The Synergistic Effects of Resveratrol combined with Resistant Training on Exercise Performance and Physiological Adaption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Experiment Design
2.3. Anaerobic Exercise Training and Capacity Test
2.4. Aerobic Exercise Endurance Performance Test
2.5. Forelimb Grip Strength
2.6. Fatigue-Associated Biochemical Variables
2.7. Clinical Biochemical Profiles
2.8. Body Composition and Glycogen Content Analysis
2.9. Immunohistochemical Staining
2.10. Histopathology
2.11. Statistical Analysis
3. Results
3.1. The Effects of Climb Training and Resveratrol on Grip Strength
3.2. The Effects of Climb Training and Resveratrol on Anaerobic Exercise Performance
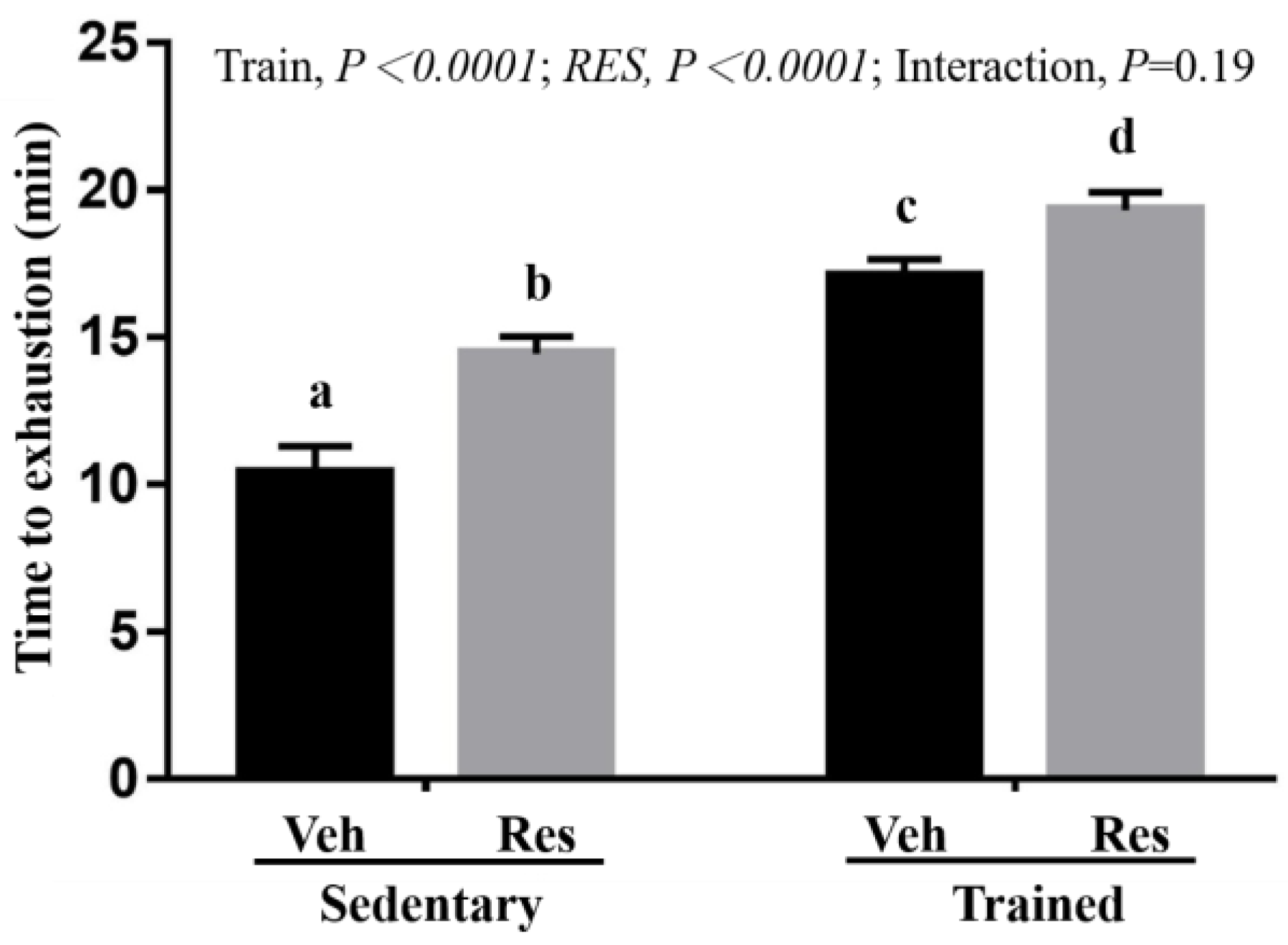
3.3. The Effects of Climb Training and Resveratrol on Aerobic Exercise Performance
3.4. The Effects of Climb Training and Resveratrol on Fatigue-Associated Biochemistries
3.5. The Effects of Climb Training and Resveratrol on Clinical Biochemistries
3.6. The Effects of Climb Training and Resveratrol on Tissue Glycogen Contents
3.7. The Effects of Climb Training and Resveratrol on Growth and Body Composition
3.8. The Effects of Climb Training and Resveratrol on Histological Observation
3.9. The Effects of Climb Training and Resveratrol on Muscle Types and Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kulkarni, S.S.; Cantó, C. The molecular targets of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Sales, J.M.; Resurreccion, A.V. Resveratrol in peanuts. Crit. Rev. Food. Sci. Nutr. 2014, 54, 734–770. [Google Scholar] [CrossRef] [PubMed]
- Romero-Pérez, A.I.; Ibern-Gómez, M.; Lamuela-Raventós, R.M.; de La Torre-Boronat, M.C. Piceid, the major resveratrol derivative in grape juices. J. Agric. Food Chem. 1999, 47, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Biagi, M.; Bertelli, A.A. Wine, alcohol and pills: What future for the French paradox? Life Sci. 2015, 131, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jia, B.; Song, X.; Kong, Q.Y.; Wu, M.L.; Qiu, Z.W.; Li, H.; Liu, J. Preventive Potential of Resveratrol in Carcinogen-Induced Rat Thyroid Tumorigenesis. Nutrients 2018, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Marques, B.C.A.A.; Trindade, M.; Aquino, J.C.F.; Cunha, A.R.; Gismondi, R.O.; Neves, M.F.; Oigman, W. Beneficial effects of acute trans-resveratrol supplementation in treated hypertensive patients with endothelial dysfunction. Clin. Exp. Hypertens. 2018, 40, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, Z.; Ke, L.; Li, Z.; Li, W.; Zhang, Z.; Zhou, Y.; Feng, X.; Zhu, W. Resveratrol improves glucose uptake in insulin-resistant adipocytes via Sirt1. J. Nutr. Biochem. 2018, 55, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Peñalver, P.; Belmonte-Reche, E.; Adán, N.; Caro, M.; Mateos-Martín, M.L.; Delgado, M.; González-Rey, E.; Morales, J.C. Alkylated resveratrol prodrugs and metabolites as potential therapeutics for neurodegenerative diseases. Eur. J. Med. Chem. 2018, 146, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Li, S.; Lin, C.C. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors 2018, 44, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J. The mechanisms of muscle hypertrophy and their application to resistance training. J. Strength Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef] [PubMed]
- Flack, K.D.; Davy, K.P.; Hulver, M.W.; Winett, R.A.; Frisard, M.I.; Davy, B.M. Aging, resistance training, and diabetes prevention. J. Aging Res. 2010, 2011, 127315. [Google Scholar] [CrossRef] [PubMed]
- Siparsky, P.N.; Kirkendall, D.T.; Garrett, W.E. Muscle Changes in Aging: Understanding Sarcopenia. Sports Health 2014, 6, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, C.; Damas, F.; Nóbrega, S.R.; Ugrinowitsch, C.; Lixandrão, M.E.; Marcelino Eder Dos Santos, L.; Libardi, C.A. High-frequency resistance training does not promote greater muscular adaptations compared to low frequencies in young untrained men. Eur. J. Sport Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Westcott, W.L. Resistance training is medicine: Effects of strength training on health. Curr. Sports Med. Rep. 2012, 11, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Buckner, S.L.; Jessee, M.B.; Dankel, S.J.; Mattocks, K.T.; Abe, T.; Loenneke, J.P. Resistance exercise and sports performance: The minority report. Med. Hypotheses 2018, 113, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.S.; Faigenbaum, A.D.; Stone, M.H.; Oliver, J.L.; Jeffreys, I.; Moody, J.A.; Brewer, C.; Pierce, K.C.; McCambridge, T.M.; Howard, R.; et al. Position statement on youth resistance training: The 2014 International Consensus. Br. J. Sports Med. 2014, 48, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.E.; Huang, W.C.; Liao, C.C.; Chang, Y.K.; Kan, N.W.; Huang, C.C. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 2013, 18, 4689–4702. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; McCrory, J.L.; Kearcher, K.; Vickers, A.; Frear, B.; Gilleland, D.L.; Bonner, D.E.; Thomas, J.M.; Donley, D.A.; Lively, M.W.; et al. Resveratrol enhances exercise-Induced cellular and functional adaptations of skeletal muscle in older men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Gliemann, L.; Schmidt, J.F.; Olesen, J.; Biensø, R.S.; Peronard, S.L.; Grandjean, S.U.; Mortensen, S.P.; Nyberg, M.; Bangsbo, J.; Pilegaard, H.; et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J. Physiol. 2013, 591, 5047–5059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornberger, T.A., Jr.; Farrar, R.P. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can. J. Appl. Physiol. 2004, 29, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Conover, C.A.; Bale, L.K.; Nair, K.S. Comparative gene expression and phenotype analyses of skeletal muscle from aged wild-type and PAPP-A-deficient Mice. Exp. Gerontol. 2016, 80, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.J.; Huang, W.C.; Chiu, C.C.; Liu, Y.L.; Chiu, W.C.; Chiu, C.H.; Chiu, Y.S.; Huang, C.C. Capsaicin supplementation reduces physical fatigue and improves exercise performance in mice. Nutrients 2016, 8, 648. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Huang, C.C.; Chuang, H.L.; Chen, W.C.; Hsu, M.C. Cornu cervi pantotrichum supplementation improves physiological adaptions during intensive endurance training. J. Vet. Med. Sci. 2017, 79, 674–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, C.Y.; Chen, Y.M.; Hsu, Y.J.; Huang, C.C.; Sung, H.C.; Chen, S.S. Supplementation with Hualian No. 4 wild bitter gourd (Momordica charantia Linn. var. abbreviata ser.) extract increases anti-fatigue activities and enhances exercise performance in mice. J. Vet. Med. Sci. 2017, 79, 1110–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musa, T.H.; Li, W.; Xiaoshan, L.; Guo, Y.; Wenjuan, Y.; Xuan, Y.; YuePu, P.; Pingmin, W. Association of normative values of grip strength with anthropometric variables among students, in Jiangsu Province. Homo 2018, 69, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Prestes, J.; Leite, R.D.; Pereira, G.B.; Shiguemoto, G.E.; Bernardes, C.F.; Asano, R.Y.; Sales, M.M.; Bartholomeu Neto, J.; Perez, S.E. Resistance training and glycogen content in ovariectomized rats. Int. J. Sports Med. 2012, 33, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Campo, D.J.; Martínez-Guardado, I.; Olcina, G.; Marín-Pagán, C.; Martínez-Noguera, F.J.; Carlos-Vivas, J.; Alcaraz, P.E.; Rubio, J.Á. Effect of high-intensity resistance circuit-based training in hypoxia on aerobic performance and repeat sprint ability. Scand. J. Med. Sci. Sports 2018. [Google Scholar] [CrossRef] [PubMed]
- Hellyer, N.J.; Nokleby, J.J.; Thicke, B.M.; Zhan, W.Z.; Sieck, G.C.; Mantilla, C.B. Reduced ribosomal protein s6 phosphorylation after progressive resistance exercise in growing adolescent rats. J. Strength Cond. Res. 2012, 26, 16571666. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Soto, M.G.; Valdez-Ortiz, R.; López Alvarenga, J.C.; Espinosa-Cuevas Mde, L. Effect of resistance exercises on the indicators of muscle reserves and handgrip strength in adult patients on hemodialysis. J. Ren. Nutr. 2016, 26, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.Y.; Zhao, K.X.; Xiao, Q. Effect of resveratrol on forelimb grip strength and myofibril structure in aged rats. Nan Fang Yi Ke Da Xue Xue Bao 2017, 37, 1405–1409. [Google Scholar] [PubMed]
- Wilson, G.J.; Newton, R.U.; Murphy, A.J.; Humphries, B.J. The optimal training load for the development of dynamic athletic performance. Med. Sci. Sports Exerc. 1993, 25, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Paavolainen, L.; Häkkinen, K.; Hämäläinen, I.; Nummela, A.; Rusko, H. Explosive-strength training improves 5-km running time by improving running economy and muscle power. J. Appl. Physiol. 1999, 86, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.; Knapp, K.; Lackie, A.; Lewry, C.; Horvey, K.; Benko, C.; Trinh, J.; Butcher, S. Multimodal high-intensity interval training increases muscle function and metabolic performance in females. Appl. Physiol. Nutr. Metab. 2015, 40, 1157–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, S.; Ahn, N.; Kim, S.; Byun, J.; Joo, Y.; Kim, S.; Jung, Y.; Park, S.; Hwang, I.; Kim, K. The effect of ladder-climbing exercise on atrophy/hypertrophy-related myokine expression in middle-aged male Wistar rats. J. Physiol. Sci. 2015, 65, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Cairns, S.P. Lactic acid and exercise performance: Culprit or friend? Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, D.; Lund, M.T.; Scheuer, C.M.; Dehlbaek, M.S.; Dideriksen, S.G.; Abildskov, C.V.; Christensen, K.K.; Dohlmann, T.L.; Larsen, S.; Vigelsø, A.H.; Dela, F.; et al. High-intensity interval training improves insulin sensitivity in older individuals. Acta Physiol. 2018, 222, e13009. [Google Scholar] [CrossRef] [PubMed]
- Lekli, I.; Ray, D.; Das, D.K. Longevity nutrients resveratrol, wines and grapes. Genes Nutr. 2010, 5, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Momken, I.; Stevens, L.; Bergouignan, A.; Desplanches, D.; Rudwill, F.; Chery, I.; Zahariev, A.; Zahn, S.; Stein, T.P.; Sebedio, J.L.; et al. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 2011, 25, 3646–3660. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Castro, L.A.; Schiborr, C.; David, F.; Ehrt, H.; Voggel, J.; Sus, N.; Behnam, D.; Bosy-Westphal, A.; Frank, J. The Oral bioavailability of trans-resveratrol from a grapevine-shoot extract in healthy humans is significantly increased by micellar solubilization. Mol. Nutr. Food Res. 2018, 62, e1701057. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]







| F Values for Two-Way ANOVA | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | Sed + Vehicle | Sed + RES | Train + Vehicle | Train + RES | Main Effect of RES | Main Effect of Climb | Interaction (RES × Climb) |
| GLU (mg/dL) | 103 ± 5 | 116 ± 11 | 124 ± 9 | 123 ± 11 | 0.45 | 2.16 | 0.5 |
| LACT (mmol/L) | 4.9 ± 0.2 b | 4.5 ± 0.2 b | 4.7 ± 0.2 b | 3.5 ± 0.2 a | 19.84 * | 9.79 * | 4.77 * |
| BUN (mg/dL) | 25.8 ± 0.8 | 23.9 ± 0.6 | 23.7 ± 1 | 23.9 ± 1 | 0.95 | 1.44 | 1.5 |
| CK (U/L) | 395 ± 65 | 349 ± 62 | 459 ± 73 | 364 ± 52 | 1.03 | 0.47 | 0.1 |
| NH3 (umol/L) | 97 ± 46 a,b | 90 ± 78 a | 106 ± 28 b | 85 ± 59 a | 8.02 * | 0.16 | 2.24 |
| F Values for Two-Way ANOVA | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | Sed + Vehicle | Sed + RES | Trained + Vehicle | Trained + RES | Main Effect of RES | Main Effect of Climb | Interaction (RES × Climb) |
| AST (U/L) | 129 ± 12 | 149 ± 22 | 116 ± 8 | 174 ± 37 | 3 | 0.07 | 0.68 |
| ALT (U/L) | 44 ± 3 a | 78 ± 12 b | 50 ± 5 a,b | 77 ± 17 b | 7.9 * | 0.06 | 0.1 |
| TG (mg/dL) | 114 ± 5 | 118 ± 9 | 124 ± 12 | 125 ± 9 | 0.06 | 0.81 | 0.02 |
| CK (U/L) | 400 ± 48 | 384 ± 83 | 360 ± 36 | 349 ± 61 | 0.05 | 0.4 | 0 |
| LDH (U/L) | 565 ± 46 | 672 ± 78 | 547 ± 28 | 634 ± 59 | 3.07 | 0.26 | 0.03 |
| BUN (mg/dL) | 24.5 ± 0.6 | 25.4 ± 1.1 | 25.8 ± 0.6 | 26.5 ± 1.3 | 0.64 | 1.5 | 0.01 |
| CREA (mg/dL) | 0.4 ± 0.01 | 0.4 ± 0.01 | 0.4 ± 0.01 | 0.4 ± 0.01 | 0 | 0 | 2 |
| UA (mg/dL) | 1.83 ± 0.14 a | 2.91 ± 0.31 b | 3.05 ± 0.39 b,c | 3.90 ± 0.40 c | 8.79 * | 11.53 * | 0.12 |
| ALB (g/dL) | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.8 ± 0.1 | 2.8 ± 0.1 | 0.66 | 0.27 | 0.14 |
| TP (g/dL) | 5.4 ± 0.1 | 5.4 ± 0.1 | 5.5 ± 0.1 | 5.4 ± 0.1 | 0.67 | 0.05 | 0.12 |
| GLU | 148 ± 8 | 142 ± 10 | 157 ± 10 | 143 ± 6 | 1.34 | 0.28 | 0.25 |
| F Values for Two-Way ANOVA | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Sed + Vehicle | Sed + RES | Trained + Vehicle | Trained + RES | Main Effect of RES | Main Effect of Climb | Interaction (RES × Climb) |
| Initial BW (g) | 31.0 ± 0.3 | 30.4 ± 0.3 | 30.3 ± 0.2 | 30.7 ± 0.4 | --- | --- | --- |
| Final BW (g) | 38.5 ± 0.4 | 37.7 ± 0.6 | 38.4 ± 0.6 | 38.5 ± 0.6 | 0.16 | 0.21 | 0.31 |
| Food intake (g/day) | 7.28 ± 0.2 a | 7.84 ± 0.2 c | 7.6 ± 0.2 b | 7.2 ± 0.2 a | 0.46 | 5.6* | 44.69 * |
| Water intake (mL/day) | 9.5 ± 0.3 a | 10.14 ± 0.2 b | 10.73 ± 0.2 c | 10.64 ± 0.2 c | 7.9 * | 77.81 * | 14.27 * |
| Liver (g) | 2.04 ± 0.17 | 2.04 ± 0.16 | 2.15 ± 0.12 | 2.10 ± 0.16 | 0.1 | 1.31 | 0.17 |
| Muscle (g) | 0.38 ± 0.05 | 0.36 ± 0.06 | 0.38 ± 0.05 | 0.39 ± 0.07 | 1.25 | 0.77 | 0.52 |
| MT (g) | 0.46 ± 0.02 a | 0.48 ± 0.02 a,b | 0.51 ± 0.02 b,c | 0.53 ± 0.02 c | 0.822 | 10.1 * | 0.01 |
| Heart (g) | 0.17 ± 0.04 | 0.18 ± 0.04 | 0.18 ± 0.05 | 0.18 ± 0.04 | 0.56 | 0.2 | 0.2 |
| Lung (g) | 0.26 ± 0.07 | 0.23 ± 0.07 | 0.24 ± 0.05 | 0.23 ± 0.06 | 1.25 | 0.77 | 0.52 |
| Kidney (g) | 0.68 ± 0.06 | 0.67 ± 0.07 | 0.68 ± 0.07 | 0.68 ± 0.08 | 0.46 | 0.03 | 0.03 |
| EFP (g) | 0.39 ± 0.11 | 0.35 ± 0.14 | 0.39 ± 0.12 | 0.34 ± 0.12 | 0.98 | 0 | 0.01 |
| BAT (g) | 0.11 ± 0.04 | 0.10 ± 0.06 | 0.10 ± 0.04 | 0.16 ± 0.13 | 0.48 | 1 | 1.22 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kan, N.-W.; Lee, M.-C.; Tung, Y.-T.; Chiu, C.-C.; Huang, C.-C.; Huang, W.-C. The Synergistic Effects of Resveratrol combined with Resistant Training on Exercise Performance and Physiological Adaption. Nutrients 2018, 10, 1360. https://doi.org/10.3390/nu10101360
Kan N-W, Lee M-C, Tung Y-T, Chiu C-C, Huang C-C, Huang W-C. The Synergistic Effects of Resveratrol combined with Resistant Training on Exercise Performance and Physiological Adaption. Nutrients. 2018; 10(10):1360. https://doi.org/10.3390/nu10101360
Chicago/Turabian StyleKan, Nai-Wen, Mon-Chien Lee, Yu-Tang Tung, Chien-Chao Chiu, Chi-Chang Huang, and Wen-Ching Huang. 2018. "The Synergistic Effects of Resveratrol combined with Resistant Training on Exercise Performance and Physiological Adaption" Nutrients 10, no. 10: 1360. https://doi.org/10.3390/nu10101360






