Glycaemic Index of Maternal Dietary Carbohydrate Differentially Alters Fto and Lep Expression in Offspring in C57BL/6 Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. In Vitro GI Testing of the Diets
2.3. Animals
2.4. Food Intake and Weight Monitoring
2.5. Glucose and Insulin Tolerance Tests
2.6. Mouse Sacrifice, Blood and Tissue Collection
2.7. Quantitative Real-Time PCR (qRT-PCR)
2.8. Plasma Analysis
2.9. Statistical Analysis
3. Results
3.1. Rate of Starch Digestion in Vitro
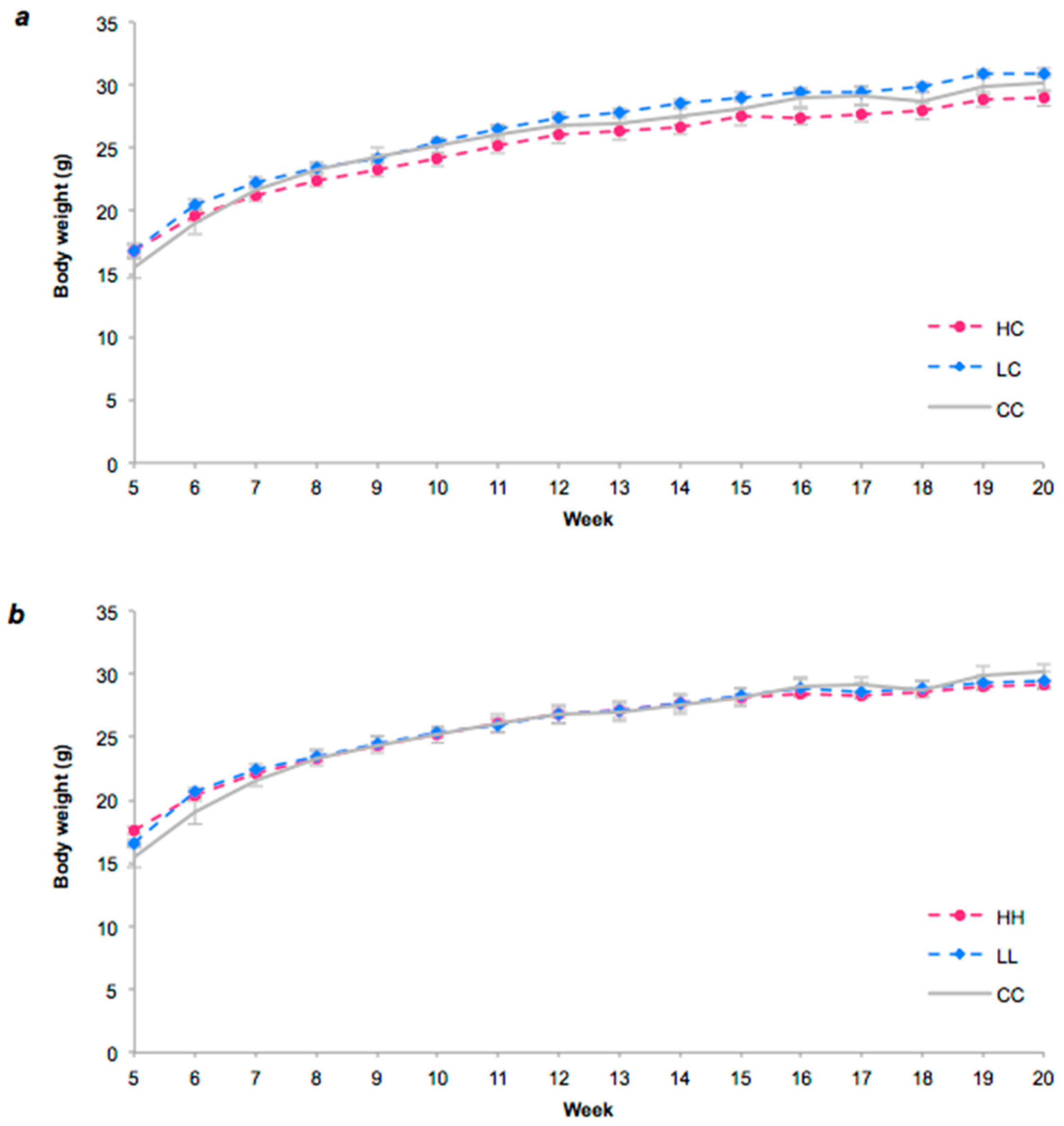
3.2. Food Intake and Body Weight
3.3. Glucose and Insulin Tolerance Tests
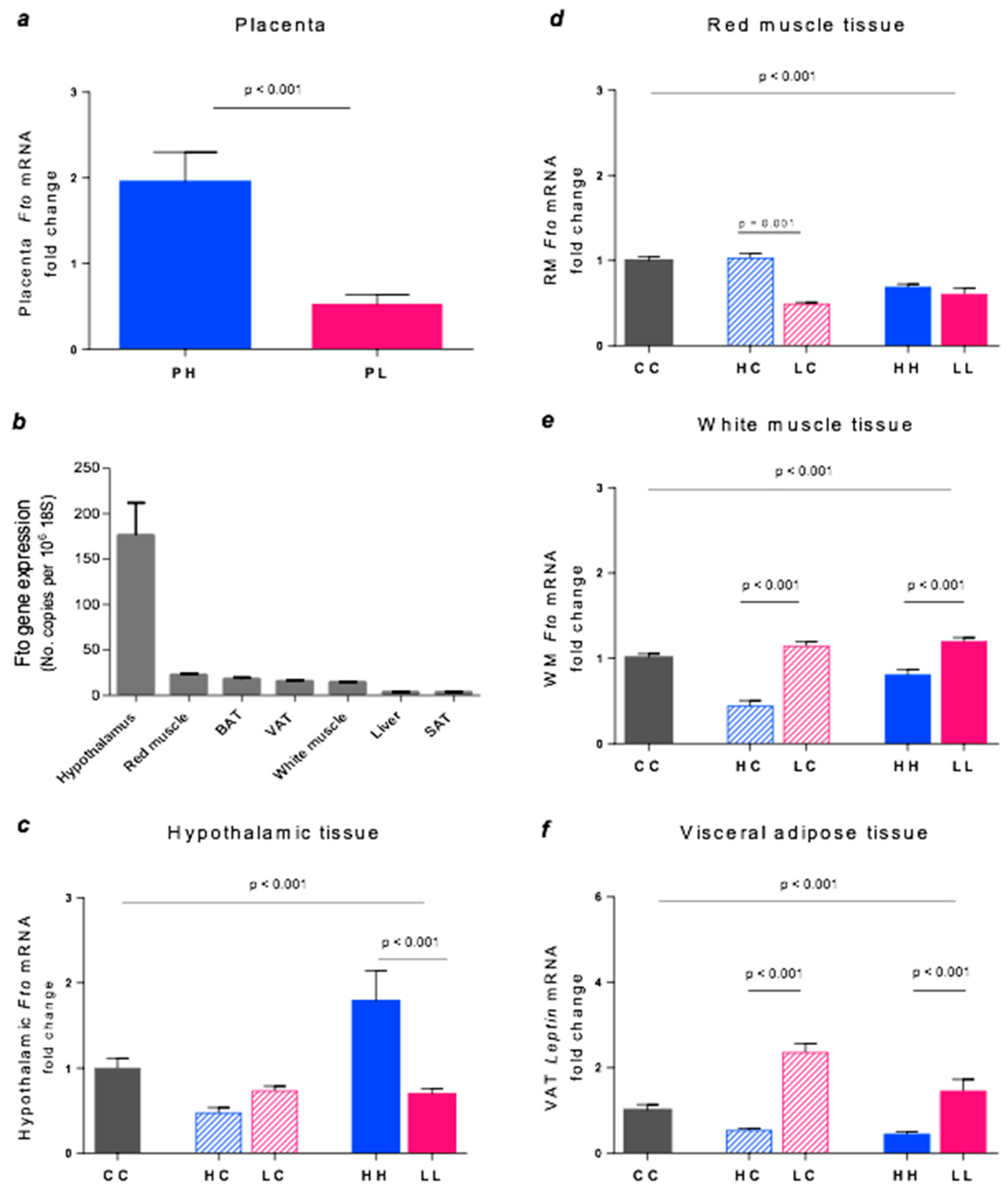
3.4. Fto Gene Expression
3.5. Leptin Gene Expression
3.6. Hypothalamic Appetite Gene Expression
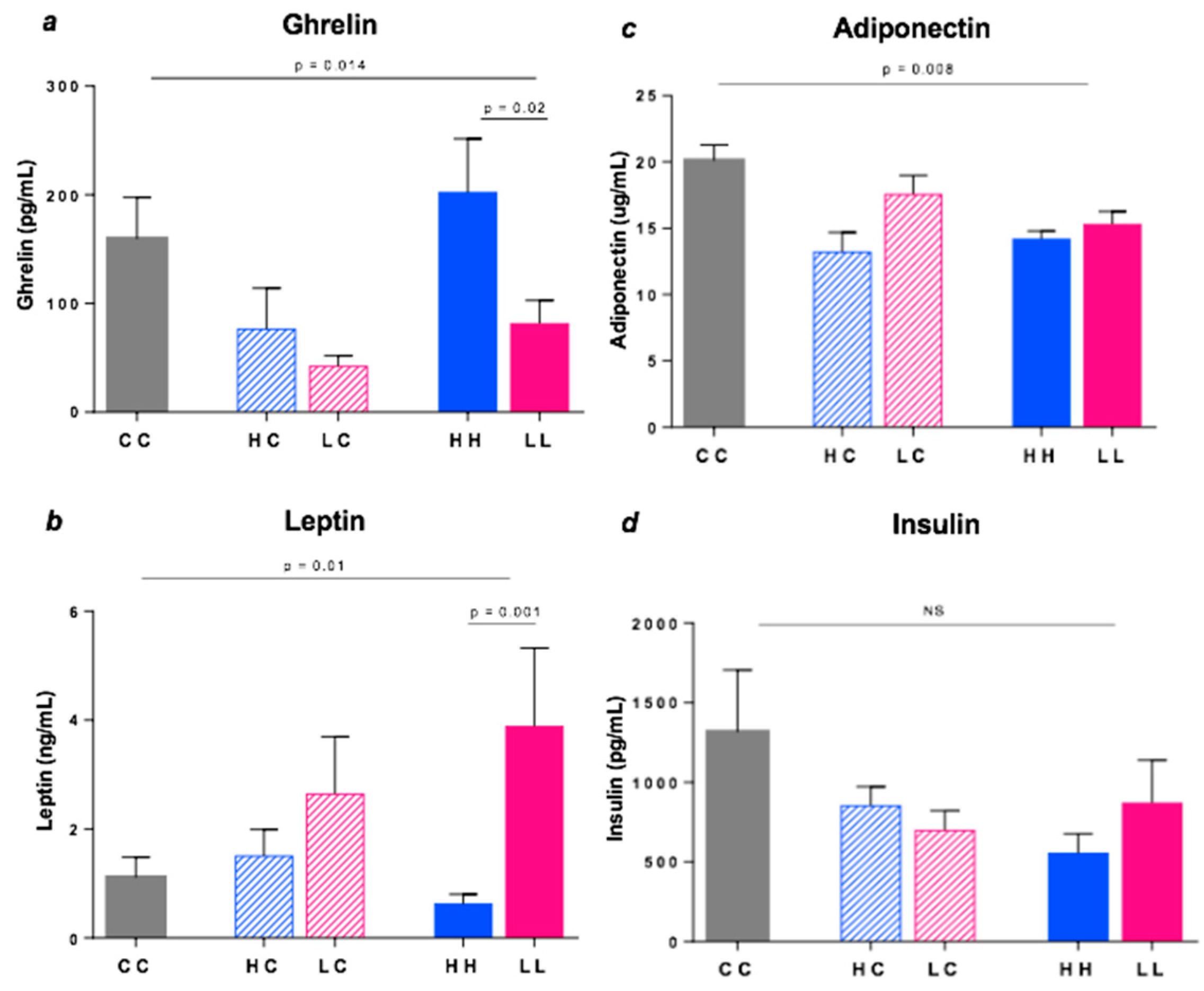
3.7. Plasma Hormones
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rivera, J.A.; de Cossio, T.G.; Pedraza, L.S.; Aburto, T.C.; Sanchez, T.G.; Martorell, R. Childhood and adolescent overweight and obesity in latin america: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 321–332. [Google Scholar] [CrossRef]
- Fowden, A.L.; Giussani, D.A.; Forhead, A.J. Endocrine and metabolic programming during intrauterine development. Early Hum. Dev. 2005, 81, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Hay, W.W. Recent observations on the regulation of fetal metabolism by glucose. J. Physiol. 2006, 572, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; McIntyre, H.D.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Eng. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef]
- Farah, N.; Hogan, J.; O’Dwyer, V.; Stuart, B.; Kennelly, M.; Turner, A.J. Influence of maternal glycemia on intrauterine fetal adiposity distribution after a normal oral glucose tolerance test at 28 weeks gestation. Exp. Diabetes Res. 2011. [Google Scholar] [CrossRef] [PubMed]
- McGowan, C.A.; McAuliffe, F.M. The influence of maternal glycaemia and dietary glycaemic index on pregnancy outcome in healthy mothers. Br. J. Nutr. 2010, 104, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, J.M.; Walsh, J.M.; Byrne, J.; Molloy, E.J.; McAuliffe, F.M. Impact of maternal diet on neonatal anthropometry: A randomized controlled trial. Pediatr. Obes. 2015, 10, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Scholl, T.O.; Chen, X.; Khoo, C.S.; Lenders, C. The dietary glycemic index during pregnancy: Influence on infant birth weight, fetal growth, and biomarkers of carbohydrate metabolism. Am. J. Epidemiol. 2004, 159, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Moses, R.G.; Luebcke, M.; Davis, W.S.; Coleman, K.J.; Tapsell, L.C.; Petocz, P.; Brand-Miller, J.C. Effect of a low-glycemic-index diet during pregnancy on obstetric outcomes. Am. J. Clin. Nutr. 2006, 84, 807–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, J.M.; Kellett, J.E.; Balsells, M.; García-Patterson, A.; Hadar, E.; Solà, I.; Gich, I.; van der Beek, E.M.; Castañeda-Gutiérrez, E.; Heinonen, S.; et al. Gestational diabetes mellitus and diet: A systematic review and meta-analysis of randomized controlled trials examining the impact of modified dietary interventions on maternal glucose control and neonatal birth weight. Diabetes Care 2018, 41, 1346. [Google Scholar] [CrossRef] [PubMed]
- Razquin, C.; Marti, A.; Martinez, J.A. Evidences on three relevant obesogenes: Mc4r, fto and ppar gamma. Approaches for personalized nutrition. Mol. Nutr. Food Res. 2011, 55, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Shuldiner, A.R. Obesity genes and gene-environment-behavior interactions: Recommendations for a way forward. Obesity 2008, 16, S79–S81. [Google Scholar] [CrossRef] [PubMed]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.B.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the fto gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Gulati, P.; Yeo, G.S.H. The biology of fto: From nucleic acid demethylase to amino acid sensor. Diabetologia 2013, 56, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Melka, M.G.; Gillis, J.; Bernard, M.; Abrahamowicz, M.; Chakravarty, M.M.; Leonard, G.T.; Perron, M.; Richer, L.; Veillette, S.; Banaschewski, T.; et al. FTo, obesity and the adolescent brain. Hum. Mol. Genet. 2013, 22, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.P. The hypothalamus, hormones, and hunger: alterations in human obesity and illness. Prog. Brain Res. 2006, 153, 57–73. [Google Scholar] [PubMed]
- Kizirian, N.V.; Kong, Y.; Muirhead, R.; Brodie, S.; Garnett, S.P.; Petocz, P.; Sim, K.A.; Celermajer, D.S.; Louie, J.C.; Markovic, T.P.; et al. Effects of a low-glycemic index diet during pregnancy on offspring growth, body composition, and vascular health: a pilot randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Kizirian, N.V.; Markovic, T.P.; Muirhead, R.; Brodie, S.; Garnett, S.P.; Louie, J.C.; Petocz, P.; Ross, G.P.; Brand-Miller, J.C. Macronutrient Balance and Dietary Glycemic Index in Pregnancy Predict Neonatal Body Composition. Nutrients 2016, 8, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.; Nicholson, P.; Thorburn, A.; Truswell, A. Food processing and the glycemic index. Am. J. Clin. Nutr. 1985, 42, 1192–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 26642:2010 Food Products–Determination of the Glycaemic Index (GI) and Recommendation for Food Classification. Available online: https://www.iso.org/obp/ui/#iso:std:iso:26642:ed-1:v1:en (accessed on 14 September 2018).
- Becker, G.F.; Passos, E.P.; Moulin, C.C. Short-term effects of a hypocaloric diet with low glycemic index and low glycemic load on body adiposity, metabolic variables, ghrelin, leptin, and pregnancy rate in overweight and obese infertile women: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.S.; Lindroos, A.K.; Kreutzer, M.; Larsen, T.M.; Astrup, A.; van Baak, M.A.; Handjieva-Darlenska, T.; Hlavaty, P.; Kafatos, A.; Kohl, A.; et al. Dietary strategy to manipulate ad libitum macronutrient intake, and glycaemic index, across eight european countries in the diogenes study. Obes. Rev. 2010, 11, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.M.; Dalskov, S.; van Baak, M.; Jebb, S.; Kafatos, A.; Pfeiffer, A.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunesova, M.; Holst, C.; et al. The diet, obesity and genes (diogenes) dietary study in eight european countries-a comprehensive design for long-term intervention. Obes. Rev. 2010, 11, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Aliaga, I.; Marvin-Guy, L.F.; Wang, P.; Wagniere, S.; Mansourian, R.; Fuerholz, A.; Saris, W.H.; Astrup, A.; Mariman, E.C.; Kussmann, M. Mechanisms of weight maintenance under high- and low-protein, low-glycaemic index diets. Mol. Nutr. Food Res. 2011, 55, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.M.; Wolever, T.M.; O’Connor, D.L.; Nisenbaum, R.; Josse, R.G. Effect of a low glycaemic index diet on blood glucose in women with gestational hyperglycaemia. Diabetes Res. Clin. Pract. 2011, 91, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Horan, M.K.; McGowan, C.A.; Gibney, E.R.; Donnelly, J.M.; McAuliffe, F.M. Maternal low glycaemic index diet, fat intake and postprandial glucose influences neonatal adiposity-secondary analysis from the rolo study. Nutr. J. 2014, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.J.; Smart, C.E.; Steil, G.M.; Brand-Miller, J.C.; King, B.; Wolpert, H.A. Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: Implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care 2015, 38, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.G.; Senior, M.A.; Bell-Anderson, S.K. Metabolic effects of high glycaemic index diets: A systematic review and meta-analysis of feeding studies in mice and rats. Nutrients 2017, 9, 646. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Rosén, L.; Wierup, N.; Östman, E.; Björck, I.; Holm, C. A low glycaemic diet improves oral glucose tolerance but has no effect on β-cell function in c57bl/6j mice. Diabetes Obes. Metable. 2010, 12, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Englyst, K.N.; Englyst, H.N.; Hudson, G.J.; Cole, T.J.; Cummings, J.H. Rapidly available glucose in foods: An in vitro measurement that reflects the glycemic response. Am. J. Clin. Nutr. 1999, 69, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, S.; Blair, A.R.; Deluca, N.; Fam, B.C.; Proietto, J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metable. 2008, 295, E1323–E1332. [Google Scholar] [CrossRef] [PubMed]
- Ayala, J.E.; Samuel, V.T.; Morton, G.J.; Obici, S.; Croniger, C.M.; Shulman, G.I.; Wasserman, D.H.; McGuinness, O.P. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis. Model. Mech. 2010, 3, 525–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolever, T.M.S. Effect of blood sampling schedule and method of calculating the area under the curve on validity and precision of glycaemic index values. Br. J. Nutr. 2004, 91, 295–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gekas, C.; Rhodes, K.E.; Mikkola, H.K.A. Isolation and visualization of mouse placental hematopoietic stem cells. Cur. Protoc. Stem Cell Biol. 2008, 6, 2A.8.1–2A.8.14. [Google Scholar] [CrossRef] [PubMed]
- Gekas, C.; Rhodes, K.E.; Mikkola, H.K. Isolation and analysis of hematopoietic stem cells from the placenta. J. Vis. Exp. 2008, 16, 742. [Google Scholar] [CrossRef] [PubMed]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real-time PCR data. BMC Bioinformatics 2006, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, A.; Sanna, S.; Chen, W.-M.; Uda, M.; Albai, G.; Strait, J.; Najjar, S.; Nagaraja, R.; Orrú, M.; Usala, G.; et al. Genome-wide association scan shows genetic variants in the fto gene are associated with obesity-related traits. PLoS Genet. 2007, 3, e115. [Google Scholar] [CrossRef] [PubMed]
- Kielar, D.; Clark, J.S.C.; Ciechanowicz, A.; Kurzawski, G.; Sulikowski, T.; Naruszewicz, M. Leptin receptor isoforms expressed in human adipose tissue. Metabolism 1998, 47, 844–847. [Google Scholar] [CrossRef]
- Church, C.; Moir, L.; McMurray, F.; Girard, C.; Banks, G.T.; Teboul, L.; Wells, S.; Brüning, J.C.; Nolan, P.M.; Ashcroft, F.M.; et al. Overexpression of fto leads to increased food intake and results in obesity. Nat. Genet. 2010, 42, 1086. [Google Scholar] [CrossRef] [PubMed]
- Karra, E.; O’Daly, O.G.; Choudhury, A.I.; Yousseif, A.; Millership, S.; Neary, M.T.; Scott, W.R.; Chandarana, K.; Manning, S.; Hess, M.E.; et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J. Clin. Investig. 2013, 123, 3539–3551. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, R.; Hawkins, M.; Barzilai, N.; Rossetti, L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 1998, 393, 684. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.E. The drive to regain is mainly in the brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1297–R1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlak, D.B.; Kushner, J.; Ludwig, D. Effects of dietary glycaemic index on adiposity, glucose homoeostasis, and plasma lipids in animals. Lancet 2004, 364, 778–785. [Google Scholar] [CrossRef]
- Byrnes, S.E.; Miller, J.C.B.; Denyer, G.S. Amylopectin starch promotes the development of insulin resistance in rats. J. Nutr. 1995, 125, 1430–1437. [Google Scholar] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sideratou, T.; Atkinson, F.; Campbell, G.J.; Petocz, P.; Bell-Anderson, K.S.; Brand-Miller, J. Glycaemic Index of Maternal Dietary Carbohydrate Differentially Alters Fto and Lep Expression in Offspring in C57BL/6 Mice. Nutrients 2018, 10, 1342. https://doi.org/10.3390/nu10101342
Sideratou T, Atkinson F, Campbell GJ, Petocz P, Bell-Anderson KS, Brand-Miller J. Glycaemic Index of Maternal Dietary Carbohydrate Differentially Alters Fto and Lep Expression in Offspring in C57BL/6 Mice. Nutrients. 2018; 10(10):1342. https://doi.org/10.3390/nu10101342
Chicago/Turabian StyleSideratou, Theodora, Fiona Atkinson, Grace J. Campbell, Peter Petocz, Kim S. Bell-Anderson, and Jennie Brand-Miller. 2018. "Glycaemic Index of Maternal Dietary Carbohydrate Differentially Alters Fto and Lep Expression in Offspring in C57BL/6 Mice" Nutrients 10, no. 10: 1342. https://doi.org/10.3390/nu10101342




