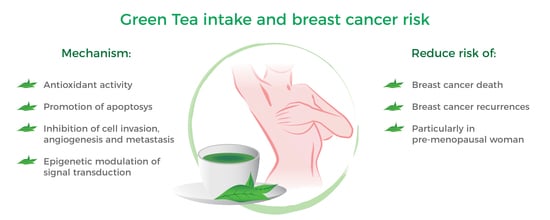
Green Tea Consumption and Risk of Breast Cancer and Recurrence—A Systematic Review and Meta-Analysis of Observational Studies
Abstract
:1. Introduction
2. Material and Methods
2.1. Inclusion Criteria
2.2. Data Extraction
2.3. Quality Evaluation
2.4. Statistical Analysis
2.5. Sensitivity Analysis
3. Results
3.1. Literature Search
3.2. Characteristics of Included Studies
3.3. Sensitivity Analysis
Study Design
3.4. Outcome: BC Diagnosis and BC Recurrences
3.5. Quality Score
3.6. Amount of Green Tea Intake
3.7. Menopausal Status
4. Discussion
Strengths and Limitations of the Study
5. Conclusion
Supplementary Materials
Author contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Namita, P.; Mukesh, R.; Vijay, K.J. Camellia Sinensis (Green Tea): A Review. Glob. J. Pharmacol. 2012, 6, 52–59. [Google Scholar]
- Saeed, M.; Naveed, M.; Arif, M.; Kakar, M.U.; Manzoor, R.; Abd El-Hack, M.E.; Alagawany, M.; Tiwari, R.; Khandia, R.; Munjal, A.; et al. Green tea (Camellia sinensis) and l-theanine: Medicinal values and beneficial applications in humans-A comprehensive review. Biomed. Pharmacother. 2017, 95, 1260–1275. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.L.; Yuan, J.M.; Koh, W.P.; Yu, M.C. Green tea, black tea and breast cancer risk: A meta-analysis of epidemiological studies. Carcinogenesis 2006, 27, 1310–1315. [Google Scholar] [CrossRef]
- Bhattacharyyaa, N.; Sethb, S.; Tudub, B.; Tamulyc, P.; Janaa, A.; Ghosha, D.; Bandyopadhyayb, R.; Bhuyand, M. Monitoring of black tea fermentation process using electronic nose. J. Food Eng. 2007, 80, 1146–1156. [Google Scholar] [CrossRef]
- Juneja, L.R.; Kapoor, M.P.; Okubo, T.; Rao, T. Green Tea Polyphenols: Nutraceuticals of Modern Life; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Rätsch, C. The Encyclopedia of Psychoactive Plants: Ethnopharmacology and Its Applications; Park Street Press: Rochester, VT, USA, 2004. [Google Scholar]
- Weisburger, J.H.; Chung, F.L. Mechanisms of chronic disease causation by nutritional factors and tobacco products and their prevention by tea polyphenols. Food Chem. Toxicol. 2002, 40, 1145–1154. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). World Tea Production and Trade. Current and Future Development; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2015. [Google Scholar]
- Sumpio, B.E.; Cordova, A.C.; Berke-Schlessel, D.W.; Qin, F.; Chen, Q.H. Green tea, the “Asian paradox,” and cardiovascular disease. J. Am. Coll. Surg. 2006, 202, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, C.J.; Farnworth, E.R. A review of latest research findings on the health promotion properties of tea. J. Nutr. Biochem. 2001, 12, 404–421. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Gimenez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Yuan, J.M.; Sun, C.; Butler, L.M. Tea and cancer prevention: Epidemiological studies. Pharmacol. Res. 2011, 64, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.S.; Wang, H.; Li, G.X.; Yang, Z.; Guan, F.; Jin, H. Cancer prevention by tea: Evidence from laboratory studies. Pharmacol. Res. 2011, 64, 113–122. [Google Scholar] [CrossRef]
- Fon Sing, M.; Yang, W.S.; Gao, S.; Gao, J.; Xiang, Y.B. Epidemiological studies of the association between tea drinking and primary liver cancer: A meta-analysis. Eur. J. Cancer Prev. 2011, 20, 157–165. [Google Scholar] [CrossRef]
- Mann, C.D.; Neal, C.P.; Garcea, G.; Manson, M.M.; Dennison, A.R.; Berry, D.P. Phytochemicals as potential chemopreventive and chemotherapeutic agents in hepatocarcinogenesis. Eur. J. Cancer Prev. 2009, 18, 13–25. [Google Scholar] [CrossRef]
- Yiannakopoulou, E.C. Interaction of green tea catechins with breast cancer endocrine treatment: A systematic review. Pharmacology 2014, 94, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.A.; Khan, T.M.; Lee, L.H. The Effect of Green Tea Consumption on Prostate Cancer Risk and Progression: A Systematic Review. Nutr. Cancer 2017, 69, 353–364. [Google Scholar] [CrossRef]
- Fujiki, H.; Watanabe, T.; Sueoka, E.; Rawangkan, A.; Suganuma, M. Cancer Prevention with Green Tea and Its Principal Constituent, EGCG: From Early Investigations to Current Focus on Human Cancer Stem Cells. Mol. Cells 2018, 41, 73–82. [Google Scholar] [CrossRef]
- Sur, S.; Panda, C.K. Molecular aspects of cancer chemopreventive and therapeutic efficacies of tea and tea polyphenols. Nutrition 2017, 43–44, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Nucci, D.; Vannini, S.; Villarini, M.; Moretti, M. In vitro Biological Effects of Sulforaphane (SFN), Epigallocatechin-3-gallate (EGCG), and Curcumin on Breast Cancer Cells: A Systematic Review of the Literature. Nutr. Cancer 2017, 69, 969–978. [Google Scholar] [CrossRef]
- Gianfredi, V.; Vannini, S.; Moretti, M.; Villarini, M.; Bragazzi, N.L.; Izzotti, A.; Nucci, D. Sulforaphane and Epigallocatechin Gallate Restore Estrogen Receptor Expression by Modulating Epigenetic Events in the Breast Cancer Cell Line MDA-MB-231: A Systematic Review and Meta-Analysis. J. Nutrigenet. Nutrigenom. 2017, 10, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef]
- Rashidi, B.; Malekzadeh, M.; Goodarzi, M.; Masoudifar, A.; Mirzaei, H. Green tea and its anti-angiogenesis effects. Biomed. Pharmacother. 2017, 89, 949–956. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Winters, S.; Martin, C.; Murphy, D.; Shokar, N.K. Breast Cancer Epidemiology, Prevention, and Screening. Prog. Mo. Biol. Transl. Sci. 2017, 151, 1–32. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [Green Version]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Brown, P.; Brunnhuber, K.; Chalkidou, K.; Chalmers, I.; Clarke, M.; Fenton, M.; Forbes, C.; Glanville, J.; Hicks, N.J.; Moody, J.; et al. How to formulate research recommendations. BMJ 2006, 333, 804–806. [Google Scholar] [CrossRef] [Green Version]
- Wong, W.C.; Cheung, C.S.; Hart, G.J. Development of a quality assessment tool for systematic reviews of observational studies (QATSO) of HIV prevalence in men having sex with men and associated risk behaviours. Themes Epidemiol. 2008, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [Green Version]
- Buitrago-Lopez, A.; Sanderson, J.; Johnson, L.; Warnakula, S.; Wood, A.; Di Angelantonio, E.; Franco, O.H. Chocolate consumption and cardiometabolic disorders: Systematic review and meta-analysis. BMJ 2011, 343, d4488. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Arthur, R.; Kirsh, V.A.; Rohan, T.E. Associations of coffee, tea and caffeine intake with risk of breast, endometrial and ovarian cancer among Canadian women. Cancer Epidemiol. 2018, 56, 75–82. [Google Scholar] [CrossRef]
- Ronco, A.L.; Stefani, E.D.; Mendoza, B.; Vazquez, A.; Abbona, E.; Sanchez, G.; Rosa, A.D. Mate and Tea Intake, Dietary Antioxidants and Risk of Breast Cancer: A Case-Control Study. Asian Pac. J. Cancer Prev. 2016, 17, 2923–2933. [Google Scholar] [CrossRef]
- Bhoo-Pathy, N.; Peeters, P.H.; Uiterwaal, C.S.; Bueno-de-Mesquita, H.B.; Bulgiba, A.M.; Bech, B.H.; Overvad, K.; Tjonneland, A.; Olsen, A.; Clavel-Chapelon, F.; et al. Coffee and tea consumption and risk of pre- and postmenopausal breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Breast Cancer Res. 2015, 17, 15. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.K.; Sandin, S.; Strom, P.; Lof, M.; Adami, H.O.; Weiderpass, E. Prospective study of breast cancer in relation to coffee, tea and caffeine in Sweden. Int. J. Cancer 2015, 137, 1979–1989. [Google Scholar] [CrossRef] [Green Version]
- Touvier, M.; Druesne-Pecollo, N.; Kesse-Guyot, E.; Andreeva, V.A.; Fezeu, L.; Galan, P.; Hercberg, S.; Latino-Martel, P. Dual association between polyphenol intake and breast cancer risk according to alcohol consumption level: A prospective cohort study. Breast Cancer Res. Treat. 2013, 137, 225–236. [Google Scholar] [CrossRef]
- Fagherazzi, G.; Touillaud, M.S.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Romieu, I. No association between coffee, tea or caffeine consumption and breast cancer risk in a prospective cohort study. Public Heal. Nutr. 2011, 14, 1315–1320. [Google Scholar] [CrossRef] [Green Version]
- Boggs, D.A.; Palmer, J.R.; Stampfer, M.J.; Spiegelman, D.; Adams-Campbell, L.L.; Rosenberg, L. Tea and coffee intake in relation to risk of breast cancer in the Black Women’s Health Study. Cancer Causes Control 2010, 21, 1941–1948. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Gao, Y.T.; Chow, W.H.; Shu, X.O.; Li, H.; Yang, G.; Cai, Q.; Rothman, N.; Cai, H.; Shrubsole, M.J.; et al. Urinary polyphenols and breast cancer risk: Results from the Shanghai Women’s Health Study. Breast Cancer Res. Treat. 2010, 120, 693–702. [Google Scholar] [CrossRef]
- Luo, J.F.; Gao, Y.T.; Chow, W.H.; Shu, X.O.; Li, H.L.; Yang, G.; Cai, Q.Y.; Li, G.L.; Rothman, N.; Cai, H.; et al. Urinary polyphenols, glutathione S-transferases copy number variation, and breast cancer risk: Results from the Shanghai women’s health study. Mol. Carcinog. 2012, 51, 379–388. [Google Scholar] [CrossRef]
- Pathy, N.B.; Peeters, P.; van Gils, C.; Beulens, J.W.J.; van der Graaf, Y.; Bueno-de-Mesquita, B.; Bulgiba, A.; Uiterwaal, C.S. Cofee and tea intake and risk of breast cancer. Breast Cancer Res. Treat. 2010, 121, 461–467. [Google Scholar] [CrossRef]
- Michels, K.B.; Holmberg, L.; Bergkvist, L.; Wolk, A. Coffee, tea, and caffeine consumption and breast cancer incidence in a cohort of Swedish women. Ann. Epidemiol. 2002, 12, 21–26. [Google Scholar] [CrossRef]
- Kumar, N.; Titus-Ernstoff, L.; Newcomb, P.A.; Trentham-Dietz, A.; Anic, G.; Egan, K.M. Tea consumption and risk of breast cancer. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 341–345. [Google Scholar] [CrossRef]
- Ganmaa, D.; Willett, W.C.; Li, T.Y.; Feskanich, D.; van Dam, R.M.; Lopez-Garcia, E.; Hunter, D.J.; Holmes, M.D. Coffee, tea, caffeine and risk of breast cancer: A 22-year follow-up. Int. J. Cancer 2008, 122, 2071–2076. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Doyle, T.J.; Kushi, L.H.; Sellers, T.A.; Hong, C.P.; Folsom, A.R. Tea consumption and cancer incidence in a prospective cohort study of postmenopausal women. Am. J. Epidemiol. 1996, 144, 175–182. [Google Scholar] [CrossRef]
- McLaughlin, C.C.; Mahoney, M.C.; Nasca, P.C.; Metzger, B.B.; Baptiste, M.S.; Field, N.A. Breast cancer and methylxanthine consumption. Cancer Causes Control 1992, 3, 175–178. [Google Scholar] [CrossRef]
- Wang, Y.; Gapstur, S.M.; Gaudet, M.M.; Peterson, J.J.; Dwyer, J.T.; McCullough, M.L. Evidence for an Association of Dietary Flavonoid Intake with Breast Cancer Risk by Estrogen Receptor Status Is Limited. J. Nutr. 2014, 144, 1603–1611. [Google Scholar] [CrossRef] [Green Version]
- Crew, K.D.; Brown, P.; Greenlee, H.; Bevers, T.B.; Arun, B.; Hudis, C.; McArthur, H.L.; Chang, J.; Rimawi, M.; Vornik, L.; et al. Phase IB Randomized, Double-Blinded, Placebo-Controlled, Dose Escalation Study of Polyphenon E in Women with Hormone Receptor-Negative Breast Cancer. Cancer Prev. Res. 2012, 5, 1144–1154. [Google Scholar] [CrossRef]
- Crew, K.D.; Ho, K.A.; Brown, P.; Greenlee, H.; Bevers, T.B.; Arun, B.; Sneige, N.; Hudis, C.; McArthur, H.L.; Chang, J.; et al. Effects of a green tea extract, Polyphenon E, on systemic biomarkers of growth factor signalling in women with hormone receptor-negative breast cancer. J. Hum. Nutr. Diet. 2015, 28, 272–282. [Google Scholar] [CrossRef]
- Lazzeroni, M.; Guerrieri-Gonzaga, A.; Gandini, S.; Johansson, H.; Serrano, D.; Cazzaniga, M.; Aristarco, V.; Macis, D.; Mora, S.; Caldarella, P.; et al. A Presurgical Study of Lecithin Formulation of Green Tea Extract in Women with Early Breast Cancer. Cancer Prev. Res. 2017, 10, 363–369. [Google Scholar] [CrossRef]
- Samavat, H.; Dostal, A.M.; Wang, R.W.; Bedell, S.; Emory, T.H.; Ursin, G.; Torkelson, C.J.; Gross, M.D.; Le, C.T.; Yu, M.C.; et al. The Minnesota Green Tea Trial (MGTT), a randomized controlled trial of the efficacy of green tea extract on biomarkers of breast cancer risk: Study rationale, design, methods, and participant characteristics. Cancer Causes Control 2015, 26, 1405–1419. [Google Scholar] [CrossRef]
- Samavat, H.; Ursin, G.; Emory, T.H.; Lee, E.; Wang, R.W.; Torkelson, C.J.; Dostal, A.M.; Swenson, K.; Le, C.T.; Yang, C.S.; et al. A Randomized Controlled Trial of Green Tea Extract Supplementation and Mammographic Density in Postmenopausal Women at Increased Risk of Breast Cancer. Cancer Prev. Res. 2017, 10, 710–718. [Google Scholar] [CrossRef]
- Chen, X.; Lu, W.; Zheng, Y.; Gu, K.; Chen, Z.; Zheng, W.; Shu, X.O. Exercise, tea consumption, and depression among breast cancer survivors. J. Clin. Oncol. 2010, 28, 991–998. [Google Scholar] [CrossRef]
- Stendell-Hollis, N.R.; Thomson, C.A.; Thompson, P.A.; Bea, J.W.; Cussler, E.C.; Hakim, I.A. Green tea improves metabolic biomarkers, not weight or body composition: A pilot study in overweight breast cancer survivors. J. Hum. Nutr. Diet. 2010, 23, 590–600. [Google Scholar] [CrossRef]
- Zhu, W.Q.; Jia, L.; Chen, G.X.; Zhao, H.X.; Sun, X.R.; Meng, X.J.; Zhao, X.G.; Xing, L.G.; Yu, J.M.; Zheng, M.Z. Epigallocatechin-3-gallate ameliorates radiation-induced acute skin damage in breast cancer patients undergoing adjuvant radiotherapy. Oncotarget 2016, 7, 48607–48613. [Google Scholar] [CrossRef] [Green Version]
- Mayo Clinic. Drinking coffee and tea doesn’t raise breast cancer risk. Mayo Clinic Women’s Healthsource 2008, 12, 3. [Google Scholar]
- Iwasaki, M.; Inoue, M.; Sasazuki, S.; Miura, T.; Sawada, N.; Yamaji, T.; Shimazu, T.; Willett, W.C.; Tsugane, S. Plasma tea polyphenol levels and subsequent risk of breast cancer among Japanese women: A nested case-control study. Breast Cancer Res. Treat. 2010, 124, 827–834. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, J.; Xie, X.; Holman, C.D.J. Dietary intakes of mushrooms and green tea combine to reduce the risk of breast cancer in Chinese women. Int. J. Cancer 2009, 124, 1404–1408. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Tsubono, Y.; Nakaya, N.; Suzuki, Y.; Koizumi, Y.; Tsuji, I. Green tea and the risk of breast cancer: Pooled analysis of two prospective studies in Japan. Br. J. Cancer 2004, 90, 1361–1363. [Google Scholar] [CrossRef]
- Inoue, M.; Tajima, K.; Mizutani, M.; Iwata, H.; Iwase, T.; Miura, S.; Hirose, K.; Hamajima, N.; Tominaga, S. Regular consumption of green tea and the risk of breast cancer recurrence: Follow-up study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC), Japan. Cancer Lett. 2001, 167, 175–182. [Google Scholar] [CrossRef]
- Iwasaki, M.; Inoue, M.; Sasazuki, S.; Sawada, N.; Yamaji, T.; Shimazu, T.; Willett, W.C.; Tsugane, S. Green tea drinking and subsequent risk of breast cancer in a population-based cohort of Japanese women. Breast Cancer Res. 2010, 12, R88. [Google Scholar] [CrossRef]
- Iwasaki, M.; Mizusawa, J.; Kasuga, Y.; Yokoyama, S.; Onuma, H.; Nishimura, H.; Kusama, R.; Tsugane, S. Green tea consumption and breast cancer risk in Japanese women: A case-control study. Nutr. Cancer 2014, 66, 57–67. [Google Scholar] [CrossRef]
- Key, T.J.; Sharp, G.B.; Appleby, P.N.; Beral, V.; Goodman, M.T.; Soda, M.; Mabuchi, K. Soya foods and breast cancer risk: A prospective study in Hiroshima and Nagasaki, Japan. Br. J. Cancer 1999, 81, 1248–1256. [Google Scholar] [CrossRef]
- Nagano, J.; Kono, S.; Preston, D.L.; Mabuchi, K. A prospective study of green tea consumption and cancer incidence, Hiroshima and Nagasaki (Japan). Cancer Causes Control 2001, 12, 501–508. [Google Scholar] [CrossRef]
- Nakachi, K.; Suemasu, K.; Suga, K.; Takeo, T.; Imai, K.; Higashi, Y. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Jpn. J. Cancer Res. 1998, 89, 254–261. [Google Scholar] [CrossRef]
- Inoue, M.; Robien, K.; Wang, R.; Van Den Berg, D.J.; Koh, W.P.; Yu, M.C. Green tea intake, MTHFR/TYMS genotype and breast cancer risk: The Singapore Chinese Health Study. Carcinogenesis 2008, 29, 1967–1972. [Google Scholar] [CrossRef]
- Li, M.; Tse, L.A.; Chan, W.C.; Kwok, C.H.; Leung, S.L.; Wu, C.; Yu, W.C.; Yu, I.T.; Yu, C.H.; Wang, F.; et al. Evaluation of breast cancer risk associated with tea consumption by menopausal and estrogen receptor status among Chinese women in Hong Kong. Cancer Epidemiol. 2016, 40, 73–78. [Google Scholar] [CrossRef]
- Shrubsole, M.J.; Lu, W.; Chen, Z.; Shu, X.O.; Zheng, Y.; Dai, Q.; Cai, Q.; Gu, K.; Ruan, Z.X.; Gao, Y.T.; et al. Drinking green tea modestly reduces breast cancer risk. J. Nutr. 2009, 139, 310–316. [Google Scholar] [CrossRef]
- Yuan, J.M.; Koh, W.P.; Sun, C.L.; Lee, H.P.; Yu, M.C. Green tea intake, ACE gene polymorphism and breast cancer risk among Chinese women in Singapore. Carcinogenesis 2005, 26, 1389–1394. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Holman, C.D.; Huang, J.P.; Xie, X. Green tea and the prevention of breast cancer: A case-control study in Southeast China. Carcinogenesis 2007, 28, 1074–1078. [Google Scholar] [CrossRef]
- Wu, A.H.; Yu, M.C.; Tseng, C.C.; Hankin, J.; Pike, M.C. Green tea and risk of breast cancer in Asian Americans. Int. J. Cancer 2003, 106, 574–579. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Gu, C.; Chen, D.; Yu, B.; He, J. Oxidative stress-induced diseases and tea polyphenols. Oncotarget 2017, 8, 81649–81661. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Zhao, L.; Gao, M.; Loor, J.J. Tea polyphenols protect bovine mammary epithelial cells from hydrogen peroxide-induced oxidative damage in vitro. J. Anim. Sci. 2018, 96, 4159–4172. [Google Scholar] [CrossRef]
- Ogunleye, A.A.; Xue, F.; Michels, K.B. Green tea consumption and breast cancer risk or recurrence: A meta-analysis. Breast Cancer Res. Treat. 2010, 119, 477–484. [Google Scholar] [CrossRef]
- Parks, R.G.; Tabak, R.G.; Allen, P.; Baker, E.A.; Stamatakis, K.A.; Poehler, A.R.; Yan, Y.; Chin, M.H.; Harris, J.K.; Dobbins, M.; et al. Enhancing evidence-based diabetes and chronic disease control among local health departments: A multi-phase dissemination study with a stepped-wedge cluster randomized trial component. Implement. Sci. 2017, 12, 122. [Google Scholar] [CrossRef]
- Horakova, D.; Bouchalova, K.; Cwiertka, K.; Stepanek, L.; Vlckova, J.; Kollarova, H. Risks and protective factors for triple negative breast cancer with a focus on micronutrients and infections. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2018, 162, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Schroder, L.; Marahrens, P.; Koch, J.G.; Heidegger, H.; Vilsmeier, T.; Phan-Brehm, T.; Hofmann, S.; Mahner, S.; Jeschke, U.; Richter, D.U. Effects of green tea, matcha tea and their components epigallocatechin gallate and quercetin on MCF7 and MDA-MB-231 breast carcinoma cells. Oncol. Rep. 2018. [Google Scholar] [CrossRef]
- Wei, R.; Mao, L.; Xu, P.; Zheng, X.; Hackman, R.M.; Mackenzie, G.G.; Wang, Y. Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models. Food Funct. 2018, 9, 5682–5696. [Google Scholar] [CrossRef]







| Search Strategy | Details |
|---|---|
| Inclusion criteria | P: general adult population (male and female) I: questionnaire or interview assessing green tea consumption C: higher vs. lower green tea consumption O: risk of breast cancer (if any) S: original research article (case-control studies, cohort studies, cross-sectional studies) |
| Exclusion criteria | P: pediatric population I: no administration of questionnaire O: other outcomes S: review article, expert opinion, comments, abstract, letters, article with no quantitative information or details |
| Language filter | English |
| Time filter | None (from inception) |
| Database | PubMed/Medline; Scopus; Web of Science |
| Author, Year | No in Analysis | Age (years) | Baseline | Study Period | Study Design | Instrument | Outcome | Green Tea Intake | OR RR HR (CI 95%) | p-Value | Country | QS/6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li M. et al. 2016 | Case = 756 Control = 789 | 20–84 y | BC diagnosis All stage | 2011–2014 | Case-control | In-person interview | recurrence | 3 cups/day | 1.2 (0.8–1.78) | 0.38 | China | 4 |
| Iwasaki M. et al. 2014 | Case = 369 Control = 369 | 20–74 y | BC diagnosis 1–2 stage | 2001–2005 | Case-control | FFQ validated | recurrence | 600 mL/day | 1.27 (0.75–2.14) | 0.20 | Japan | 5 |
| Iwasaki M. et al. 2010 | 67,422 | 40–69 y | Healthy women | 1990–1994 Follow-up 1995–1998 | Prospective cohort | FFQ (self-administered) not validated | BC | 5 cups/day | 1.12 (0.81–1.56) | 0.60 | Japan | 5 |
| Shrubsole J. M. et al. 2009 | Case = 3454 Control = 3474 | 25–70 y | BC diagnosis | 1996–2005 | Case-control | FFQ validated | recurrence | 148 ± 124 g/mo | 0.88 (0.79–0.98) | n.a. | China | 4 |
| Inoue M. et al. 2008 | 678 | 45–74 y | Healthy women | 1993–1998 | Prospective cohort | 24 h food recalls validated | BC | 174.6 ± 75.2 µg/day | 1.00 (0.82–1.22) | 0.41 | China | 4 |
| Zhang M. et al. 2007 | Case = 1009 Control = 1009 | 20–87 y | BC diagnosis | 2004–2005 | Case-control | FFQ validated | recurrence | 4 cups/day | 0.57 (0.47–0.69) | 0.001 | China | 6 |
| Yuan J.M. et al. 2005 | 665 | 45–74 y | Healthy women | 1993–1998 | Prospective cohort | In-person interview | BC | Weekly | 0.91 (0.66–1.26) | n.a. | China | 4 |
| Suzuki Y. et al. 2004 (a) | 17,353 | >40 y (cohort I) | Healthy women | 1984–1990 | Prospective cohort | FFQ validated (self-administered) | BC | 5 cups/day | 0.96 (0.50–1.86) | 0.51 | Japan | 6 |
| Suzuki Y. et al. 2004 (b) | 24,769 | 40–64 y (cohort II) | Healthy women | 1984–1990 | Prospective cohort | FFQ validated (self-administered) | BC | 5 cups/day | 0.85 (0.43–1.669) | 0.95 | Japan | 6 |
| Wu H. A. et al. 2003 | Case = 501 Control = 594 | 25–74 y | BC diagnosis | 1995–1998 | Case-control | In-person interview | recurrence | 85.7 mL/day | 0.61 (0.40–0.93) | 0.01 | USA | 4 |
| Inoue M. et al. 2001 | 1160 | Mean age 51.5 y | BC diagnosis 1–2 stage | 1990–1997 | Follow-up | FFQ (self-administered) not validated | recurrence | 6 cups/day | 0.68 (0.4–1.16) | 0.72 | Japan | 3 |
| Nagano J. et al. 2001 | 38,540 | Mean age 54.8 y | Healthy women | 1979–1981 | Prospective cohort | FFQ (self-administered) not validated | BC and other cancers | 5 cups/day | 1.0 (0.67–1.6) | 0.80 | Japan | 3 |
| Key T.J. et al. 1999 | 34,759 | 40–80 y | Healthy women | 1969–1981 | Prospective cohort | FFQ not validated | BC | 5 cups/day | 0.86 (0.62–1.21) | 0.284 | Japan | 3 |
| Nakachi K. et al. 1998 | 472 | Mean age 49.7±11.2 | BC diagnosis 1–2–3 stage | 1984–1993 | Follow-up | FFQ not validated | recurrence | 8 cups/day | 0.775 (0.53–1.13) | 0.15 | Japan | 4 |
| Premenopausal | Postmenopausal | |||
|---|---|---|---|---|
| Author, Year | N in Analysis | OR RR HR (CI 95%) | N in Analysis | OR RR HR (CI 95%) |
| Li M. et al. 2016 | 267 | OR 0.62 (0.40–0.97) | 405 | OR 1.40 (1.00–1.96) |
| Iwasaki M. et al. 2014 | 79 | OR 1.10 (0.54–2.23) | 212 | OR 1.42 (0.71–2.85) |
| Iwasaki M. et al. 2010 | 81 | HR 0.97 (0.66–1.41) | 70 | HR 1.08 (0.75–1.55) |
| Shrubsole M. et al. 2009 | 1302 | 0R 0.87 (0.76–1.00) | 799 | OR 0.88 (0.74–1.04) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gianfredi, V.; Nucci, D.; Abalsamo, A.; Acito, M.; Villarini, M.; Moretti, M.; Realdon, S. Green Tea Consumption and Risk of Breast Cancer and Recurrence—A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2018, 10, 1886. https://doi.org/10.3390/nu10121886
Gianfredi V, Nucci D, Abalsamo A, Acito M, Villarini M, Moretti M, Realdon S. Green Tea Consumption and Risk of Breast Cancer and Recurrence—A Systematic Review and Meta-Analysis of Observational Studies. Nutrients. 2018; 10(12):1886. https://doi.org/10.3390/nu10121886
Chicago/Turabian StyleGianfredi, Vincenza, Daniele Nucci, Angela Abalsamo, Mattia Acito, Milena Villarini, Massimo Moretti, and Stefano Realdon. 2018. "Green Tea Consumption and Risk of Breast Cancer and Recurrence—A Systematic Review and Meta-Analysis of Observational Studies" Nutrients 10, no. 12: 1886. https://doi.org/10.3390/nu10121886