, 433, 410–413. [Google Scholar]
- Tilman, D.; Reich, P.B.; Knops, J.M.H. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 2006, 441, 629–632. [Google Scholar]
- Benton, T.G.; Vickery, J.A.; Wilson, J.D. Farmland biodiversity: Is habitat heterogeneity the key? Trends Ecol. Evol 2003, 18, 182–188. [Google Scholar]
- Burnett, M.R.; August, P.V.; Brown, J.H., Jr.; Killingbeck, K.T. The influence of geomorphological heterogeneity on biodiversity I. A patch-scale perspective. Conserv. Biol 1998, 12, 363–370. [Google Scholar]
- Nichols, W.F.; Killingbeck, K.T.; August, P.V. The influence of geomorphological heterogeneity on Biodiversity II. A Landscape Perspective. Conserv. Biol 1998, 12, 371–379. [Google Scholar]
- Ellis, E.C.; Ramankutty, N. Putting people in the map: Anthropogenic biomes of the world. Front. Ecol. Environ 2007, 6, 439–447. [Google Scholar]
- Medan, D.; Torretta, J.P.; Hodara, K.; de la Fuente, E.B.; Montaldo, N.H. Effects of agriculture expansion and intensification on the vertebrate and invertebrate diversity in the Pampas of Argentina. Biodivers. Conserv 2011, 20, 3077–3100. [Google Scholar]
- Cerezo, A.; Conde, M.C.; Poggio, S.L. Pasture area and landscape heterogeneity are key determinants of bird diversity in intensively managed farmland. Biodivers. Conserv 2011, 20, 2649–2667. [Google Scholar]
- Jauni, M.; Hyvönen, T. Positive diversity-invasibility relationships across multiple scales in Finnish agricultural habitats. Biol. Invasions 2011, 14, 1379–1391. [Google Scholar]
- Tognetti, P.M.; Chaneton, E.J.; Omacini, M.; Trebino, H.J.; León, R.J.C. Exotic vs. native plant dominance over 20 years of old-field succession on set-aside farmland in Argentina. Biol. Conserv 2010, 143, 2494–2503. [Google Scholar]
- Poggio, S.L.; Chaneton, E.J.; Ghersa, C.M. Landscape complexity differentially affects alpha, beta and gamma diversities of plants occurring in fencerows and crop fields. Biol. Conserv 2010, 143, 2477–2486. [Google Scholar]
- Larsen, T.H. Upslope range shifts of andean dung beetles in response to deforestation: compounding and confounding effects of microclimatic change. Biotropica 2012, 44, 82–89. [Google Scholar]
- Strassburg, B.B.N.; Rodrigues, A.S.L.; Gusti, M.; Balmford, A.; Fritz, S.; Obersteiner, M.; Turner, R.K.; Brooks, T.M. Impacts of incentives to reduce emissions from deforestation on global species extinctions. Nature Clim. Change 2012, 2, 350–355. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Koppen-Geiger climate classification updated. Meteorologische Zeitschrift 2006, 15, 259–263. [Google Scholar]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on Earth. BioScience 2001, 51, 933–938. [Google Scholar]
- Sanderson, E.W.; Jaiteh, M.; Levy, M.A.; Redford, K.H.; Wannebo, A.V.; Woolmer, G. The human footprint and the last of the wild. BioScience 2002, 52, 891–904. [Google Scholar]
- Alcaraz-Segura, D.; Paruelo, J.M.; Cabello, J. Baseline characterization of major Iberian vegetation types based on the NDVI dynamics. Plant Ecol 2009, 202, 13–29. [Google Scholar]
- Virginia, R.A.; Wall, D.H.; Levin, S.A. Principles of Ecosystem Function. In Encyclopedia of Biodiversity; Academic Press: San Diego, CA, USA, 2001; pp. 345–352. [Google Scholar]
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.M.; Tucker, C.J.; Stenseth, N.C. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trend. Ecol. Evol 2005, 20, 503–510. [Google Scholar]
- Paruelo, J.M.; Epstein, H.E.; Lauenroth, W.K.; Burke, I.C. ANPP estimates from NDVI for the Central Grassland Region of the United States. Ecology 1997, 78, 953–958. [Google Scholar]
- Monfreda, C.; Ramankutty, N.; Foley, J.A. Farming the planet: 2. Geographic distribution of crop areas, yields, physiological types and net primary production in the year 2000. Glob. Biogeochem. Cy 2008, 22, 1–19. [Google Scholar]
- Ramankutty, N.; Evan, A.T.; Monfreda, C.; Foley, J.A. Farming the planet: 1. Geographic distribution of global agricultural lands in the year 2000. Glob. Biogeochem. Cy 2008, 22, 1–19. [Google Scholar]
- Lehner, B.; Verdin, K.; Jarvis, A. HydroSHEDS Technical Documentation; Version 1.0; World Wildlife Fund US: Washington, DC, USA, 2008. [Google Scholar]
- Crawley, M.J. GLIM for Ecologists; Blackwell Scientific Publications: Oxford, UK, 1993. [Google Scholar]
- Ricci, V. Fitting Distributions with R; CRAN: Vienna, Austria, 2005; p. 24. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology: Developments in Environmental Modelling; Elsevier Publishers: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Higgins, M.A.; Asner, G.P.; Perez, E.; Elespuru, N.; Tuomisto, H.; Ruokolainen, K.; Alonso, A. Use of Landsat and SRTM data to detect broad-scale biodiversity patterns in Northwestern Amazonia. Remote Sens 2012, 4, 2401–2418. [Google Scholar]
- Nagendra, H.; Rocchini, D.; Ghate, R.; Sharma, B.; Pareeth, S. Assessing plant diversity in a dry tropical forest: Comparing the utility of Landsat and IKONOS satellite images. Remote Sens 2010, 2, 478–496. [Google Scholar]
- Leutner, B.F.; Reineking, B.; Müller, J.; Bachmann, M.; Beierkuhnlein, C.; Dech, S.; Wegmann, M. Modelling forest α-diversity and floristic composition—On the added value of LiDAR plus hyperspectral remote sensing. Remote Sens 2012, 4, 2818–2845. [Google Scholar]
- Rocchini, D.; Balkenhol, N.; Carter, G.A.; Foody, G.M.; Gillespie, T.W.; He, K.S.; Kark, S.; Levin, N.; Lucas, K.; Luoto, M. Remotely sensed spectral heterogeneity as a proxy of species diversity: recent advances and open challenges. Ecol. Inform 2010, 5, 318–329. [Google Scholar]
- Rocchini, D.; McGlinn, D.; Ricotta, C.; Neteler, M.; Wohlgemuth, T. Landscape complexity and spatial scale influence the relationship between remotely sensed spectral diversity and survey-based plant species richness. J. Veg. Sci 2011, 22, 688–698. [Google Scholar]
- Rocchini, D. Commentary on Krishnaswamy et al.—Quantifying and mapping biodiversity and ecosystem services: Utility of a multi-season NDVI based Mahalanobis distance surrogate. Remote Sens. Environ 2009, 113, 904–906. [Google Scholar]
- Krishnaswamy, J.; Bawa, K.S.; Ganeshaiah, K.N.; Kiran, M.C. Quantifying and mapping biodiversity and ecosystem services: Utility of a multi-season NDVI based Mahalanobis distance surrogate. Remote Sens. Environ 2009, 113, 857–867. [Google Scholar]
- Eisele, A.; Lau, I.; Hewson, R.; Carter, D.; Wheaton, B.; Ong, C.; Cudahy, T.J.; Chabrillat, S.; Kaufmann, H. Applicability of the thermal infrared spectral region for the prediction of soil properties across semi-arid agricultural landscapes. Remote Sens 2012, 4, 3265–3286. [Google Scholar]
- Nemani, R.R.; Running, S.W. Land cover characterization using multitemporal red, near-IR and thermal-IR data from NOAA/AVHRR. Ecol. Appl 1997, 7, 79–90. [Google Scholar]
- McKnight, M.W.; White, P.S.; McDonald, R.I.; Lamoreux, J.F.; Sechrest, W.; Ridgely, R.S.; Stuart, S.N. Putting beta-diversity on the map: Broad-scale congruence and coincidence in the extremes. PLoS Biol 2007, 5, e272. [Google Scholar]
- Woodward, F.I.; Williams, B.G. Climate and plant distribution at global and local scales. Plant Ecolog 1987, 69, 189–197. [Google Scholar]
- Holdridge, L.R. Determination of world plant formations from simple climatic data. Science 1947, 105, 367. [Google Scholar]
- Archibold, O.W. Ecology of World Vegetation; Chapman & Hall Ltd: London, UK, 1995. [Google Scholar]
- Morrone, J.J. Biogeografía de América Latina y el Caribe; M&T–Manuales & Tesis SEA: Zaragoza, Spain, 2001. [Google Scholar]
- Volante, J.N.; Alcaraz-Segura, D.; Mosciaro, M.J.; Viglizzo, E.F.; Paruelo, J.M. Ecosystem functional changes associated with land clearing in NW Argentina. Agric. Ecosyst. Environ 2011, 154, 12–22. [Google Scholar]
- Liras, E.; Cabello, J.; Alcaraz-Segura, D.; Paruelo, J.; Maestre, F.T. Patrones Espaciales del Funcionamiento de los Ecosistemas: Efectos del Cambio en la Cobertura y el Uso del Suelo. In Analisis Espacial en Ecología, Métodos y Aplicaciones; Asociación Española de Ecología Terrestre: Alicante, 2008; pp. 717–730. [Google Scholar]
- Segurado, P.; Araújo, M.B.; Kunin, W.E. Consequences of spatial autocorrelation for niche-based models. J. Appl. Ecol 2006, 43, 433–444. [Google Scholar]
- Diniz-Filho, J.A.F.; Bini, L.M.; Hawkins, B.A. Spatial autocorrelation and red herrings in geographical ecology. Glob. Ecol. Biogeogr 2003, 12, 53–64. [Google Scholar]
Appendix
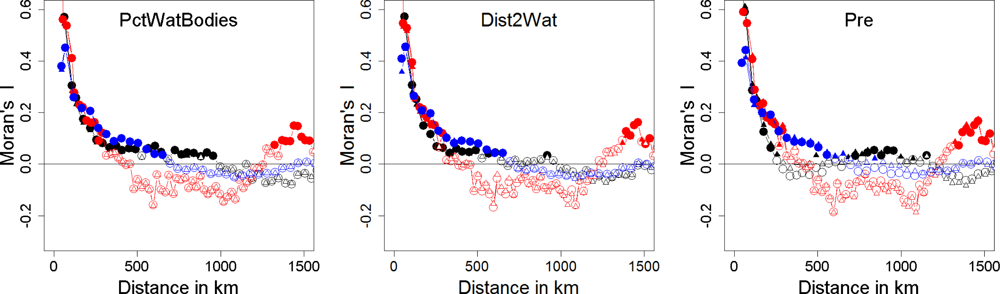



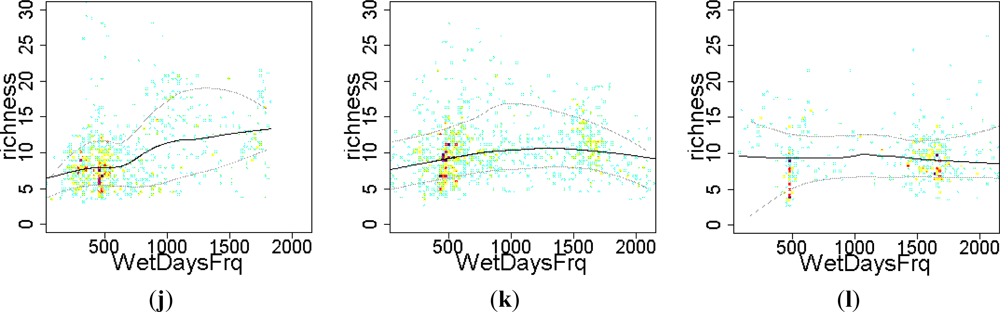
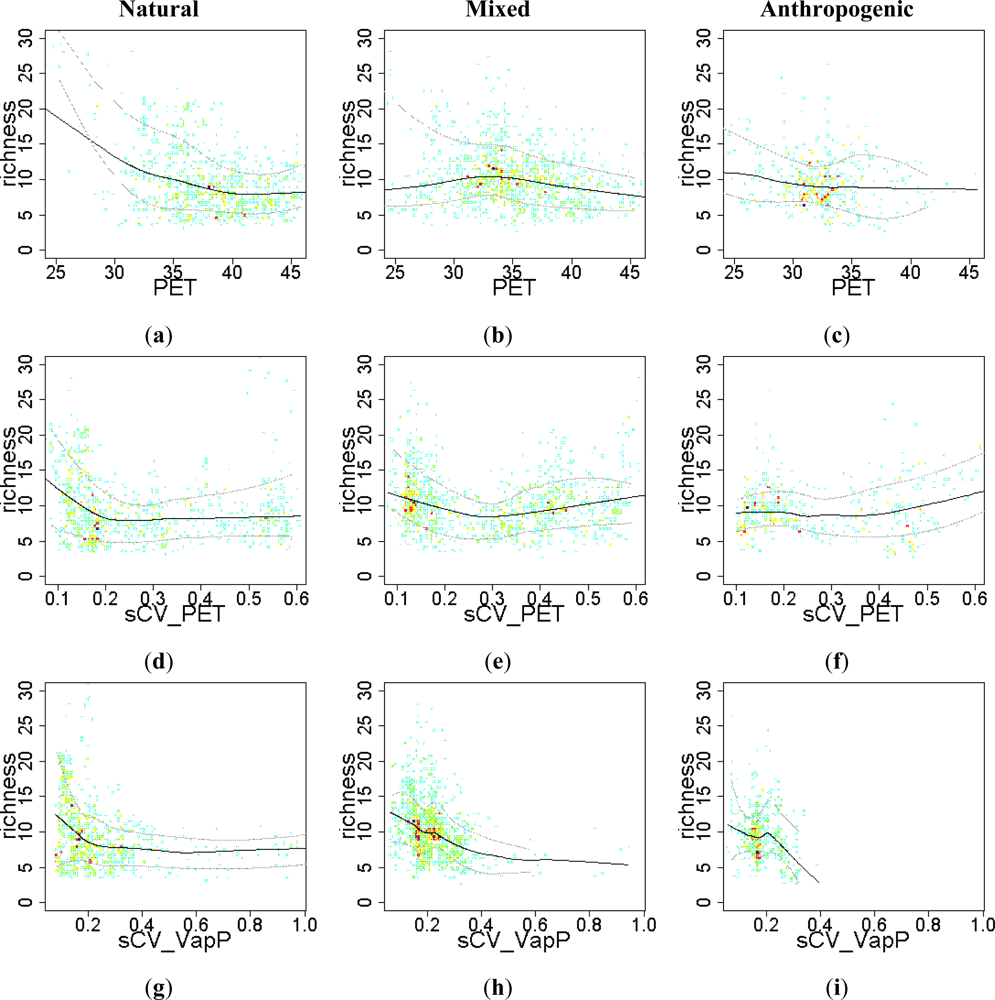

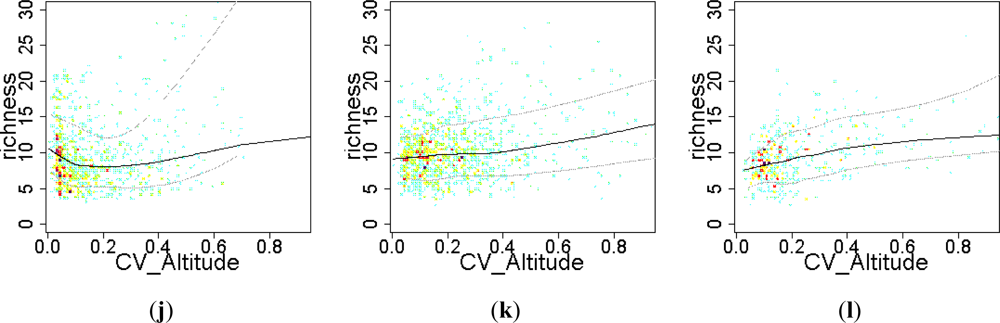
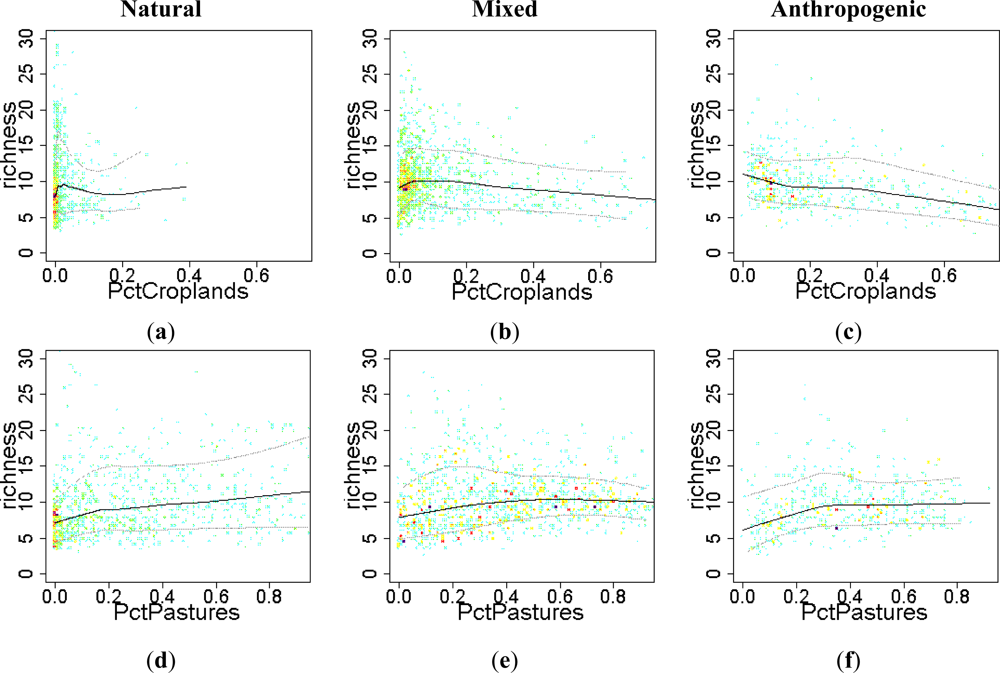
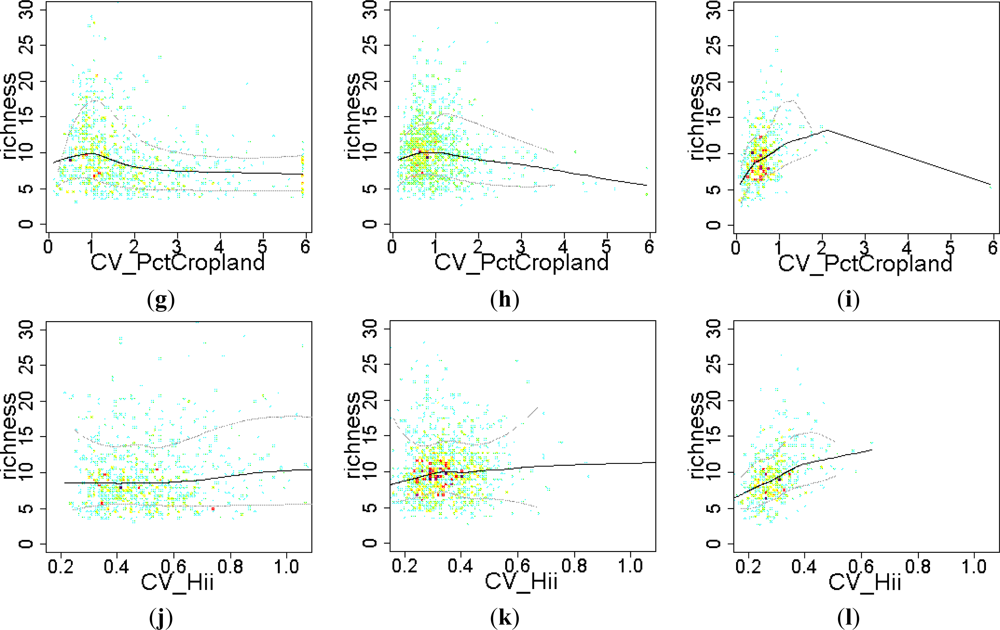
| Explained Deviance | Natural.x1 | Natural.x2 | Mix.x1 | Mix.x2 | Anthropogenic.x1 | Anthropogenic.x2 | |
|---|---|---|---|---|---|---|---|
| Water | %Water Bodies | 0.09 | 0.11 | 0.06 | 0.06 | 0.13 | 0.13 |
| Dist2Wat | 0.15 | 0.17 | 0.01 | 0.03 | 0.04 | 0.06 | |
| Precipitation | 0.14 | 0.24 | 0.03 | 0.10 | |||
| WetDaysFrq | 0.15 | 0.16 | 0.01 | 0.06 | |||
| sCV_Pre | 0.09 | 0.09 | 0.05 | ||||
| Water - Energy | PET | 0.19 | 0.22 | 0.05 | 0.05 | 0.03 | 0.03 |
| sCV_PET | 0.01 | 0.10 | 0.12 | 0.05 | |||
| sCV_VapP | 0.07 | 0.12 | 0.11 | 0.11 | 0.05 | 0.06 | |
| Energy | Temperature | 0.03 | 0.08 | 0.02 | |||
| DTR | 0.13 | 0.13 | 0.01 | 0.04 | 0.04 | 0.05 | |
| sCV_Tmp | 0.01 | 0.02 | 0.01 | 0.03 | |||
| sCV_DTR | 0.09 | 0.12 | 0.04 | 0.05 | |||
| sCV_FrostFrq | 0.02 | 0.02 | 0.03 | 0.03 | |||
| Relief | CV_Altitude | 0.02 | 0.02 | 0.06 | 0.06 | 0.18 | 0.20 |
| Human influence | %Croplands | 0.02 | 0.02 | 0.15 | 0.15 | ||
| %Pastures | 0.06 | 0.07 | 0.01 | 0.03 | 0.04 | 0.07 | |
| HII | 0.01 | ||||||
| CV_%Cropland | 0.07 | 0.07 | 0.02 | 0.06 | 0.16 | ||
| CV_%Pastures | 0.05 | 0.05 | 0.01 | ||||
| CV_HII | 0.01 | 0.01 | 0.14 | 0.15 | |||
| AIC | Natural.x1 | Natural.x2 | Mix.x1 | Mix.x2 | Anthropogenic.x1 | Anthropogenic.x2 | |
|---|---|---|---|---|---|---|---|
| Water | %Water Bodies | 5182 | 5151 | 6132 | 6127 | 2460 | 2462 |
| Dist2Wat | 5083 | 5046 | 6201 | 6172 | 2515 | 2507 | |
| Precipitation | 5086 | 4908 | 6176 | 6074 | 2537 | 2531 | |
| WetDaysFrq | 5076 | 5053 | 6207 | 6129 | 2536 | 2537 | |
| sCV_Pre | 5192 | 5191 | 6218 | 6146 | 2538 | 2538 | |
| Water - Energy | PET | 5011 | 4943 | 6144 | 6141 | 2522 | 2522 |
| sCV_PET | 5331 | 5174 | 6221 | 6045 | 2530 | 2512 | |
| sCV_VapP | 5217 | 5125 | 6049 | 6049 | 2506 | 2504 | |
| Energy | Temperature | 5286 | 5199 | 6213 | 6200 | 2536 | 2533 |
| DTR | 5120 | 5114 | 6206 | 6162 | 2514 | 2513 | |
| sCV_Tmp | 5335 | 5305 | 6200 | 6186 | 2538 | 2539 | |
| sCV_DTR | 5186 | 5128 | 6154 | 6150 | 2534 | 2534 | |
| sCV_FrostFrq | 5314 | 5306 | 6223 | 6222 | 2522 | 2524 | |
| Relief | CV_Altitude | 5315 | 5312 | 6129 | 6130 | 2428 | 2423 |
| Human influence | %Croplands | 5346 | 5348 | 6195 | 6193 | 2452 | 2450 |
| %Pastures | 5234 | 5230 | 6206 | 6177 | 2517 | 2500 | |
| HII | 5346 | 5331 | 6223 | 6216 | 2537 | 2532 | |
| CV_%Cropland | 5214 | 5214 | 6213 | 6194 | 2503 | 2445 | |
| CV_%Pastures | 5264 | 5261 | 6211 | 6212 | 2538 | 2540 | |
| CV_HII | 5331 | 5324 | 6221 | 6223 | 2456 | 2449 |
Share and Cite
Alcaraz-Segura, D.; Paruelo, J.M.; Epstein, H.E.; Cabello, J. Environmental and Human Controls of Ecosystem Functional Diversity in Temperate South America. Remote Sens. 2013, 5, 127-154. https://doi.org/10.3390/rs5010127
Alcaraz-Segura D, Paruelo JM, Epstein HE, Cabello J. Environmental and Human Controls of Ecosystem Functional Diversity in Temperate South America. Remote Sensing. 2013; 5(1):127-154. https://doi.org/10.3390/rs5010127
Chicago/Turabian StyleAlcaraz-Segura, Domingo, José M. Paruelo, Howard E. Epstein, and Javier Cabello. 2013. "Environmental and Human Controls of Ecosystem Functional Diversity in Temperate South America" Remote Sensing 5, no. 1: 127-154. https://doi.org/10.3390/rs5010127
APA StyleAlcaraz-Segura, D., Paruelo, J. M., Epstein, H. E., & Cabello, J. (2013). Environmental and Human Controls of Ecosystem Functional Diversity in Temperate South America. Remote Sensing, 5(1), 127-154. https://doi.org/10.3390/rs5010127







