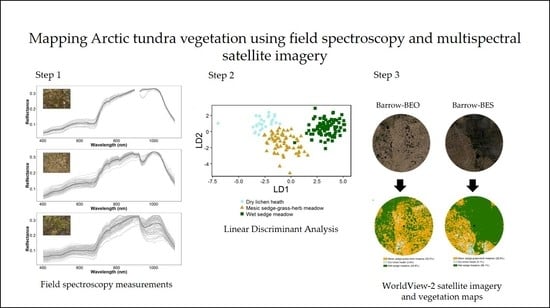
Mapping Arctic Tundra Vegetation Communities Using Field Spectroscopy and Multispectral Satellite Data in North Alaska, USA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Vegetation Data
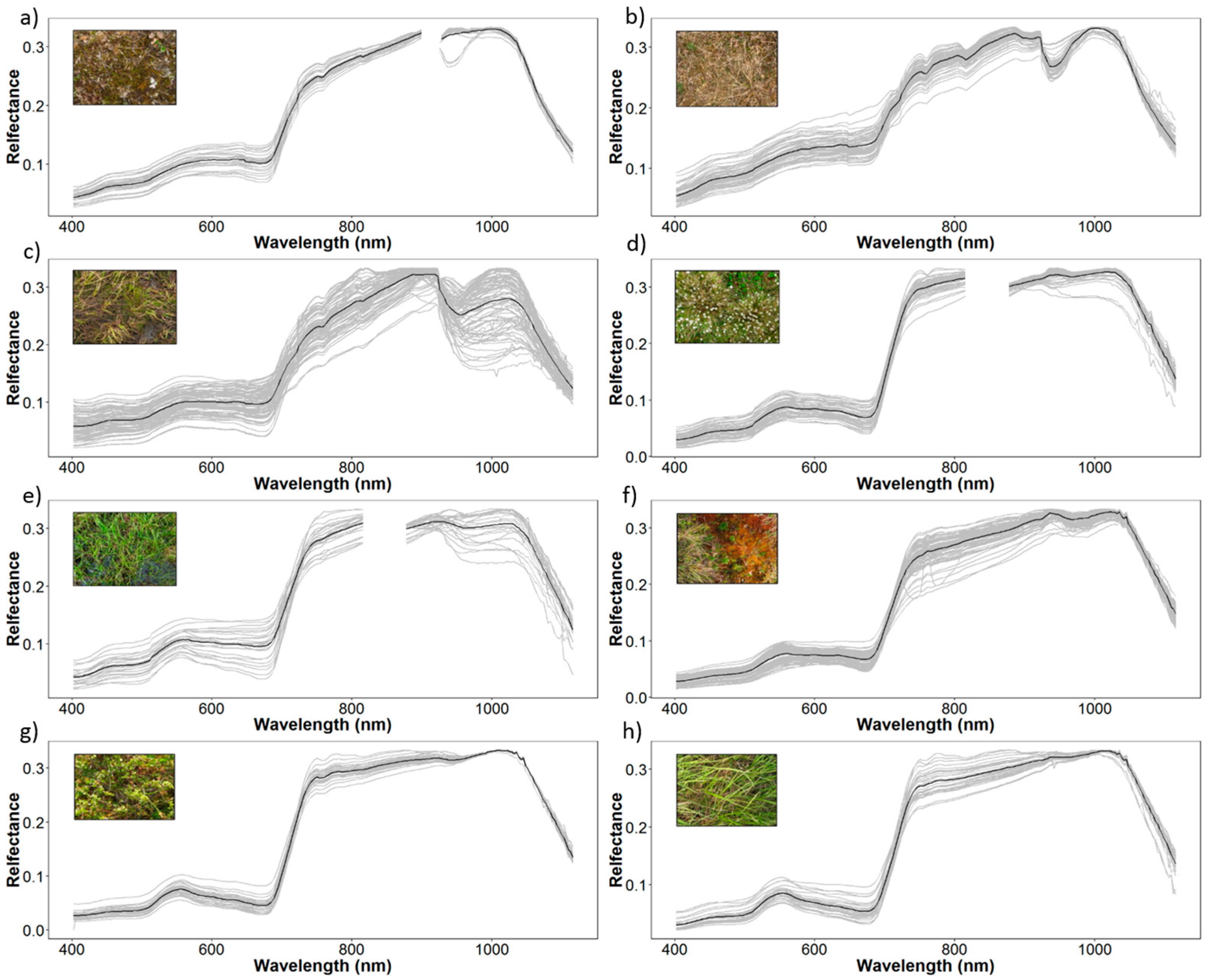
2.3. Field Spectroscopy Data Collection and Pre-Processing
2.4. Satellite Data Collection and Pre-Processing
2.5. Vegetation Indices
2.6. Spectral Separability and Image Classification
3. Results
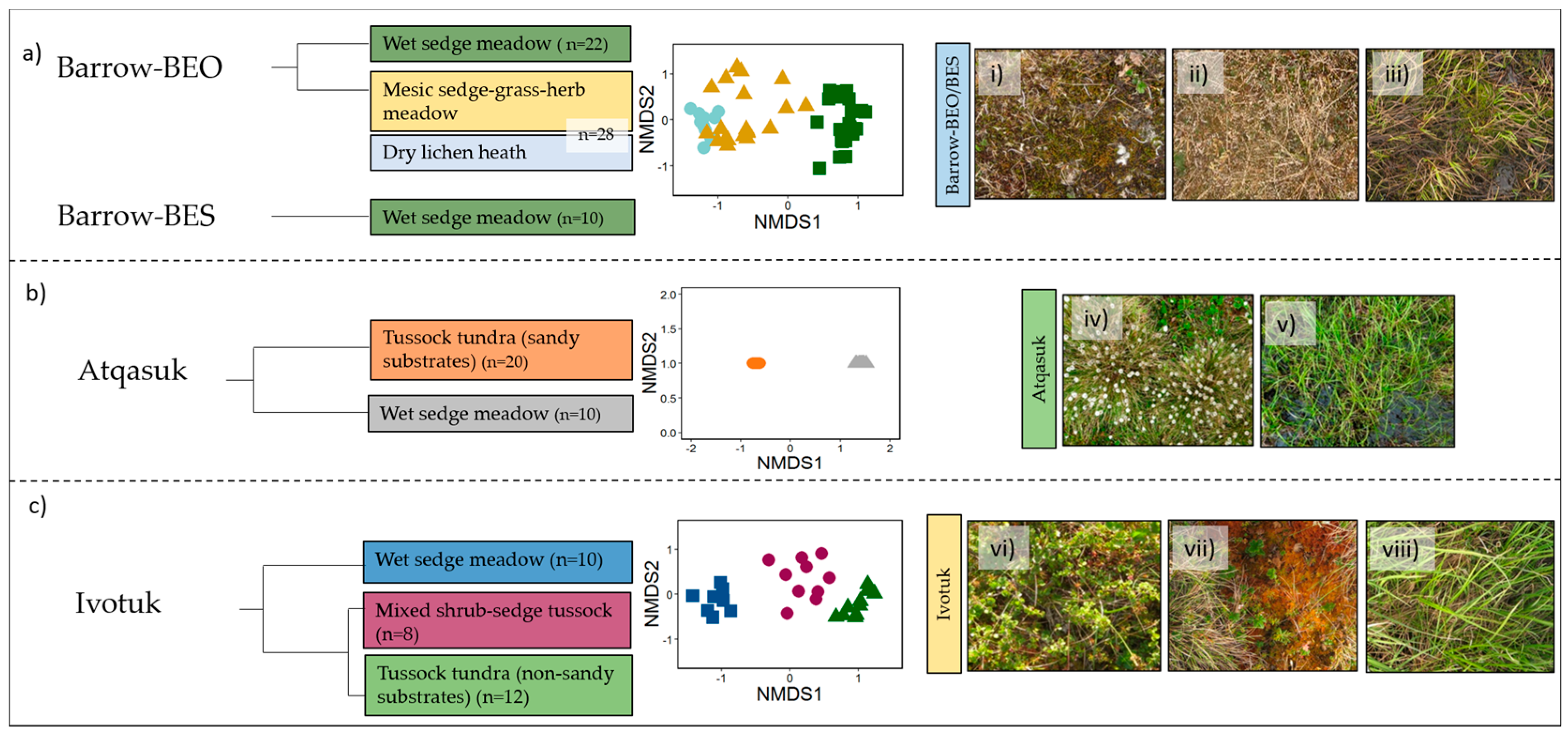
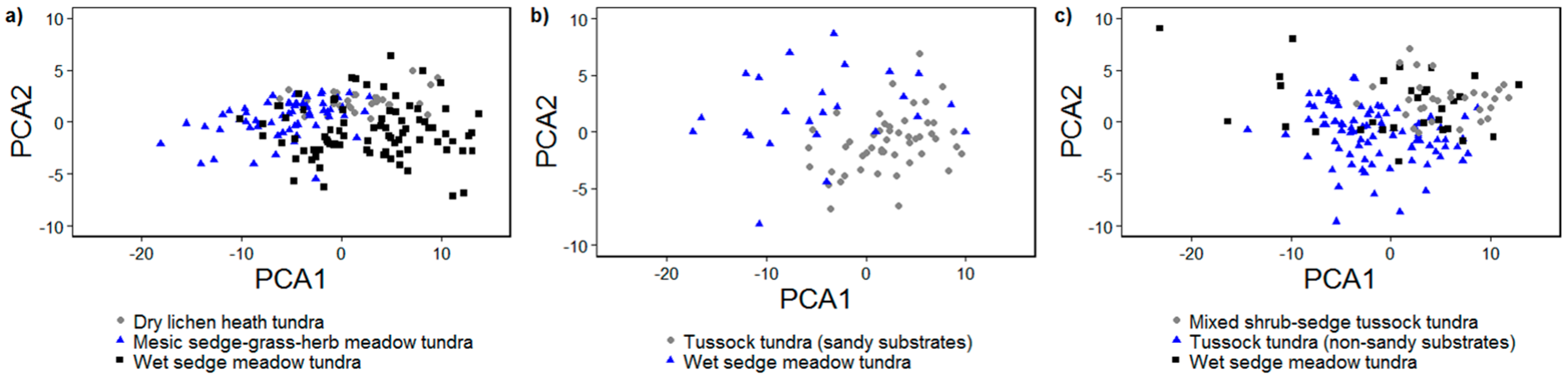
3.1. Vegetation Communities
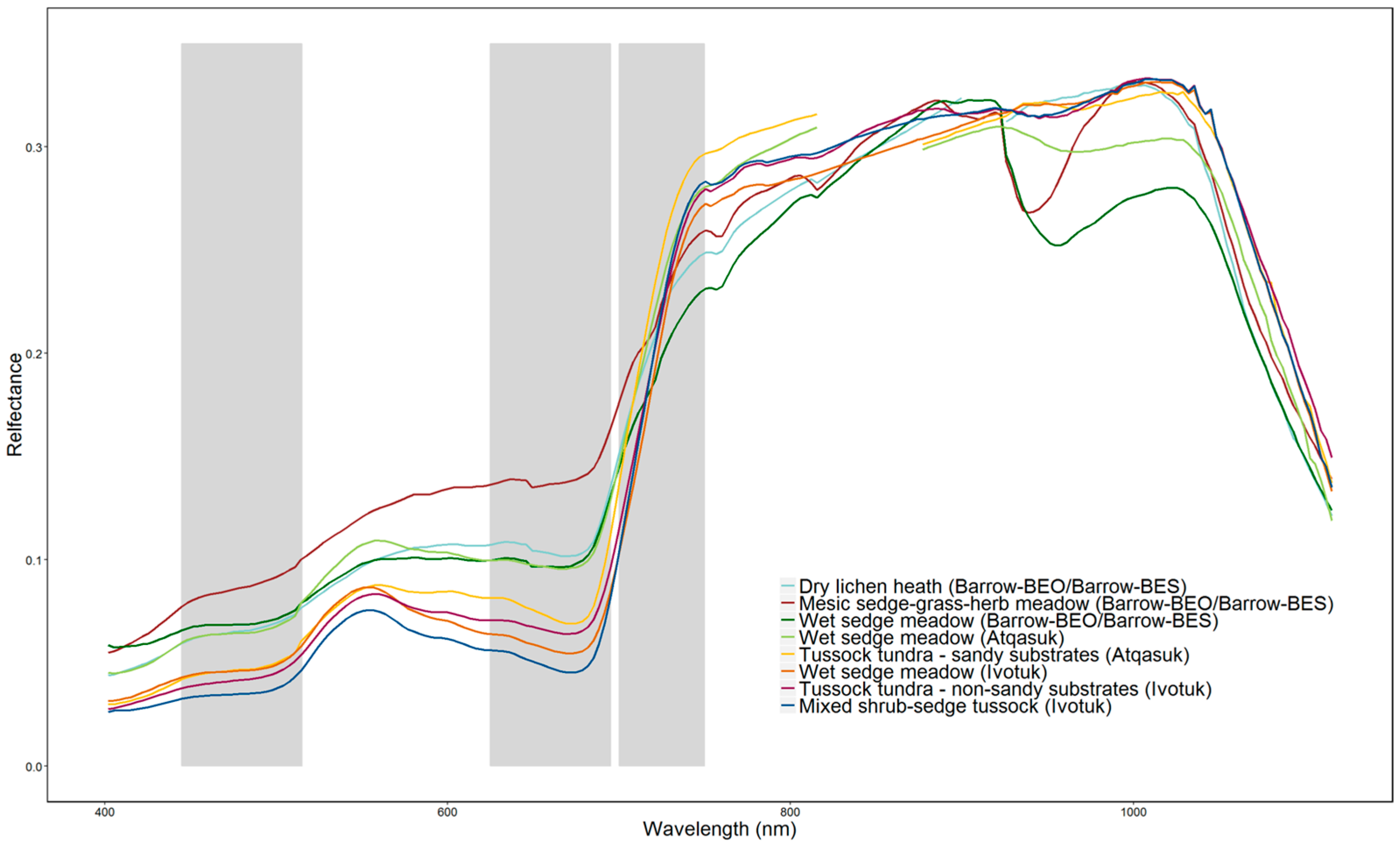
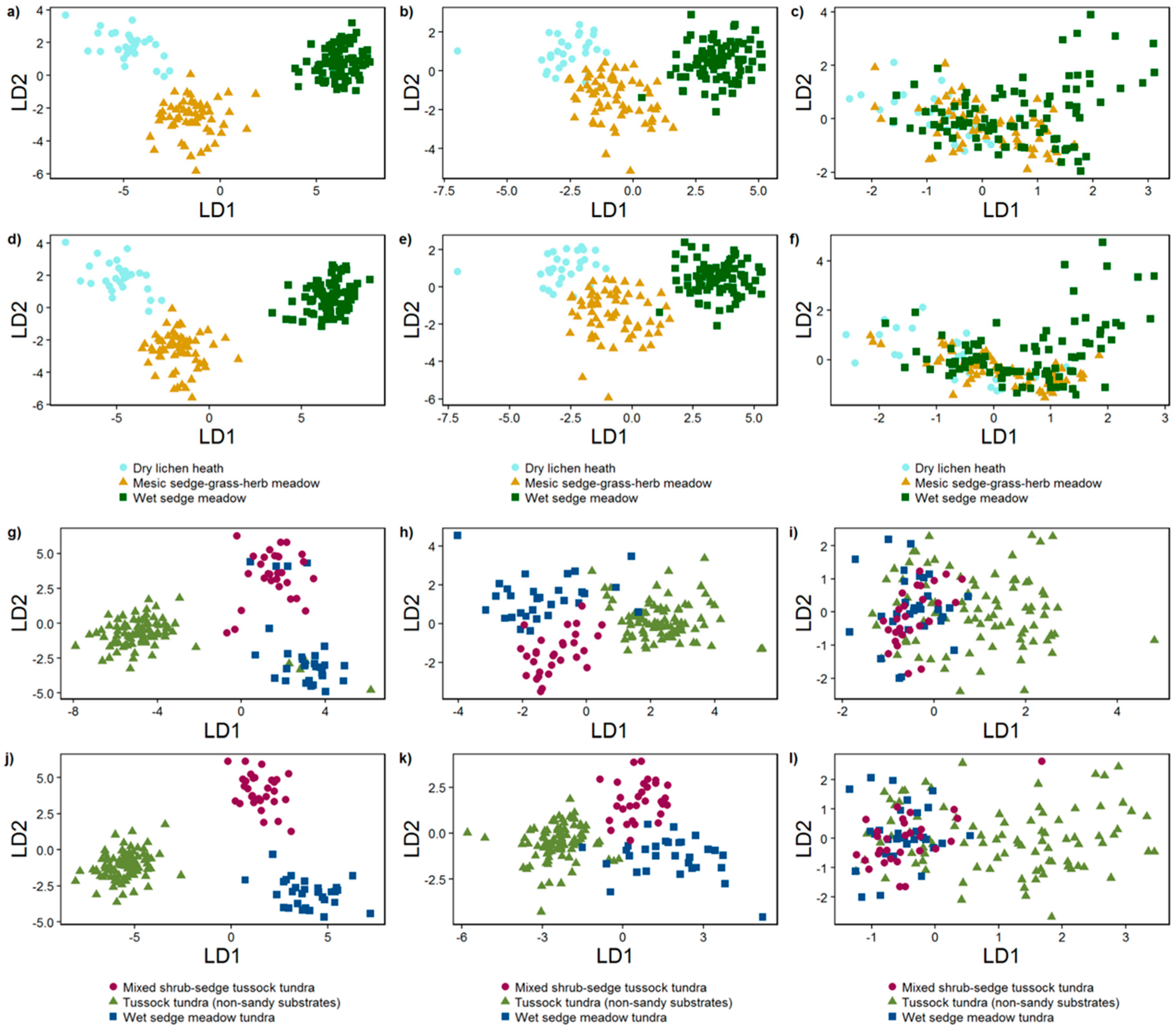
3.2. Spectral Separability in Tundra Vegetation Communities
3.2.1. Principal Components Analysis
3.2.2. Linear Discriminant Analysis
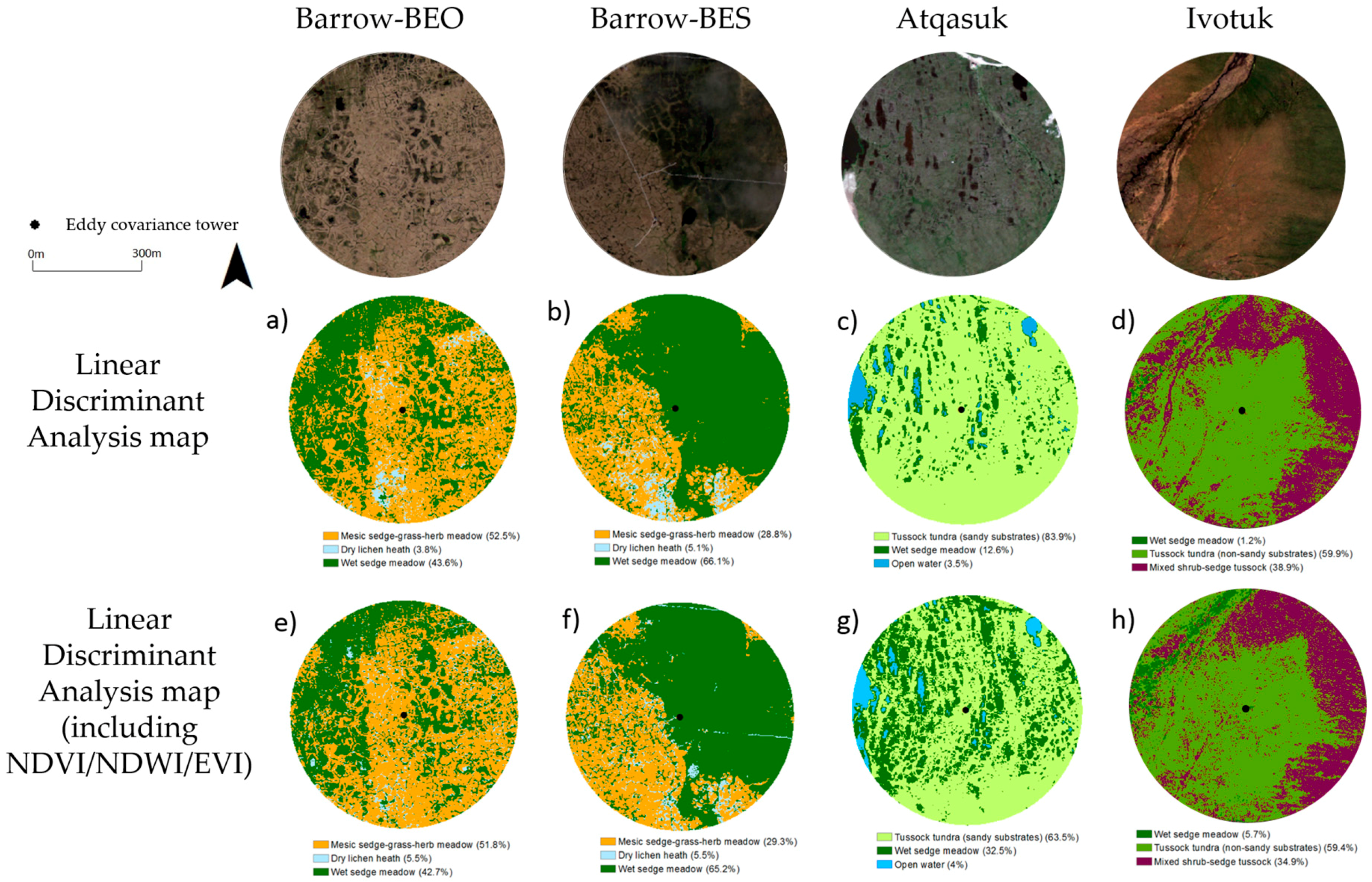
3.2.3. Vegetation Map Validation
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oechel, W.C.; Vourlitis, G.L.; Hastings, S.J.; Zulueta, R.C.; Hinzman, L.; Kane, D. Acclimation of ecosystem CO2 exchange in the Alaskan Arctic in response to decadal climate warming. Nature 2000, 406, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S.; Sturm, M.; Serreze, M.C.; McFadden, J.P.; Key, J.R.; Lloyd, A.H.; McGuire, A.D.; Rupp, T.S.; Lynch, A.H.; Schimel, J.P.; et al. Role of land-surface changes in arctic summer warming. Science 2005, 310, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Huemmrich, K.F.; Gamon, J.A.; Tweedie, C.E.; Entcheva Campbell, P.K.; Landis, D.R.; Middleton, E.M. Arctic tundra vegetation functional types based on photosynthetic physiology and optical properties. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2013, 6, 265–275. [Google Scholar] [CrossRef]
- Huemmrich, K.F.; Gamon, J.A.; Tweedie, C.E.; Oberbauer, S.F.; Kinoshita, G.; Houston, S.; Kuchy, A.; Hollister, R.D.; Kwon, H.; Mano, M.; et al. Remote sensing of tundra gross ecosystem productivity and light use efficiency under varying temperature and moisture conditions. Remote Sens. Environ. 2010, 114, 481–489. [Google Scholar] [CrossRef]
- Zeng, H.; Jia, G.; Epstein, H. Recent changes in phenology over the northern high latitudes detected from multi-satellite data. Environ. Res. Lett. 2011, 6, 45508–45518. [Google Scholar] [CrossRef]
- Ju, J.; Masek, J.G. The vegetation greenness trend in Canada and US Alaska from 1984–2012 Landsat data. Remote Sens. Environ. 2016, 176, 1–16. [Google Scholar] [CrossRef]
- Starr, G.; Oberbauer, S.F.; Ahlquist, L.E. The photosynthetic response of Alaskan tundra plants to increased season length and soil warming. Arct. Antarct. Alp. Res. 2008, 40, 181–191. [Google Scholar] [CrossRef]
- Walker, D.A.; Jia, G.J.; Epstein, H.E.; Raynolds, M.K.; Chapin, F.S., III; Copas, C.; Hinzman, L.D.; Knudson, J.A.; Maier, H.A.; Michaelson, G.J.; et al. Vegetation-soil-thaw-depth relationships along a low-arctic bioclimate gradient, Alaska: Synthesis of information from the ATLAS studies. Permafr. Periglac. Process. 2003, 14, 103–123. [Google Scholar] [CrossRef]
- Tape, K.; Sturm, M.; Racine, C. The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Glob. Chang. Biol. 2006, 12, 686–702. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Forbes, B.C.; Wilmking, M.; Hallinger, M.; Lantz, T.; Blok, D.; Tape, K.D.; Marcias-Fauria, M.; Sass-Klassen, U.; Lévesque, E.; et al. Shrub expansion in tundra ecosystems: Dynamics, impacts and research priorities. Environ. Res. Lett. 2011, 6, 1–15. [Google Scholar] [CrossRef]
- Serreze, M.C.; Walsh, J.E.; Chapin, F.S., III; Osterkamp, T.; Dyurgerov, M.; Romanovsky, V.; Oechel, W.C.; Morison, J.; Zhang, T.; Barry, R.G. Observational evidence of recent change in the northern high-latitude environment. Clim. Chang. 2000, 46, 159–207. [Google Scholar] [CrossRef]
- Zona, D.; Lipson, D.A.; Richards, J.H.; Phoenix, G.K.; Liljedahl, A.; Ueyama, M.; Sturtevant, C.S.; Oechel, W.C. Delayed responses of an Arctic ecosystem to an extreme summer: Impacts on net ecosystem exchange and vegetation functioning. Biogeosciences 2014, 11, 1–12. [Google Scholar] [CrossRef]
- Zhang, W.; Miller, P.A.; Smith, B.; Wania, R.; Koenigk, T.; Döscher, R. Tundra shrubification and tree-line advance amplify arctic climate warming: Results from an individual-based dynamic vegetation model. Environ. Res. Lett. 2013, 8, 1–10. [Google Scholar] [CrossRef]
- King, J.Y.; Reeburgh, W.S.; Regli, S.K. Methane emission and transport by arctic sedges in Alaska: Results of a vegetation removal experiment. J. Geophys. Res. 1998, 103, 29083–29092. [Google Scholar] [CrossRef]
- Davidson, S.J.; Sloan, V.L.; Phoenix, G.K.; Wagner, R.; Fisher, J.P.; Oechel, W.C.; Zona, D. Vegetation type dominates the spatial variability in CH4 emissions across multiple Arctic tundra landscapes. Ecosystems 2016, 19, 1116–1132. [Google Scholar] [CrossRef]
- Stow, D.A.; Hope, A.; McGuire, D.; Verbyla, D.; Gamon, J.; Huemmrich, F.; Houston, S.; Racine, C.; Sturm, M.; Tape, K.; et al. Remote sensing of vegetation and land-cover change in Arctic tundra ecosystems. Remote Sens. Environ. 2004, 89, 281–308. [Google Scholar] [CrossRef]
- Fox, A.M.; Huntley, B.; Lloyd, C.R.; Williams, M.; Baxter, R. Net ecosystem exchange over heterogeneous Arctic tundra: Scaling between chamber and eddy covariance measurements. Glob. Biogeochem. Cycles 2008, 22, 1–15. [Google Scholar] [CrossRef]
- Lantz, T.C.; Gergel, S.E.; Kokelj, S.V. Spatial heterogeneity in the shrub tundra ecotone in the Mackenzie Delta region, Northwest Territories: Implications for Arctic environmental change. Ecosystems 2010, 13, 194–204. [Google Scholar] [CrossRef]
- Greaves, H.E.; Vierling, L.A.; Eitel, J.U.H.; Boelman, N.T.; Magney, T.S.; Prager, C.M.; Griffin, K.L. High-resolution mapping of aboveground shrub biomass in Arctic tundra using airborne LiDAR and imagery. Remote Sens. Environ. 2016, 184, 361–373. [Google Scholar] [CrossRef]
- Shaver, G.R.; Billings, W.D.; Chapin, F.S., III; Giblin, A.E.; Nadelhoffer, K.J.; Oechel, W.C.; Ratstetter, E.B. Global change and the carbon balance of Arctic Ecosystems. BioScience 1992, 42, 433–441. [Google Scholar] [CrossRef]
- Ström, L.; Ekberg, A.; Mastepanov, M.; Christensen, T.R. The effect of vascular plants on carbon turnover and methane emissions from a tundra wetland. Glob. Chang. Biol. 2003, 9, 1185–1192. [Google Scholar] [CrossRef]
- Zona, D.; Lipson, D.A.; Zulueta, R.C.; Oberbauer, S.F.; Oechel, W.C. Microtopographic controls on ecosystem functioning in the Arctic Coastal Plain. J. Geophys. Res. 2011, 116, 1–12. [Google Scholar] [CrossRef]
- Walker, D.A.; Raynolds, M.K.; Daniëls, F.J.A.; Eythor, E.; Elvebakk, A.; Gould, W.A.; Katenin, A.E.; Kholod, S.; Markon, C.J.; Melnikov, E.; et al. The circumpolar Arctic vegetation map. J. Veg. Sci. 2005, 16, 267–282. [Google Scholar] [CrossRef]
- Komárková, V.; Webber, P.J. Two low Arctic vegetation maps near Atkasook, Alaska. Arct. Alp. Res. 1980, 12, 447–472. [Google Scholar] [CrossRef]
- Stow, D.; Hope, A.; Boynton, W.; Phinn, S.; Walker, D.; Auerbach, N. Satellite-derived vegetation index and cover type maps for estimating carbon dioxide flux for Arctic tundra regions. Geomorphology 1998, 21, 313–327. [Google Scholar] [CrossRef]
- Hartley, I.P.; Hill, T.C.; Wade, T.J.; Clement, R.J.; Moncrieff, J.B.; Prieto-Blanco, A.; Disney, M.I.; Huntley, B.; Williams, M.; Howden, N.J.K.; et al. Quantifying landscape-level methane fluxes in subarctic Finland using a multiscale approach. Glob. Chang. Biol. 2015, 21, 3712–3725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, U.S.; Walker, D.A.; Raynolds, M.K.; Comiso, J.C.; Epstein, H.E.; Jia, G.; Gens, R.; Pinzon, J.E.; Tucker, C.J.; Tweedie, C.E.; et al. Circumpolar Arctic tundra vegetation change is linked to sea ice decline. Earth Interact. 2010, 14, 1–20. [Google Scholar] [CrossRef]
- Buchhorn, M.; Walker, D.A.; Heim, B.; Raynolds, M.K.; Epstein, H.E.; Schwieder, M. Ground-based hyperspectral characterization of Alaska tundra vegetation along environmental gradients. Remote Sens. 2013, 5, 3971–4005. [Google Scholar] [CrossRef] [Green Version]
- Hope, A.S.; Kimball, J.S.; Stow, D.A. The relationship between tussock tundra spectral reflectance properties and biomass and vegetation composition. Int. J. Remote Sens. 1993, 14, 1861–1874. [Google Scholar] [CrossRef]
- Raynolds, M.K.; Comiso, J.C.; Walker, D.A.; Verbyla, D. Relationship between satellite-derived land surface temperatures, arctic vegetation types, and NDVI. Remote Sens. Environ. 2008, 112, 1884–1894. [Google Scholar] [CrossRef]
- Bhatt, U.S.; Walker, D.A.; Raynolds, M.K.; Bienek, P.A.; Epstein, H.E.; Comiso, J.C.; Pinzon, J.E.; Tucker, C.T.; Polyakov, I.V. Recent declines in warming and vegetation greening trends over Pan-Arctic tundra. Remote Sens. 2013, 5, 4229–4254. [Google Scholar] [CrossRef]
- McFadden, J.P.; Chapin, F.S., III; Hollinger, D.Y. Subgrid-scale variability in the surface energy balance of arctic tundra. J. Geophys. Res. 1998, 103, 28947–28961. [Google Scholar]
- Laidler, G.J.; Treitz, P.; Atkinson, D.M. Remote sensing of Arctic vegetation relations between the NDVI, spatial resolution and vegetation cover on Boothia Peninsula, Nunavut. Arctic 2008, 61, 1–13. [Google Scholar] [CrossRef]
- Soegaard, H.; Nordstroem, C.; Friborg, T.; Hansen, B.U. Fluxes from canopy to landscape using flux data, footprint modeling, and remote sensing. Glob. Biogeochem. Cycle 2000, 14, 725–744. [Google Scholar] [CrossRef]
- Riutta, T.; Laine, J.; Aurela, M.; Rinne, J.; Vesala, T.; Laurila, T.; Haapanala, S.; Pilhatie, M.; Tuittila, E.-S. Spatial variation in plant community functions regulates carbon gas dynamics in a boreal fen ecosystem. Tellus B 2007, 59, 838–852. [Google Scholar] [CrossRef]
- Schneider, J.; Grosse, G.; Wagner, D. Land cover classification of tundra environments in the Arctic Lena Delta based on Landsat 7 ETM+ data and its application for upscaling of methane emissions. Remote Sens. Environ. 2009, 113, 380–391. [Google Scholar] [CrossRef] [Green Version]
- Tagesson, T.; Mastepanov, M.; Mölder, M.; Tamstorf, M.P.; Eklundh, L.; Smith, B.; Sigsgaard, C.; Lund, M.; Ekberg, A.; Falk, J.M.; et al. Modelling of growing season methane fluxes in a high-Arctic wet tundra ecosystem 1997–2010 using in situ and high-resolution satellite data. Tellus B 2013, 65, 1–21. [Google Scholar] [CrossRef]
- Harris, A.; Charnock, R.; Lucas, R.M. Hyperspectral remote sensing of peatland floristic gradients. Remote Sens. Environ. 2015, 162, 99–111. [Google Scholar] [CrossRef]
- Bratsch, S.N.; Epstein, E.; Bucchorn, M.; Walker, D.A. Differentiating among four Arctic tundra plant communities at Ivotuk, Alaska using field spectroscopy. Remote Sens. 2016, 8, 51. [Google Scholar] [CrossRef]
- Andrew, M.E.; Ustin, S.L. Effects of microtopography and hydrology on phenology of an invasive herb. Ecography 2009, 32, 860–870. [Google Scholar] [CrossRef]
- Santos, M.J.; Hester, E.L.; Khanna, S.; Ustin, S.L. Image spectroscopy and stable isotopes elucidate functional dissimilarity between native and nonnative plant species in the aquatic environment. New Phytol. 2012, 193, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Ustin, S.L.; Gamon, J.A. Remote sensing of plant functional types. New Phytol. 2010, 186, 795–816. [Google Scholar] [CrossRef] [PubMed]
- Roelofsen, H.D.; van Bodegom, P.M.; Kooistra, L.; Witte, J.-P.M. Trait estimation in herbaceous plant assemblages from in situ canopy spectra. Remote Sens. 2013, 5, 6323–6345. [Google Scholar] [CrossRef]
- Walker, D.A.; Daniëls, F.J.A.; Alsos, I.; Bhatt, U.S.; Breen, A.L.; Buchhorn, M.; Bültmann, H.; Druckenmiller, L.A.; Edwards, M.E.; Ehrich, D.; et al. Circumpolar Arctic vegetation: A hierarchic review and roadmap toward an internationally consistent approach to survey, archive and classify tundra plot data. Environ. Res. Lett. 2016, 11, 1–16. [Google Scholar] [CrossRef]
- Laidler, G.J.; Treitz, P. Biophysical remote sensing of arctic environments. Prog. Phys. Geogr. 2003, 27, 44–68. [Google Scholar] [CrossRef]
- Adam, E.; Mutanga, O.; Rugege, D. Multispectral and hyperspectral remote sensing for identification and mapping of wetland vegetation: A review. Wetl. Ecol. Manag. 2010, 18, 281–296. [Google Scholar] [CrossRef]
- Langford, Z.; Kumar, J.; Hoffman, F.M.; Norby, R.J.; Wullschleger, S.D.; Sloan, V.L.; Iversen, C.M. Mapping Arctic plant functional type distributions in the Barrow Environmental Observatory using WorldView-2 and LiDAR Datasets. Remote Sens. 2016, 8, 733. [Google Scholar] [CrossRef]
- Washburn, A.L. Periglacial Processes and Environments; Edward Arnold: London, UK, 1973. [Google Scholar]
- Zona, D.; Oechel, W.C.; Kochendorfer, J.; Paw, U.K.T.; Salyuk, A.N.; Olivas, P.C.; Oberbaeur, S.F.; Lipson, D.A. Methane fluxes during the initiation of a large-scale water table manipulation experiment in the Alaskan Arctic tundra. Glob. Biogeochem. Cycles 2009, 23, 1–11. [Google Scholar] [CrossRef]
- Zona, D.; Gioli, B.; Commane, R.; Lindaas, J.; Wofsy, S.C.; Miller, C.E.; Dinardo, S.J.; Dengel, S.; Sweeney, C.; Karion, A.; et al. Cold season emissions dominate the Arctic tundra methane budget. Proc. Natl. Acad. Sci. USA 2016, 113, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Oechel, W.C.; Laskowski, C.A.; Burba, G.; Gioli, B.; Kalhori, A.A.M. Annual patterns and budget of CO2 flux in an Arctic tussock tundra ecosystem. J. Geophys. Res. Biogeosci. 2014, 119, 323–339. [Google Scholar] [CrossRef]
- Hinkel, K.M.; Paetzold, F.; Nelson, F.E.; Bochkeim, J.G. Patterns of soil temperature and moisture in the active layer and upper permafrost at Barrow, Alaska: 1993–1999. Glob. Planet. Chang. 2001, 29, 293–309. [Google Scholar] [CrossRef]
- Kwon, H.J.; Oechel, W.C.; Zulueta, R.C.; Hastings, S.J. Effects of climate variability on carbon sequestration among adjacent wet sedge tundra and moist tussock tundra ecosystems. J. Geophys. Res. Biogeosci. 2006, 111, 1–18. [Google Scholar] [CrossRef]
- Edwards, E.J.; Moody, A.; Walker, D.A. Field Data Report of ATLAS Grids and Transects 1998–1999; Alaska Geobotany Center: Fairbanks, AK, USA, 2000. [Google Scholar]
- Burba, G.; Anderson, D. A Brief Practical Guide to Eddy Covariance Flux Measurements: Principles and Workflow Examples for Scientific and Industrial Applications; Li-COR Biosciences: Lincoln, NE, USA, 2010. [Google Scholar]
- Hultén, E. Flora of Alaska and Neighboring Territories; Stanford University Press: Palo Alto, CA, USA, 1968. [Google Scholar]
- Vitt, D.H.; Marsh, J.E.; Bovey, R.B. Mosses, Lichens, and Ferns of Northwest North America; Lone Pine: Edmonton, AB, Canada, 1998. [Google Scholar]
- Walker, D.A.; Kuss, P.; Epstein, H.E.; Kade, A.N.; Vonlanthen, C.M.; Raynolds, M.K.; Daniëls, F.J.A. Vegetation of zonal patterned-ground ecosystems along the North America Arctic bioclimate gradient. Appl. Veg. Sci. 2011, 14, 440–463. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria, 2013. Available online: http://www.R-project.org/ (accessed on 1 March 2016).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Gavin, L.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. vegan: Community Ecology Package. 2013. R package version 2.0–10. Available online: http://CRAN.R-project.org/package=vegan (accessed on 1 March 2016).
- Chapin, F.S.; Bret-Harte, M.S.; Hobbie, S.E.; Zhong, H. Plant functional types as predictors of transient responses of Arctic vegetation to global change. J. Veg. Sci. 1996, 7, 347–358. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite-1 Symposium, Washington, DC, USA, 10–14 December 1974.
- Gao, B.-C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Walker, D.A.; Epstein, H.E.; Jia, G.J.; Balser, A.; Copass, C.; Edwards, E.J.; Gould, W.A.; Hollingsworth, J.; Knudson, J.; Maier, H.A.; et al. Phytomass, LAI, and NDVI in northern Alaska: Relationships to summer warmth, soil pH, plant functional types, and extrapolation to the circumpolar Arctic. J. Geophys. Res. 2003, 108, 1–16. [Google Scholar] [CrossRef]
- Gao, X.; Huete, A.R.; Ni, W.; Miura, T. Optical-biophysical relationships of vegetation spectra without background contamination. Remote Sens. Environ. 2000, 74, 609–620. [Google Scholar] [CrossRef]
- Rocha, A.V.; Shaver, G.R. Advantages of a two band EVI calculated from solar and photosynthetically active radiation fluxes. Agric. For. Meteorol. 2009, 149, 1560–1563. [Google Scholar] [CrossRef]
- Goswami, S.; Gamon, J.A.; Tweedie, C.E. Surface hydrology of an arctic ecosystem: Multiscale analysis of a flooding and draining experiment using spectral reflectance. J. Geophys. Res. Biogeosci. 2011, 116, 1–14. [Google Scholar] [CrossRef]
- Singh, A. Digital change detection techniques using remotely-sensed data. Int. J. Remote Sens. 1989, 10, 989–1003. [Google Scholar] [CrossRef]
- Schwaller, M.R. A geobotanical investigation based on linear discriminant and profile analyses of airborne thematic mapper simulator data. Remote Sens. Environ. 1987, 23, 23–34. [Google Scholar] [CrossRef]
- Bandos, T.V.; Bruzzone, L.; Camps-Valls, G. Classification of hyperspectral images with regularized linear discriminant analysis. IEEE Trans. Geosci. Remote Sens. 2009, 47, 862–873. [Google Scholar] [CrossRef]
- Gong, P.; Pu, R.; Yu, B. Conifer species recognition: An exploratory analysis of in situ hyperspectral data. Remote Sens. Environ. 1997, 62, 189–200. [Google Scholar] [CrossRef]
- Clark, M.L.; Roberts, D.A.; Clark, D.B. Hyperspectral discrimination of tropical rain forest tree species at leaf to crown scales. Remote Sens. Environ. 2005, 96, 375–398. [Google Scholar] [CrossRef]
- Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Hollister, R.D.; Webber, P.J.; Tweedie, C.E. The response of Alaskan arctic tundra to experimental warming: Differences between short- and long-term responses. Glob. Chang. Biol. 2005, 11, 525–536. [Google Scholar] [CrossRef]
- Muster, S.; Langer, M.; Heim, B.; Westermann, S.; Boike, J. Subpixel heterogeneity of ice-wedge polygonal tundra: A multi-scale analysis of land cover and evapotranspiration in the Lena River Delta, Siberia. Tellus B 2012, 64, 1–19. [Google Scholar] [CrossRef]
- Schapeman-Strub, G.; Limpens, J.; Menken, M.; Bartholomeus, H.M.; Schaepman, M.E. Towards spatial assessment of carbon sequestration in peatlands: Spectroscopy based estimation of fractional cover of three plant functional types. Biogeosciences 2009, 6, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Gates, D.M.; Keegan, H.J.; Schleter, J.C.; Weidner, V.R. Spectral properties of plants. Appl. Opt. 1965, 4, 11–20. [Google Scholar] [CrossRef]
- Ulrich, M.; Grosse, G.; Chabrillat, S.; Schirrmeister, L. Spectral characterization of periglacial surfaces and geomorphological units in the Arctic Lena Delta using field spectrometry and remote sensing. Remote Sens. Environ. 2009, 113, 1220–1235. [Google Scholar] [CrossRef] [Green Version]
- Thenkabail, P.; Smith, R.; De Pauw, E. Evaluation of narrowband and broadband vegetation indices for determining optimal hyperspectral wavebands for agricultural crop characterization. Photogramm. Eng. Remote Sens. 2002, 68, 607–621. [Google Scholar]
- Atkinson, D.M.; Treitz, P. Arctic ecological classifications derived from vegetation community and satellite spectral data. Remote Sens. 2012, 4, 3948–3971. [Google Scholar] [CrossRef]
- Shaver, G.R.; Street, L.E.; Rastetter, E.B.; van Wijk, M.T.; Williams, M. Functional convergence in regulation of net CO2 flux in heterogeneous tundra landscapes in Alaska and Sweden. J. Ecol. 2007, 95, 802–817. [Google Scholar] [CrossRef]
- La Puma, I.P.; Philippi, T.E.; Oberbauer, S.F. Relating NDVI to ecosystem CO2 exchange patterns in response to season length and soil warming manipulations in arctic Alaska. Remote Sens. Environ. 2007, 109, 225–236. [Google Scholar] [CrossRef]
- Zona, D.; Oechel, W.C.; Peterson, K.M.; Clements, R.J.; Paw U, K.T.; Ustin, S.L. Characterization of the carbon fluxes of a vegetated drained lake basin chronosequence on the Alaskan Arctic Coastal Plain. Glob. Chang. Biol. 2010, 16, 1870–1882. [Google Scholar] [CrossRef]
- Emmerton, C.A.; St. Louis, V.L.; Humphreys, E.R.; Gamon, J.A.; Barker, J.D.; Pastorello, G.Z. Net ecosystem exchange of CO2 with rapidly changing high Arctic landscapes. Glob. Chang. Biol. 2016, 22, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Kushida, K.; Kim, Y.; Tsuyuzaki, S.; Fukuda, M. Spectral vegetation indices for estimating shrub cover, green phytomass and leaf turnover in a sedge-shrub tundra. Int. J. Remote Sens. 2009, 30, 1651–1658. [Google Scholar] [CrossRef]
- Kade, A.; Bret-Harte, M.S.; Euskirchen, E.S.; Edgar, C.; Fulweber, R.A. Upscaling of CO2 fluxes from heterogeneous tundra plant communities in Arctic Alaska. J. Geophys. Res. Biogeosci. 2012, 117, 1–11. [Google Scholar] [CrossRef]
- Riedel, S.M.; Epstein, H.E.; Walker, D.A. Biotic controls over spectral indices of tundra vegetation and implications for regional scaling. Int. J. Remote Sens. 2005, 26, 2391–2405. [Google Scholar] [CrossRef]
- Hollister, R.D.; May, J.L.; Kremers, K.S.; Tweedie, C.E.; Oberbauer, S.F.; Liebig, J.A.; Botting, T.F.; Barrett, R.T.; Gregory, J.L. Warming experiments elucidate the drivers of observed directional changes in tundra vegetation. Ecol. Evol. 2015, 5, 1881–1895. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, S.; Hollister, R.D.; Johnson, D.R.; Lara, M.J.; Webber, P.J.; Tweedie, C.E. Tundra vegetation change near Barrow, Alaska (1972–2010). Environ. Res. Lett. 2012, 7, 1–10. [Google Scholar] [CrossRef]
- Frohn, R.C.; Hinkel, K.M.; Eisner, W.R. Satellite remote sensing classification of thaw lakes and drained thaw lake basins on the North Slope of Alaska. Remote Sens. Environ. 2005, 97, 116–126. [Google Scholar] [CrossRef]
- Andresen, C.G.; Lougheed, V.L. Disappearing Arctic tundra ponds: Fine-scale analysis of surface hydrology in drained thaw lake basins over a 65 year period (1948–2013). J. Geophys. Res. Biogeosci. 2015, 120, 466–479. [Google Scholar] [CrossRef]
- Liljedahl, A.K.; Boike, J.; Daanen, R.P.; Federov, A.N.; Frost, G.V.; Grosse, G.; Hinzman, L.D.; Iijma, Y.; Jorgenson, J.C.; Matveyeva, N.; et al. Pan-Arctic ice-wedge degradation in warming permafrost and influence on tundra hydrology. Nat. Geosci. 2016, 9, 312–318. [Google Scholar] [CrossRef]
- Fraser, R.H.; Olthof, I.; Lantz, T.C.; Schmitt, C. UAV photogrammetry for mapping vegetation in the low-Arctic. Arct. Sci. 2016, 2, 79–102. [Google Scholar] [CrossRef]

| Site | UniSpec DC Field Spectroscopy Measurement Collection | WorldView-2 Satellite Imagery Collection |
|---|---|---|
| Barrow-BEO/Barrow-BES | 11 July 2014 | 25 July 2014 |
| Atqasuk | 29 July 2014 | 9 July 2014 |
| Ivotuk | 16 July 2014 | 21 June 2013 |
| Barrow-BEO | Barrow-BES | Atqasuk | Ivotuk | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant Functional Type (PFT) or Category | Wet Sedge Meadow | Mesic Sedge-Grass-Herb Meadow | Dry Lichen Heath | Wet Sedge Meadow | Tussock Tundra (Sandy Substrates) | Wet Sedge Meadow | Wet Sedge Meadow | Mixed Shrub-Sedge Tussock | Tussock Tundra (Non-Sandy Substrates) | |||||||||
| freq. | % | freq. | % | freq. | % | freq. | % | freq. | % | freq. | % | freq. | % | freq. | % | freq. | % | |
| sedge | 14/20 | 4–41 | 15/20 | 1–15 | 8/10 | 0.1–1 | 5/10 | 16–28 | 17/20 | 4–41 | 9/10 | 14–60 | 9/10 | 11–65 | 9/10 | 1–40 | 9/10 | 5–70 |
| grass | 6/20 | 2–40 | 9/20 | 1.1–11 | 10/10 | 3–15 | – | – | 14/20 | 0.1–5 | - | - | - | - | 1/10 | 0.1 | - | - |
| forb | - | - | 10/20 | 0.1–10 | 6/10 | 0.1–13 | - | - | 17/20 | 3–40 | - | - | - | - | 10/10 | 1–40 | 10/10 | 5–35 |
| deciduous shrub | - | - | 3/20 | 5–10 | - | - | - | - | 13/20 | 3–31 | 3/10 | 0.1–3 | 10/10 | 5–40 | 10/10 | 3–50 | 10/10 | 0.1–6 |
| evergreen shrub | - | - | - | - | - | - | - | - | 18/20 | 6–43 | 1–10 | 0.1 | 3/10 | 0.1–6 | 10/10 | 8–75 | 10/10 | 4–20 |
| lichen | - | - | 19/20 | 0.2–25 | 10/10 | 3–27 | - | - | 18/20 | 0.7–51 | - | - | - | - | 9/10 | 0.1–27 | 9/10 | 0.1–4 |
| moss | 12/20 | 1–100 | 20/20 | 2.2–94 | 10/10 | 25–80 | 9/10 | 5–103 | 18/20 | 6–90 | 2/10 | 0.1–6 | 10/10 | 5–75 | 10/10 | 8–70 | 10/10 | 10–88 |
| bare | 2/20 | 25–50 | 3/20 | 5–10 | 4/10 | 5–20 | - | - | 11/20 | 3–10 | - | - | - | - | 5/10 | 5 | 10/10 | 5–10 |
| water | - | - | - | - | - | - | 7/10 | 30–80 | - | - | - | - | 2/10 | 5–25 | 1/10 | 5 | - | - |
| standing dead | 7/20 | 3–10 | 20/20 | 10–80 | 10/10 | 3–70 | 2/10 | 3–5 | 17/20 | 1–10 | 7/10 | 5–45 | 10/10 | 5–40 | 10/10 | 3–50 | 10/10 | 5–40 |
| Waveband | Barrow-BEO/Barrow-BES | Atqasuk | Ivotuk | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F | df | p | F | df | p | F | df | p | |
| Blue (450–510 nm) | 26.24 | 2 | <0.001 | 27.05 | 1 | <0.001 | 9.986 | 2 | <0.001 |
| Green (510–580 nm) | 43.86 | 2 | <0.001 | 28.16 | 1 | <0.001 | 7.77 | 2 | <0.001 |
| Yellow (585–625 nm) | 72.5 | 2 | <0.001 | 21.1 | 1 | <0.001 | 23.1 | 2 | <0.001 |
| Red (630–690 nm) | 74.51 | 2 | <0.001 | 24.97 | 1 | <0.001 | 45.37 | 2 | <0.001 |
| Red edge (705–745 nm) | 45.6 | 2 | <0.001 | 14.72 | 1 | <0.001 | 8.064 | 2 | <0.001 |
| Near-IR1 (770–895 nm) | 4.357 | 2 | <0.001 | 4.737 | 1 | <0.001 | 22.04 | 2 | <0.001 |
| Near-IR2 (860–1040 nm) | 43.35 | 2 | <0.001 | 15.37 | 1 | <0.001 | 0.827 | 2 | 4.39 |
| Barrow-BEO/Barrow-BES | Dry Lichen Heath | Mesic Sedge-Grass-Herb Meadow | Wet Sedge Meadow | Kappa Coefficient |
| UniSpec | 100 | 100 | 100 | 1 |
| UniSpec (+ NDVI/NDWI/EVI) | 100 | 100 | 100 | 1 |
| UniSpecWV2 | 96 | 90 | 99 | 0.92 |
| UniSpecWV2(+ NDVI/NDWI/EVI) | 100 | 92 | 99 | 0.94 |
| WorldView-2 | 61 | 52 | 43 | 0.24 |
| WorldView-2 (+ NDVI/NDWI/EVI) | 61 | 61 | 39 | 0.26 |
| Atqasuk | N/A | Tussock Tundra (Sandy Substrates) | Wet Sedge Meadow | Kappa Coefficient |
| UniSpec | n/a | 100 | 100 | 1 |
| UniSpec (+ NDVI/NDWI/EVI) | n/a | 100 | 100 | 1 |
| UniSpecWV2 | n/a | 96 | 92 | 0.88 |
| UniSpecWV2(+ NDVI/NDWI/EVI) | n/a | 98 | 96 | 0.94 |
| WorldView-2 | n/a | 86 | 92 | 0.74 |
| WorldView-2 (+ NDVI/NDWI/EVI) | n/a | 86 | 88 | 0.71 |
| Ivotuk | Mixed Shrub-Sedge Tussock | Tussock Tundra (Non-Sandy Substrates) | Wet Sedge Meadow | Kappa Coefficient |
| UniSpec | 100 | 100 | 100 | 1 |
| UniSpec (+ NDVI/NDWI/EVI) | 100 | 100 | 100 | 1 |
| UniSpecWV2 | 90 | 96 | 83 | 0.86 |
| UniSpecWV2(+ NDVI/NDWI/EVI) | 97 | 97 | 90 | 0.93 |
| WorldView-2 | 59 | 64 | 50 | 0.37 |
| WorldView-2 (+ NDVI/NDWI/EVI) | 55 | 67 | 53 | 0.4 |
| Barrow-BEO/Barrow-BES | Mesic Sedge-Grass-Herb Meadow | Dry Lichen Heath | Wet Sedge Meadow | Barrow-BEO/Barrow-BES | Mesic Sedge-Grass-Herb Meadow | Dry Lichen Heath | Wet Sedge Meadow |
|---|---|---|---|---|---|---|---|
| Mesic sedge-grass-herb meadow | 46 | 5 | 9 | Mesic sedge-grass-herb meadow | 50 | 1 | 9 |
| Dry lichen heath | 16 | 12 | 2 | Dry lichen heath | 11 | 17 | 2 |
| Wet sedge meadow | 30 | 1 | 59 | Wet sedge meadow | 31 | 10 | 49 |
| Classification accuracy | 65% | Classification accuracy | 64% | ||||
| Kappa | 0.43 | Kappa | 0.43 | ||||
| Atqasuk | Tussock Tundra (Sandy Substrates) | Wet Sedge Meadow | Atqasuk | Tussock Tundra (Sandy Substrates) | Wet Sedge Meadow | ||
| Tussock tundra (sandy substrates) | 58 | 2 | Tussock tundra (sandy substrates) | 49 | 11 | ||
| Wet sedge meadow | 9 | 21 | Wet sedge meadow | 7 | 23 | ||
| Classification accuracy | 88% | Classification accuracy | 80% | ||||
| Kappa | 0.71 | Kappa | 0.56 | ||||
| Ivotuk | Wet Sedge Meadow | Tussock Tundra (Non-Sandy Substrates) | Mixed Shrub-Sedge Tussock | Ivotuk | Wet Sedge Meadow | Tussock Tundra (Non-Sandy Substrates) | Mixed Shrub-Sedge Tussock |
| Wet sedge meadow | 4 | 40 | 14 | Wet sedge meadow | 13 | 40 | 5 |
| Tussock tundra (non-sandy substrates) | 0 | 120 | 2 | Tussock tundra (non-sandy substrates) | 1 | 111 | 11 |
| Mixed shrub-sedge tussock | 0 | 19 | 31 | Mixed shrub-sedge tussock | 0 | 6 | 44 |
| Classification accuracy | 67% | Classification accuracy | 73% | ||||
| Kappa | 0.39 | Kappa | 0.52 |
| Site | Vegetation Community | Linear Discriminant Analysis (% Cover) | Linear Discriminant Analysis + NDVI, NDWI and EVI (% Cover) |
|---|---|---|---|
| Barrow-BEO | Mesic-sedge-grass-herb meadow | 52.5 | 51.8 |
| Dry lichen heath | 3.8 | 5.5 | |
| Wet sedge meadow | 43.6 | 42.7 | |
| Barrow-BES | Mesic-sedge-grass-herb meadow | 28.8 | 29.3 |
| Dry lichen heath | 5.1 | 5.5 | |
| Wet sedge meadow | 66.1 | 65.2 | |
| Atqasuk | Tussock sedge (sandy substrates) | 83.9 | 63.5 |
| Wet sedge meadow | 12.6 | 32.5 | |
| Ivotuk | Wet sedge meadow | 1.2 | 5.7 |
| Tussock sedge (non-sandy substrates) | 59.9 | 59.4 | |
| Mixed shrub-sedge tussock | 38.9 | 34.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidson, S.J.; Santos, M.J.; Sloan, V.L.; Watts, J.D.; Phoenix, G.K.; Oechel, W.C.; Zona, D. Mapping Arctic Tundra Vegetation Communities Using Field Spectroscopy and Multispectral Satellite Data in North Alaska, USA. Remote Sens. 2016, 8, 978. https://doi.org/10.3390/rs8120978
Davidson SJ, Santos MJ, Sloan VL, Watts JD, Phoenix GK, Oechel WC, Zona D. Mapping Arctic Tundra Vegetation Communities Using Field Spectroscopy and Multispectral Satellite Data in North Alaska, USA. Remote Sensing. 2016; 8(12):978. https://doi.org/10.3390/rs8120978
Chicago/Turabian StyleDavidson, Scott J., Maria J. Santos, Victoria L. Sloan, Jennifer D. Watts, Gareth K. Phoenix, Walter C. Oechel, and Donatella Zona. 2016. "Mapping Arctic Tundra Vegetation Communities Using Field Spectroscopy and Multispectral Satellite Data in North Alaska, USA" Remote Sensing 8, no. 12: 978. https://doi.org/10.3390/rs8120978
APA StyleDavidson, S. J., Santos, M. J., Sloan, V. L., Watts, J. D., Phoenix, G. K., Oechel, W. C., & Zona, D. (2016). Mapping Arctic Tundra Vegetation Communities Using Field Spectroscopy and Multispectral Satellite Data in North Alaska, USA. Remote Sensing, 8(12), 978. https://doi.org/10.3390/rs8120978