Regulation of Adipogenesis and Thermogenesis through Mouse Olfactory Receptor 23 Stimulated by α-Cedrene in 3T3-L1 Cells
Abstract
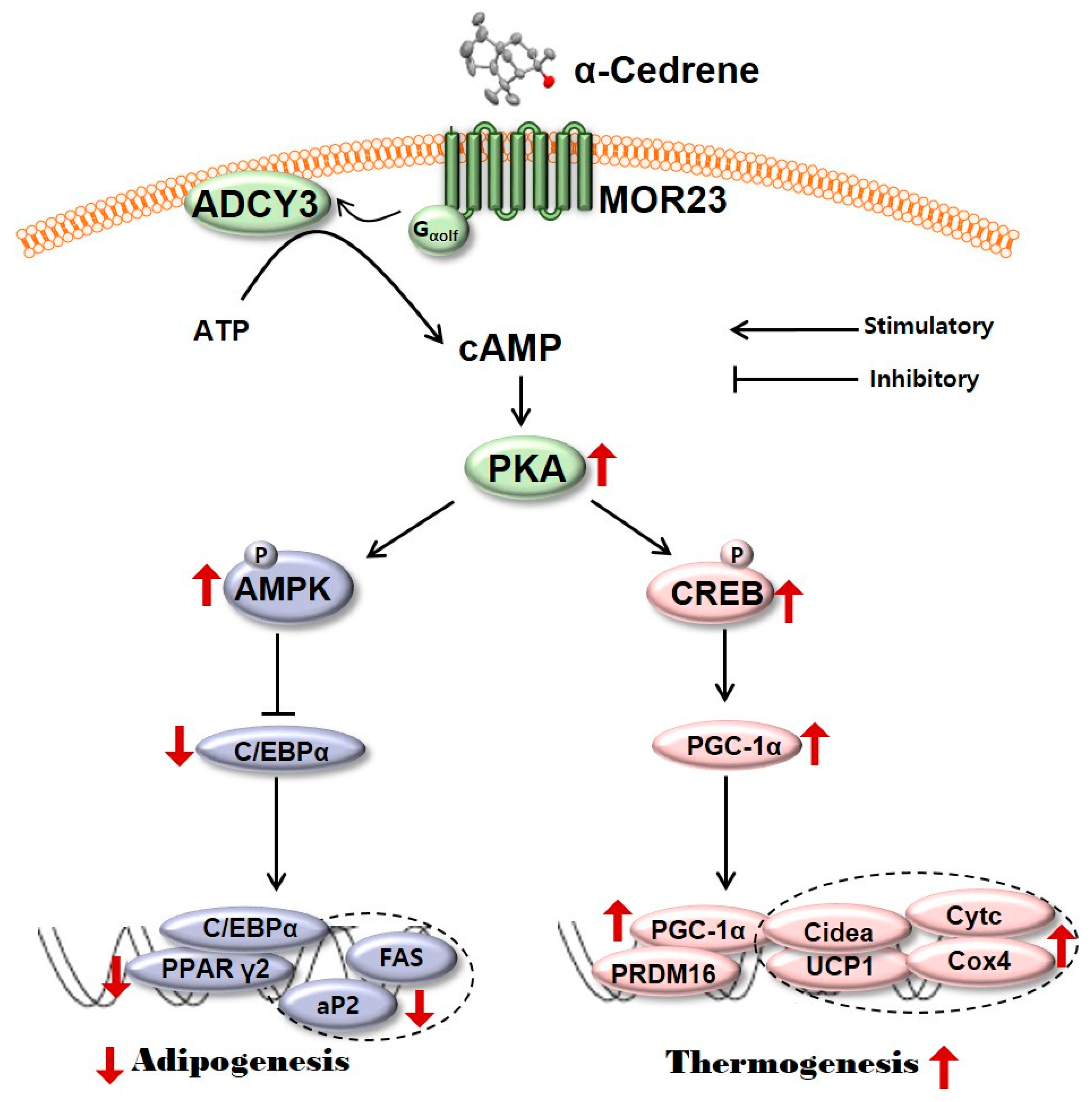
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. cAMP Assay
2.4. siRNA Knockdown
2.5. Oil Red O Staining
2.6. Measurement of the Oxygen Consumption Rate
2.7. RNA Extraction and PCR
2.8. Protein Extraction and Western Blotting Assay
2.9. Statistical Analysis
3. Results
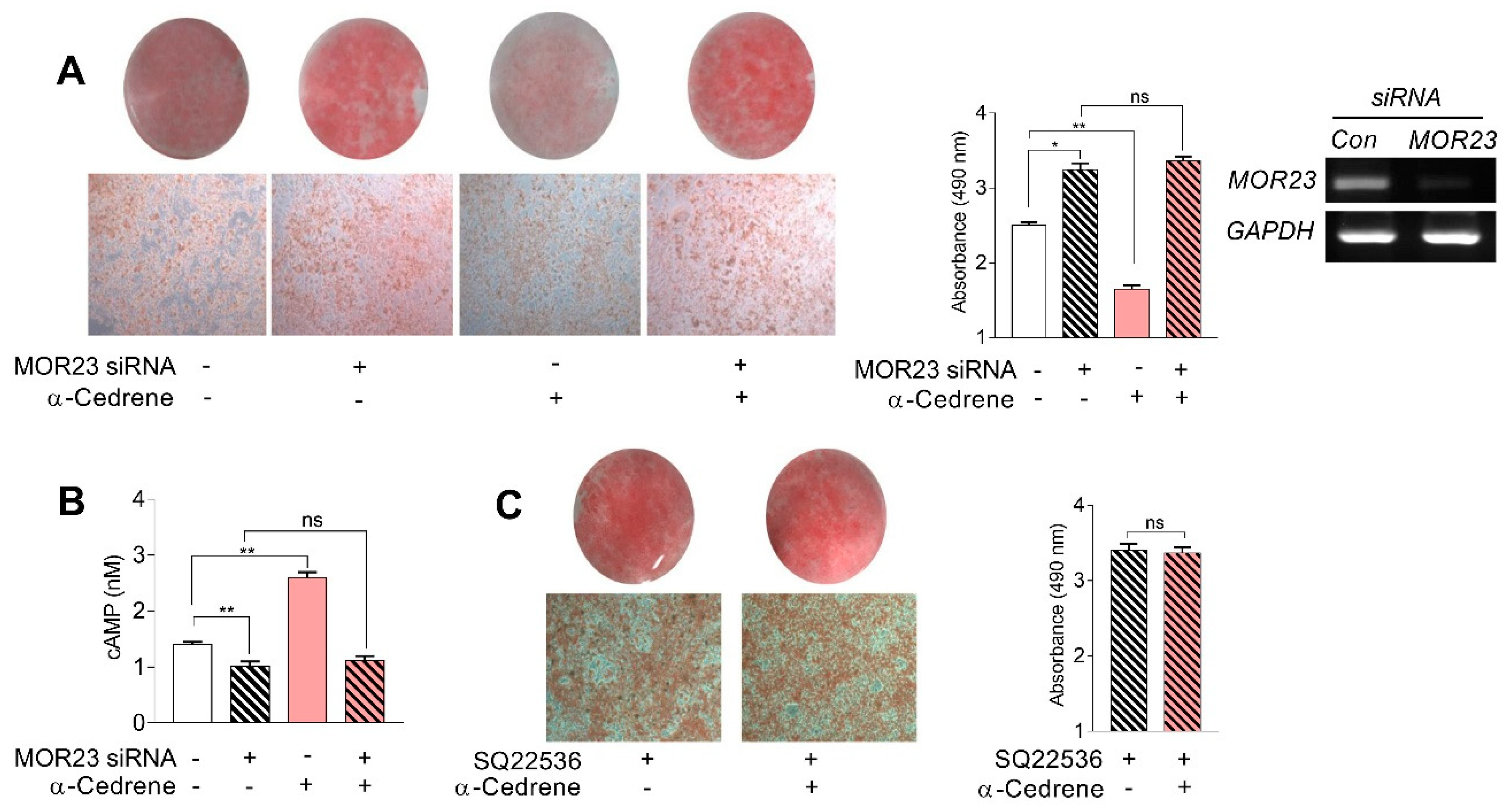
3.1. A Reduction in MOR23 Expression by Specific siRNA Increases Intracellular Lipid Accumulation
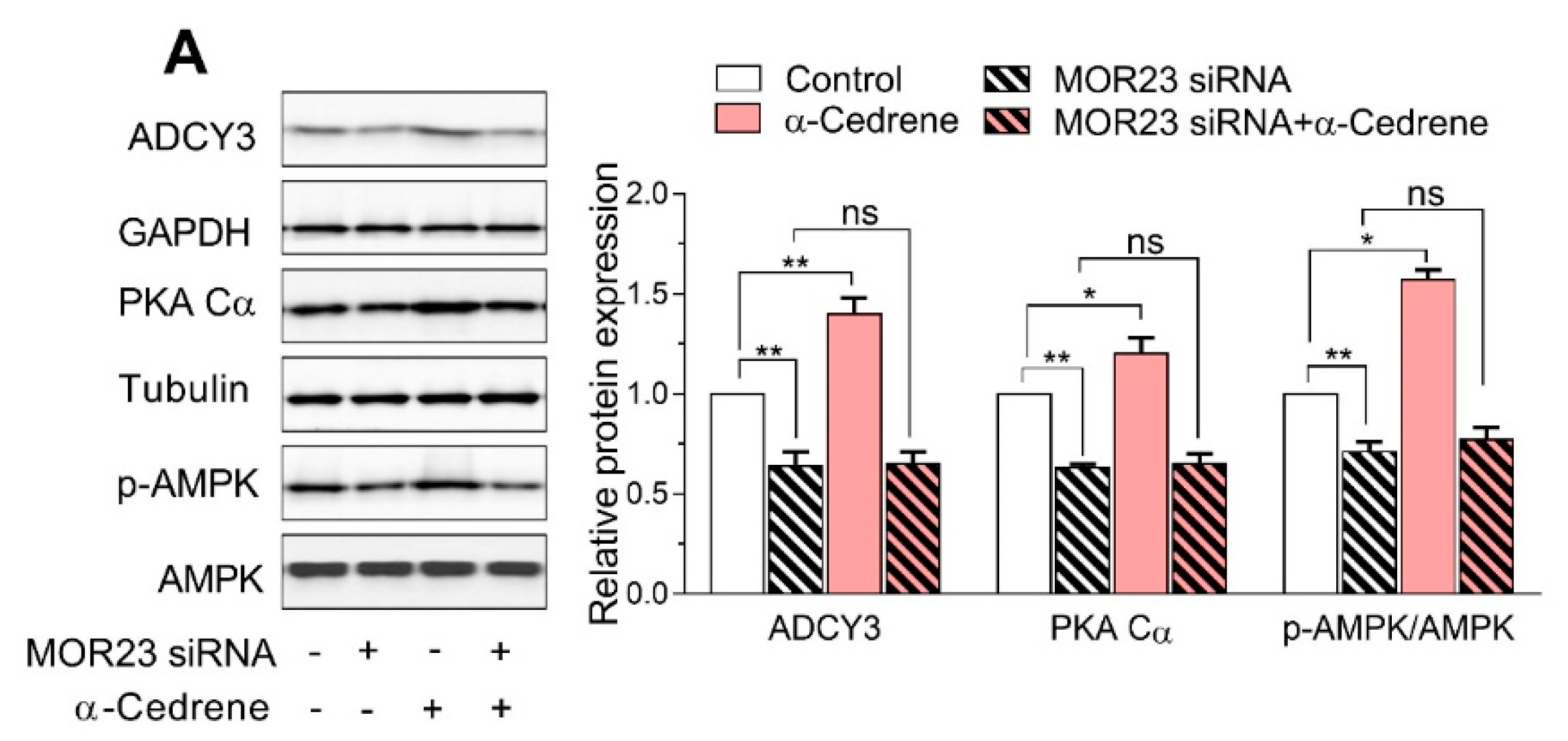
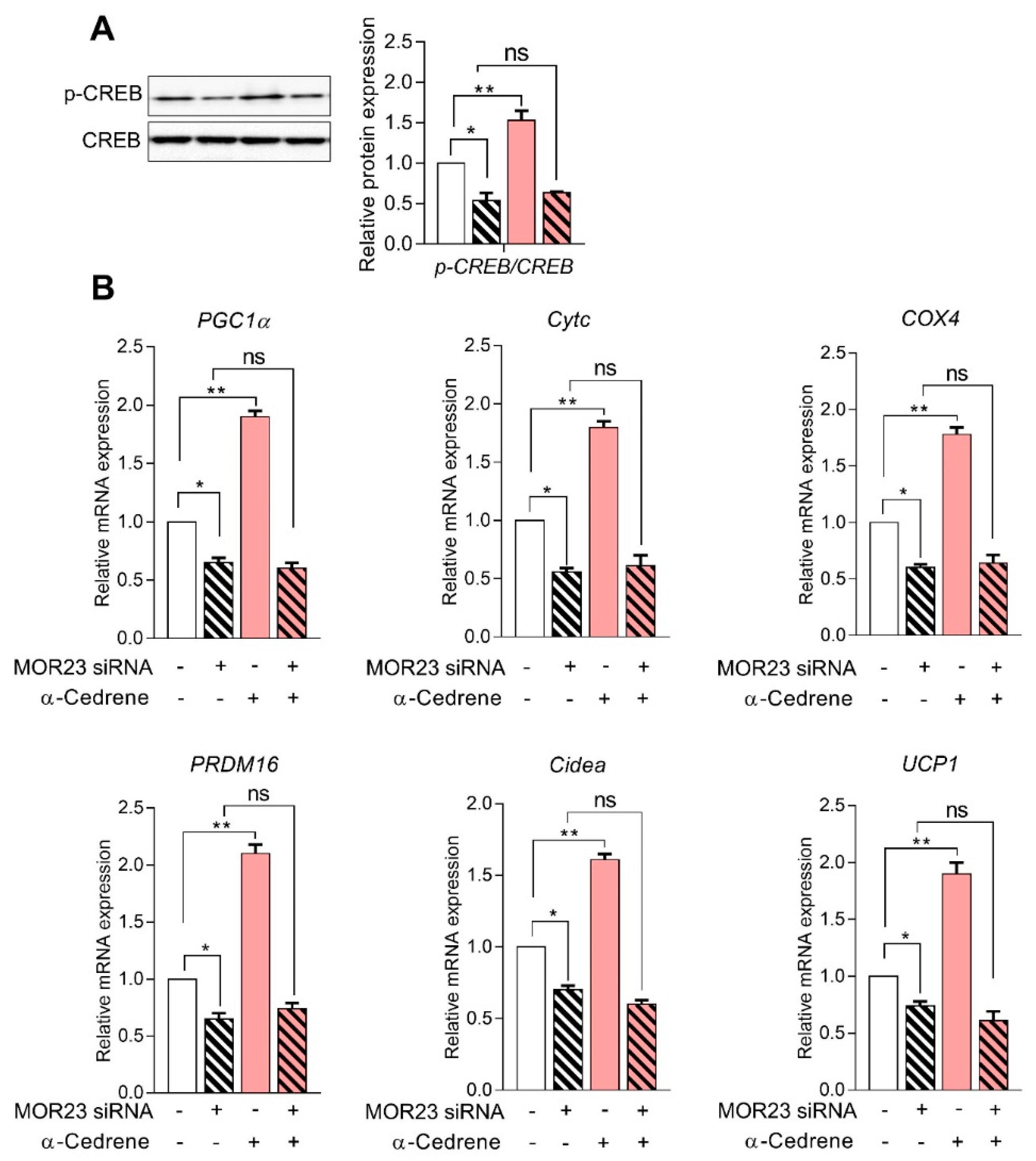
3.2. A Reduction in MOR23 Expression Impaired the cAMP Signaling Pathway and Upregulated the Expression of Adipogenic Genes
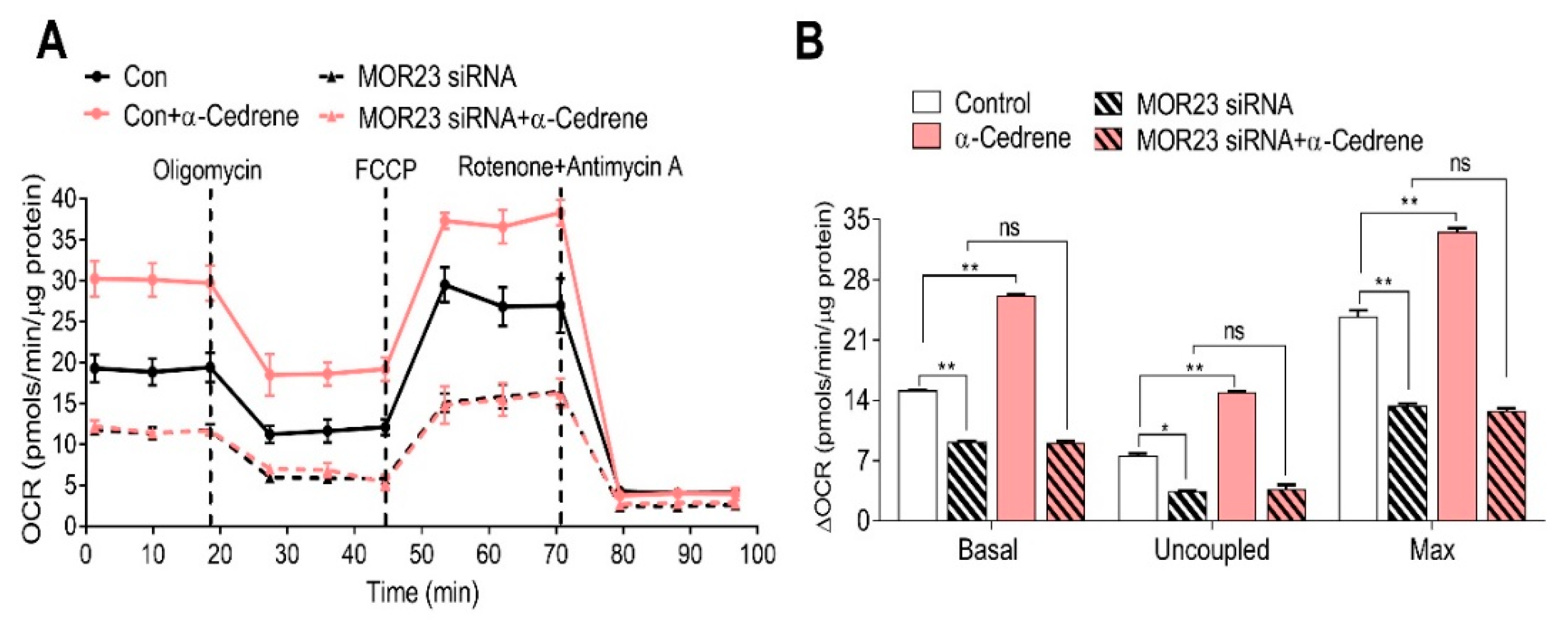
3.3. MOR23 Depletion Decreases Thermogenesis 3T3-L1 cells
3.4. MOR23 Depletion Decreases the Expression of Thermogenic and Mitochondrial Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Blad, C.C.; Tang, C.; Offermanns, S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat. Rev. Drug Discov. 2012, 11, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Buck, L.; Axel, R. A novel multigene family may encode odorant receptors—A molecular-basis for odor recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef]
- Bjarnadottir, T.K.; Gloriam, D.E.; Hellstrand, S.H.; Kristiansson, H.; Fredriksson, R.; Schioth, H.B. Comprehensive repertoire and phylogenetic analysis of the g protein-coupled receptors in human and mouse. Genomics 2006, 88, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Koo, J. Olfactory receptors in non-chemosensory tissues. BMB Rep. 2012, 45, 612–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.X.; Rey, F.; Wang, T.; et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blache, P.; Gros, L.; Salazar, G.; Bataille, D. Cloning and tissue distribution of a new rat olfactory receptor-like (ol2). Biochem. Bioph. Res. Commun. 1998, 242, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Ryu, S.E.; Min, Y.; de March, C.A.; Bushdid, C.; Golebiowski, J.; Moon, C.; Park, T. Olfactory receptor 10j5 responding to alpha-cedrene regulates hepatic steatosis via the camp-pka pathway. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Voland, P.; Kunz, L.; Prinz, C.; Gratzl, M. Enterochromaffin cells of the human gut: Sensors for spices and odorants. Gastroenterology 2007, 132, 1890–1901. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.L.; Stackhouse, B.G.; Florence, K.; Zhang, W.; Shanmugam, N.; Sesterhenn, I.A.; Zou, Z.Q.; Srikantan, V.; Augustus, M.; Roschke, V.; et al. Psgr, a novel prostate-specific gene with homology to a g protein-coupled receptor, is overexpressed in prostate cancer. Cancer Res. 2000, 60, 6568–6572. [Google Scholar] [PubMed]
- Fukuda, N.; Yomogida, K.; Okabe, M.; Touhara, K. Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J. Cell Sci. 2004, 117, 5835–5845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brock, A.; Krause, S.; Li, H.; Kowalski, M.; Goldberg, M.S.; Collins, J.J.; Ingber, D.E. Silencing hoxa1 by intraductal injection of sirna lipidoid nanoparticles prevents mammary tumor progression in mice. Sci. Transl. Med. 2014, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ergun, A.; Lawrence, C.A.; Kohanski, M.A.; Brennan, T.A.; Collins, J.J. A network biology approach to prostate cancer. Mol. Syst. Biol. 2007, 3. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.M.; Gardner, T.S. The mode-of-action by network identification (mni) algorithm: A network biology approach for molecular target identification. Nat. Protoc. 2006, 1, 2551–2554. [Google Scholar] [CrossRef] [PubMed]
- di Bernardo, D.; Thompson, M.J.; Gardner, T.S.; Chobot, S.E.; Eastwood, E.L.; Wojtovich, A.P.; Elliott, S.J.; Schaus, S.E.; Collins, J.J. Chemogenomic profiling on a genomewide scale using reverse-engineered gene networks. Nat. Biotechnol. 2005, 23, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Hur, C.G.; Park, T. Induction of olfaction and cancer-related genes in mice fed a high-fat diet as assessed through the mode-of-action by network identification analysis. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Shen, Y.; Lee, H.W.; Yu, R.; Park, T. Adenylyl cyclase 3 haploinsufficiency confers susceptibility to diet-induced obesity and insulin resistance in mice. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Kim, M.; Park, T. Alpha-cedrene, a newly identified ligand of mor23, increases skeletal muscle mass and strength. Mol. Nutr. Food Res. 2018, e1800173. [Google Scholar] [CrossRef] [PubMed]
- Massberg, D.; Hatt, H. Human olfactory receptors: Novel cellular functions outside of the nose. Physiol. Rev. 2018, 98, 1739–1763. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, J.; Golozoubova, V.; Matthias, A.; Asadi, A.; Jacobsson, A.; Cannon, B. Ucp1: The only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim. Biophys. Acta 2001, 1504, 82–106. [Google Scholar] [CrossRef]
- Jones, D.T.; Reed, R.R. Golf: An olfactory neuron specific-g protein involved in odorant signal transduction. Science 1989, 244, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Lowe, G.; Nakamura, T.; Gold, G.H. Adenylate-cyclase mediates olfactory transduction for a wide variety of odorants. Proc. Natl. Acad. Sci. USA 1989, 86, 5641–5645. [Google Scholar] [CrossRef] [PubMed]
- Spehr, M.; Hatt, H. Hor17-4 as a potential therapeutic target. Drug News Perspect. 2004, 17, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Busse, D.; Kudella, P.; Gruning, N.M.; Gisselmann, G.; Stander, S.; Luger, T.; Jacobsen, F.; Steinstrasser, L.; Paus, R.; Gkogkolou, P.; et al. A synthetic sandalwood odorant induces wound-healing processes in human keratinocytes via the olfactory receptor or2at4. J. Investig. Dermatol. 2014, 134, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.A.; Kafadar, K.A.; Pavlath, G.K. Mor23 promotes muscle regeneration and regulates cell adhesion and migration. Dev. Cell 2009, 17, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.; Kristiansen, K. The importance of dietary modulation of camp and insulin signaling in adipose tissue and the development of obesity. Ann. N. Y. Acad. Sci. 2010, 1190, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rogne, M.; Tasken, K. Compartmentalization of camp signaling in adipogenesis, lipogenesis, and lipolysis. Horm. Metab. Res. 2014, 46, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Carmen, G.Y.; Victor, S.M. Signalling mechanisms regulating lipolysis. Cell Signal. 2006, 18, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, D.; Zhou, Y.; Zhou, B.; Yang, Y.; Chen, H.; Song, J. Protein kinase a suppresses the differentiation of 3t3-l1 preadipocytes. Cell Res. 2008, 18, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.A.; Perkins, J.P.; Krebs, E.G. An adenosine 3′,5′-monophosphate-dependant protein kinase from rabbit skeletal muscle. J. Biol. Chem. 1968, 243, 3763–3765. [Google Scholar] [PubMed]
- Caretta, A.; Mucignat-Caretta, C. Protein kinase a in cancer. Cancers 2011, 3, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Mu, J.; Birnbaum, M.J. Role of amp-activated protein kinase in cyclic amp-dependent lipolysis in 3t3-l1 adipocytes. J. Biol. Chem. 2003, 278, 43074–43080. [Google Scholar] [CrossRef] [PubMed]
- McGarry, J.D.; Brown, N.F. The mitochondrial carnitine palmitoyltransferase system—From concept to molecular analysis. Eur. J. Biochem. 1997, 244, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. Ppar gamma: A nuclear regulator of metabolism, differentiation, and cell growth. J. Biol. Chem. 2001, 276, 37731–37734. [Google Scholar] [CrossRef] [PubMed]
- Catania, C.; Binder, E.; Cota, D. Mtorc1 signaling in energy balance and metabolic disease. Int. J. Obesity 2011, 35, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Zhu, T.Q.; Guan, K.L. Tsc2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Habinowski, S.A.; Witters, L.A. The effects of aicar on adipocyte differentiation of 3t3-l1 cells. Biochem. Biophys. Res. Commun. 2001, 286, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.H.; Daniel, K.W.; Robidoux, J.; Puigserver, P.; Medvedev, A.V.; Bai, X.; Floering, L.M.; Spiegelman, B.M.; Collins, S. P38 mitogen-activated protein kinase is the central regulator of cyclic amp-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell. Biol. 2004, 24, 3057–3067. [Google Scholar] [CrossRef] [PubMed]
- Peterlin, Z.; Firestein, S.; Rogers, M.E. The state of the art of odorant receptor deorphanization: A report from the orphanage. J. Gen. Physiol. 2014, 143, 527–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, H.; Chi, Q.Y.; Zhuang, H.Y.; Matsunami, H.; Mainland, J.D. Odor coding by a mammalian receptor repertoire. Sci. Signal. 2009, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yoon, Y.C.; Lee, A.S.; Kang, N.; Koo, J.; Rhyu, M.R.; Park, J.H. Expression of human olfactory receptor 10j5 in heart aorta, coronary artery, and endothelial cells and its functional role in angiogenesis. Biochem. Bioph. Res. Commun. 2015, 460, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Ryall, J.G.; Schertzer, J.D.; Alabakis, T.M.; Gehrig, S.M.; Plant, D.R.; Lynch, G.S. Intramuscular beta(2)-agonist administration enhances early regeneration and functional repair in rat skeletal muscle after myotoxic injury. J. Appl. Physiol. 2008, 105, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Harcourt, L.J.; Schertzer, J.D.; Ryall, J.G.; Lynch, G.S. Low dose formoterol administration improves muscle function in dystrophic mdx mice without increasing fatigue. Neuromuscul. Disord. 2007, 17, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Zeman, R.J.; Peng, H.; Danon, M.J.; Etlinger, J.D. Clenbuterol reduces degeneration of exercised or aged dystrophic (mdx) muscle. Muscle Nerve 2000, 23, 521–528. [Google Scholar] [CrossRef]
- Hall, J.E.; Kaczor, J.J.; Hettinga, B.P.; Isfort, R.J.; Tarnopolsky, M.A. Effects of a crf2r agonist and exercise on mdx and wildtype skeletal muscle. Muscle Nerve 2007, 36, 336–341. [Google Scholar] [CrossRef] [PubMed]

| Type | Gene Description | Sequences (5′→3′) |
|---|---|---|
| Mouse | Mouse olfactory receptor 23 (MOR23) | F: CAAGGCACACATTCCCTTGC |
| R: TTCCCATATCCTTGGCAGGC | ||
| Peroxisome proliferator-activated receptor γ2 (PPARγ2) | F: TTCGGAATCAGCTCTGTGGA | |
| R: CCATTGGGTCAGCTCTTGTG | ||
| CCAAT/enhancer binding-protein α (C/EBPα) | F: TCAGCTTACAACAGGCCAGG | |
| R: ACACAAGGCTAATGGTCCCC | ||
| Adipocyte fatty acid binding protein (aP2) | F: CATGCGACAAAGGCAGAAAT | |
| R: GTTACAAGGCAAGGAAGGGC | ||
| Fatty acid synthase (FAS) | F: CAGCCAGGAGAATCGCAGTA | |
| R: CTGCGATGAAGAGCATGGTT | ||
| Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) | F: TAAATCTGCGGGATGATGGA | |
| R: GTTTCGTTCGACCTGCGTAA | ||
| Uncoupling protein 1 (UCP1) | F: GGTTTGCACCACACTCCTG | |
| R: ACATGGACATCGCACAGCTT | ||
| PR domain containing 16 (PRDM16) | F: GGACCTTTTTGACAGCAGCA | |
| R: GGGGGCAAAGCATTTAACTC | ||
| Cytochrome c (Cytc) | F: ACACTGTGGAAAAGGGAGGC | |
| R: GCACTGGTTAACCCAAGCAA | ||
| Cytochrome c oxidase subunit 4 (COX4) | F: GGAAAACGTCTGCCGGAAA | |
| R: AAGCATCGCGGGAATCAGG | ||
| Cell death activator CIDE-A (Cidea) | F: GGAATCTGCTGAGGTTTATG | |
| R: ATCCCACAGCCTATAACAGA | ||
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | F: GTGATGGCATGGACTGTGGT | |
| R: GGAGCCAAAAGGGTCATCAT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, T.; Park, J.; Moon, C.; Park, T. Regulation of Adipogenesis and Thermogenesis through Mouse Olfactory Receptor 23 Stimulated by α-Cedrene in 3T3-L1 Cells. Nutrients 2018, 10, 1781. https://doi.org/10.3390/nu10111781
Tong T, Park J, Moon C, Park T. Regulation of Adipogenesis and Thermogenesis through Mouse Olfactory Receptor 23 Stimulated by α-Cedrene in 3T3-L1 Cells. Nutrients. 2018; 10(11):1781. https://doi.org/10.3390/nu10111781
Chicago/Turabian StyleTong, Tao, Jinju Park, Cheil Moon, and Taesun Park. 2018. "Regulation of Adipogenesis and Thermogenesis through Mouse Olfactory Receptor 23 Stimulated by α-Cedrene in 3T3-L1 Cells" Nutrients 10, no. 11: 1781. https://doi.org/10.3390/nu10111781





