Induction of Trained Innate Immunity in Human Monocytes by Bovine Milk and Milk-Derived Immunoglobulin G
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Samples and Monocyte Purification
2.2. Trained Immunity Model in Human Monocytes
2.3. Reagents
2.4. Cytokine Measurement
2.5. Statistical Analysis
3. Results
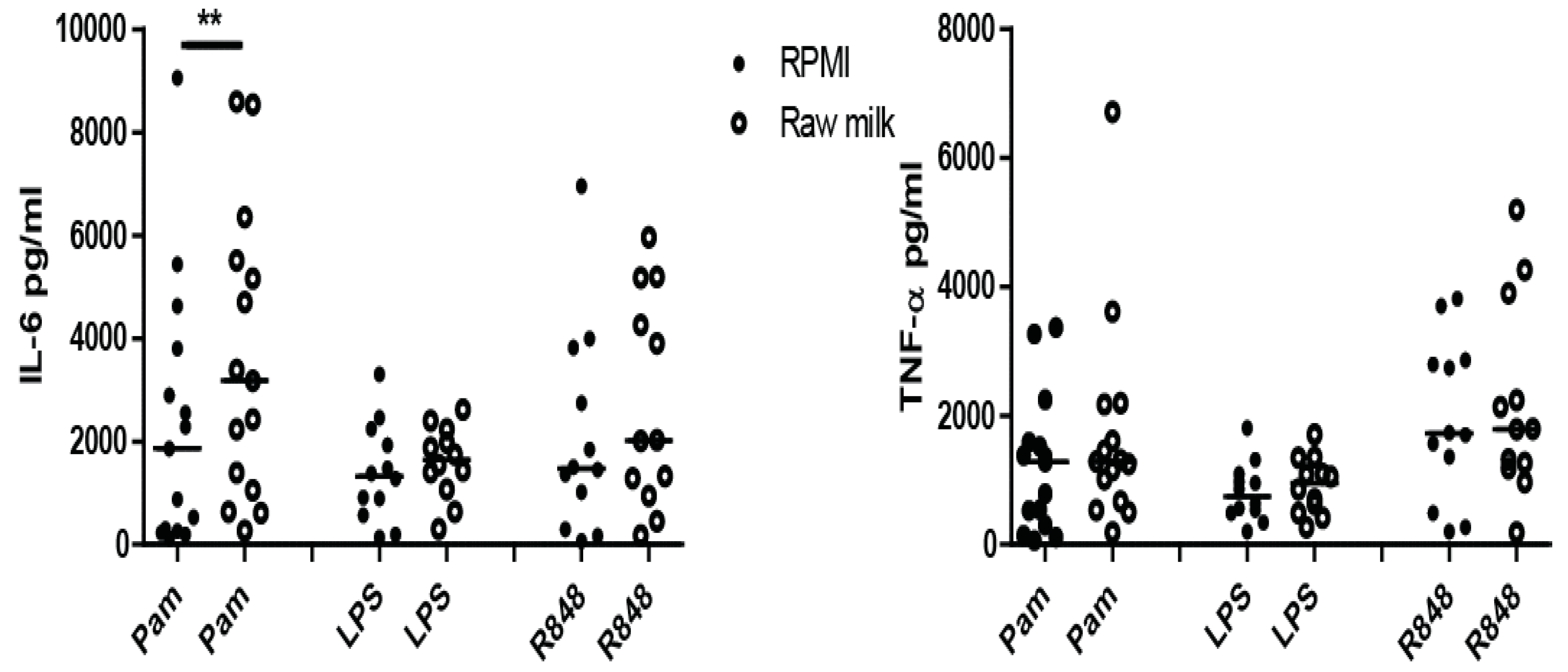
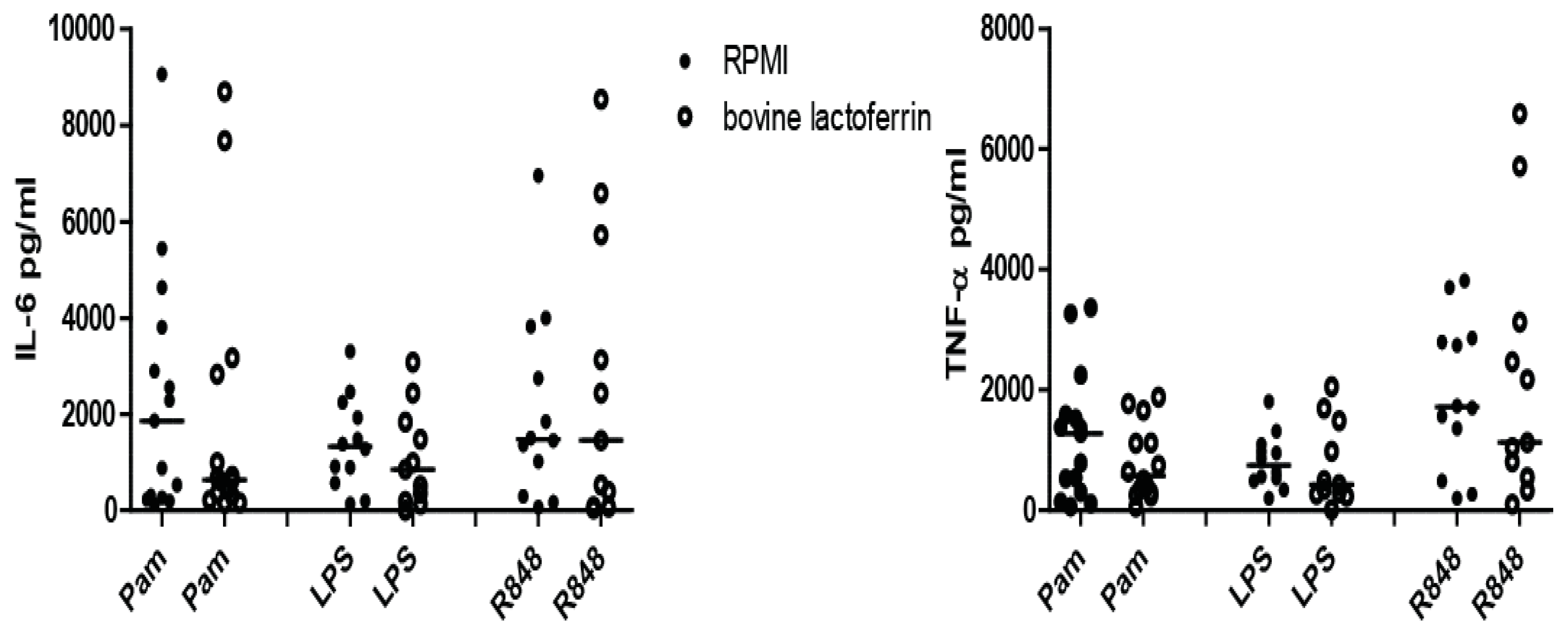
3.1. Screening of Bovine Milk and Milk Fractions for the Induction of Trained Immunity
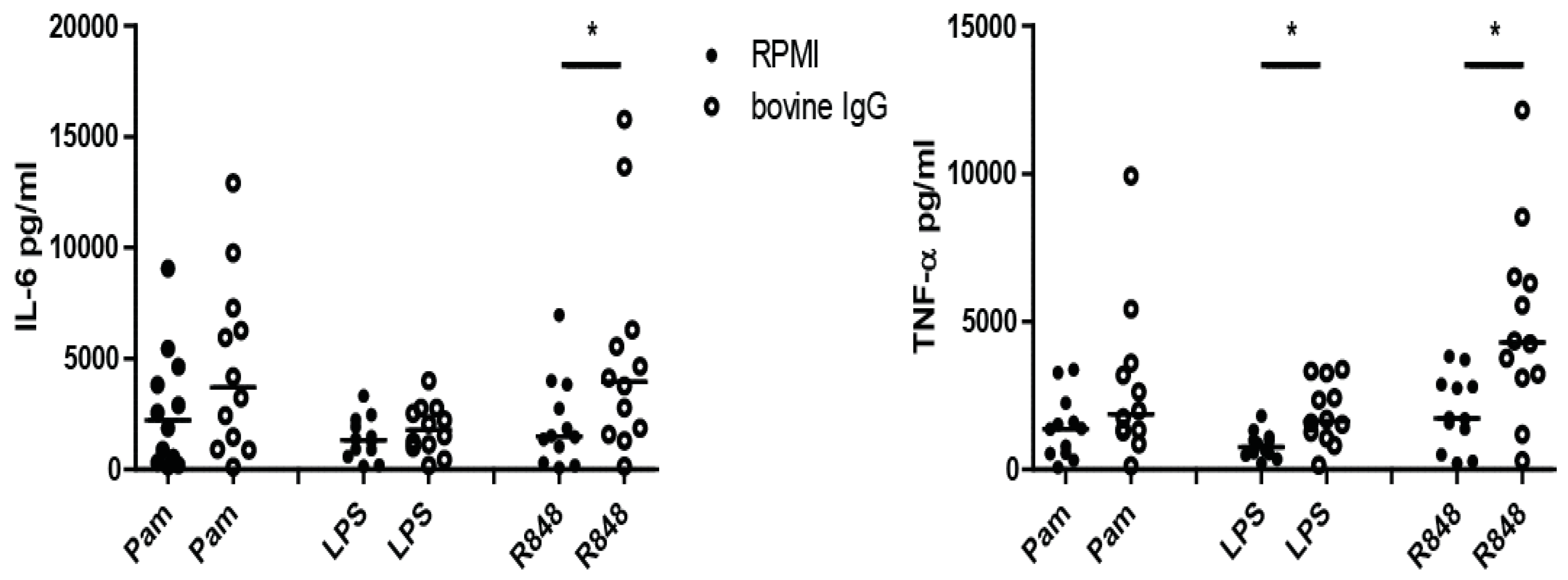
3.2. Induction of Trained Immunity by Raw Milk and Bovine Immunoglobulin G
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Netea, M.G.; Quintin, J.; Van Der Meer, J.W.M. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; van der Meer, J.W.M. Trained Immunity: An Ancient Way of Remembering. Cell Host Microbe 2017, 21, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Durrant, W.E.; Dong, X. Systemic Acquired Resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.-M.; Zhu, S.; Kachroo, P.; Kachroo, A. Signal regulators of systemic acquired resistance. Front. Plant Sci. 2015, 6, 228. [Google Scholar] [CrossRef] [PubMed]
- Reimer-Michalski, E.M.; Conrath, U. Innate immune memory in plants. Semin. Immunol. 2016, 28, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Tate, A.T.; Andolfatto, P.; Demuth, J.P.; Graham, A.L. The within-host dynamics of infection in trans-generationally primed flour beetles. Mol. Ecol. 2017, 26, 3794–3807. [Google Scholar] [CrossRef] [PubMed]
- Green, T.J.; Helbig, K.; Speck, P.; Raftos, D.A. Primed for success: Oyster parents treated with poly(I:C) produce offspring with enhanced protection against Ostreid herpesvirus type I infection. Mol. Immunol. 2016, 78, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J. Specific memory within innate immune systems. Trends Immunol. 2005, 26, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.N.; Dionne, M.S.; Shirasu-Hiza, M.; Schneider, D.S. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog. 2007, 3, e26. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.; Wiegertjes, G.F. Long-lived effects of administering β-glucans: Indications for trained immunity in fish. Dev. Comp. Immunol. 2016, 64, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Benn, C.S.; Netea, M.G.; Selin, L.K.; Aaby, P. A Small Jab—A Big Effect: Nonspecific Immunomodulation by Vaccines. Trends Immunol. 2013, 34, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Roth, A.; Ravn, H.; Napirna, B.M.; Rodrigues, A.; Lisse, I.M.; Stensballe, L.; Diness, B.R.; Lausch, K.R.; Lund, N.; et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: Beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011, 204, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Jacobs, C.; Xavier, R.J.; van der Meer, J.W.M.; van Crevel, R.; Netea, M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 2014, 155, 213–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.-J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection vai functional reprogramming of monocytes. Cell Host Microbe 2012, 16, 123–128. [Google Scholar] [CrossRef]
- Meena, D.K.; Das, P.; Kumar, S.; Mandal, S.C.; Prusty, A.K.; Singh, S.K.; Akhtar, M.S.; Behera, B.K.; Kumar, K.; Pal, A.K.; et al. Beta-glucan: An ideal immunostimulant in aquaculture (a review). Fish Physiol. Biochem. 2013, 39, 431–457. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, S.; Quintin, J.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Netea, M.G.; Riksen, N.P. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Günther, P.; Lauterbach, M.A.R.; Duewell, P.; Biswas, D.; Pelka, K.; Scholz, C.J.; Oosting, M.; Haendler, K.; Baßler, K.; et al. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell 2018, 172, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Loss, G.; Apprich, S.; Waser, M.; Kneifel, W.; Genuneit, J.; Büchele, G.; Weber, J.; Sozanska, B.; Danielewicz, H.; Horak, E.; et al. The protective effect of farm milk consumption on childhood asthma and atopy: The GABRIELA study. J. Allergy Clin. Immunol. 2011, 128, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Loss, G.; Depner, M.; Ulfman, L.H.; van Neerven, R.J.J.; Hose, A.J.; Genuneit, J.; Karvonen, A.M.; Hyvärinen, A.; Kaulek, V.; Roduit, C.; et al. Consumption of unprocessed cow’s milk protects infants from common respiratory infections. J. Allergy Clin. Immunol. 2015, 135, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Waser, M.; Michels, K.B.; Bieli, C.; Flöistrup, H.; Pershagen, G.; Von Mutius, E.; Ege, M.; Riedler, J.; Schram-Bijkerk, D.; Brunekreef, B.; et al. Inverse association of farm milk consumption with asthma and allergy in rural and suburban populations across Europe. Clin. Exp. Allergy 2006, 37, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Van Neerven, R.J.J.; Knol, E.F.; Heck, J.M.L.; Savelkoul, H.F.J. Which factors in raw cow’s milk contribute to protection against allergies? J. Allergy Clin. Immunol. 2012, 130, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindmark-Månsson, H.; Fondén, R.; Pettersson, H.E. Composition of Swedish dairy milk. Int. Dairy J. 2003, 13, 409–425. [Google Scholar] [CrossRef]
- Gaucheron, F. The minerals of milk. Reprod. Nutr. Dev. 2005, 45, 473–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heck, J.M.L.; van Valenberg, H.J.F.; Dijkstra, J.; van Hooijdonk, A.C.M. Seasonal variation in the Dutch bovine raw milk composition. J. Dairy Sci. 2009, 92, 4745–4755. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Nutritional roles of lactoferrin. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Pezo, A.; Cruz, K.; Chea-Woo, E.; Cleary, T.G. Clinical studies of lactoferrin in children. Biochem. Cell Biol. 2012, 90, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Marnila, P.; Gill, H.S. Bovine milk antibodies for health. Br. J. Nutr. 2000, 84 (Suppl. S1), S135–S146. [Google Scholar] [CrossRef]
- Ulfman, L.; Leusen, J.H.W.; Savelkoul, H.F.J.; Warner, J.O.; van Neerven, R.J.J. Effects of bovine immunoglobulins on immune function, allergy and infection. Front. Nutr. 2018, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Ifrim, D.C.; Quintin, J.; Joosten, L.A.B.; Jacobs, C.; Jansen, T.; Jacobs, L.; Gow, N.A.R.; Williams, D.L.; van der Meer, J.W.M.; Netea, M.G. Trained Immunity or Tolerance: Opposing Functional Programs Induced in Human Monocytes after Engagement of Various Pattern Recognition Receptors. Clin. Vaccine Immunol. 2014, 21, 534–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, S.; Quintin, J.; Kerstens, H.H.D.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.-C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Rice, P.J.; Ensley, H.E.; Coogan, P.S.; Kalbfleish, J.H.; Kelley, J.L.; Love, E.J.; Portera, C.A.; Ha, T.; Browder, I.W.; et al. Receptor binding and internalization of a water-soluble (1,3)-beta-d-glucan biologic response modifier in two monocyte/macrophage cell lines. J. Immunol. 1996, 156, 3418–3425. [Google Scholar] [PubMed]
- Teodorowicz, M.; Perdijk, O.; Verhoek, I.; Govers, C.; Savelkoul, H.F.J.; Tang, Y.; Wichers, H.; Broersen, K. Optimized triton X-114 assisted lipopolysaccharide (LPS) removal method reveals the immunomodulatory effect of food proteins. PLoS ONE 2017, 12, e0173778. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.J.; Grahame Hardie, D. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013, 493, 346–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.A.; Rao, N.A.; Aghajanirefah, A.; et al. MTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.W.; Novakovic, B.; ter Horst, R.; Carvalho, A.; Bekkering, S.; Lachmandas, E.; Rodrigues, F.; Silvestre, R.; Cheng, S.C.; Wang, S.Y.; et al. Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell Metab. 2016, 24, 807–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekkering, S.; Arts, R.J.W.; Novakovic, B.; Kourtzelis, I.; van der Heijden, C.D.C.C.; Li, Y.; Popa, C.D.; ter Horst, R.; van Tuijl, J.; Netea-Maier, R.T.; et al. Metabolic Induction of Trained Immunity through the Mevalonate Pathway. Cell 2018, 172, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Leentjens, J.; Quintin, J.; Gerretsen, J.; Kox, M.; Pickkers, P.; Netea, M.G. The effects of orally administered beta-glucan on innate immune responses in humans, a randomized open-label intervention pilot-study. PLoS ONE 2014, 9, e108794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Chen, X.; Yu, J.; Zen, K.; Zhang, C.Y.; Li, L. Immune modulatory function of abundant immune-related microRNAs in microvesicles from bovine colostrum. Protein Cell 2013, 4, 197–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lönnerdal, B.; Jiang, R.; Du, X. Bovine Lactoferrin Can Be Taken Up by the Human Intestinal Lactoferrin Receptor and Exert Bioactivities. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Aoki, R.; Uchida, R.; Tajima, A.; Aoki-Yoshida, A. Role of CXC chemokine receptor type 4 as a lactoferrin receptor. Biochem. Cell Biol. 2017, 95, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Baveye, S.; Elass, E.; Fernig, D.G.; Blanquart, C.; Mazurier, J.; Legrand, D. Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect. Immun. 2000, 68, 6519–6525. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Hasegawa, K.; Shindo, K.I.; Furusawa, T.; Fujino, T.; Kikugawa, K.; Nakano, H.; Takeuchi, O.; Akira, S.; Akiyama, T.; et al. Human lactoferrin activates NF-κB through the Toll-like receptor 4 pathway while it interferes with the lipopolysaccharide-stimulated TLR4 signaling. FEBS J. 2010, 277, 2051–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verschoor, C.P.; Kohli, V. Cryopreserved whole blood for the quantification of monocyte, T-cell and NK-cell subsets, and monocyte receptor expression by multi-color flow cytometry: A methodological study based on participants from the Canadian longitudinal study on aging. Cytometry Part A 2018, 93, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Coulson, S.; Beck, S.L.; Gramotnev, H.; Du, S.; Lewis, S. The clinical efficacy of a bovine lactoferrin/whey protein Ig-rich fraction (Lf/IgF) for the common cold: A double blind randomized study. Complement. Ther. Med. 2013, 21, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Triantafilou, K.; Vakakis, E.; Richer, E.A.J.; Evans, G.L.; Villiers, J.P.; Triantafilou, M. Human rhinovirus recognition in non-immune cells is mediated by Toll-like receptors and MDA-5, which trigger a synergetic pro-inflammatory immune response. Virulence 2011, 2, 22–29. [Google Scholar] [CrossRef] [PubMed]
- den Hartog, G.; Jacobino, S.; Bont, L.; Cox, L.; Ulfman, L.H.; Leusen, J.H.W.; van Neerven, R.J.J. Specificity and Effector Functions of Human RSV-Specific IgG from Bovine Milk. PLoS ONE 2014, 9, e112047. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H.; Paludan, S.R.; Kilian, M.; Ostergaard, L. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J. Leuk. Biol. 2006, 80, 267–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schijf, M.A.; Lukens, M.V.; Kruijsen, D.; Van Uden, N.O.P.; Garssen, J.; Coenjaerts, F.E.J.; Van’t Land, B.; Van Bleek, G.M. Respiratory syncytial virus induced type I IFN production by pDC is regulated by RSV-infected airway epithelial cells, RSV-exposed monocytes and virus specific antibodies. PLoS ONE 2013, 8, e81695. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.; Winston, B.; Chan, E.; Riches, D.; Gerwins, P.; Johnson, G.; PM, H. Fc gamma receptor cross-linking activates p42, p38 and JNK/SAPK mitogen-activated protein kinases in murine macrophages: Role for p42MAPK in Fc gamma receptor-stimulated TNF-alpha synthesis. J. Immunol. 1977, 158, 3433–3438. [Google Scholar] [CrossRef]
- Gais, P.; Tiedje, C.; Altmayr, F.; Gaestel, M.; Weighardt, H.; Holzmann, B. TRIF signaling stimulates translation of TNF-alpha mRNA via prolonged activation of MK2. J. Immunol. 2010, 184, 5842–5848. [Google Scholar] [CrossRef] [PubMed]
- Tartey, S.; Takeuchi, O. Pathogen recognition and Toll-like receptor targeted therapeutics in innate immune cells. Int. Rev. Immunol. 2017, 36, 57–73. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Splunter, M.; Van Osch, T.L.J.; Brugman, S.; Savelkoul, H.F.J.; Joosten, L.A.B.; Netea, M.G.; Van Neerven, R.J.J. Induction of Trained Innate Immunity in Human Monocytes by Bovine Milk and Milk-Derived Immunoglobulin G. Nutrients 2018, 10, 1378. https://doi.org/10.3390/nu10101378
Van Splunter M, Van Osch TLJ, Brugman S, Savelkoul HFJ, Joosten LAB, Netea MG, Van Neerven RJJ. Induction of Trained Innate Immunity in Human Monocytes by Bovine Milk and Milk-Derived Immunoglobulin G. Nutrients. 2018; 10(10):1378. https://doi.org/10.3390/nu10101378
Chicago/Turabian StyleVan Splunter, Marloes, Thijs L. J. Van Osch, Sylvia Brugman, Huub F. J. Savelkoul, Leo A. B. Joosten, Mihai G. Netea, and R. J. Joost Van Neerven. 2018. "Induction of Trained Innate Immunity in Human Monocytes by Bovine Milk and Milk-Derived Immunoglobulin G" Nutrients 10, no. 10: 1378. https://doi.org/10.3390/nu10101378




