Effects of Glutamine and Alanine Supplementation on Central Fatigue Markers in Rats Submitted to Resistance Training
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals
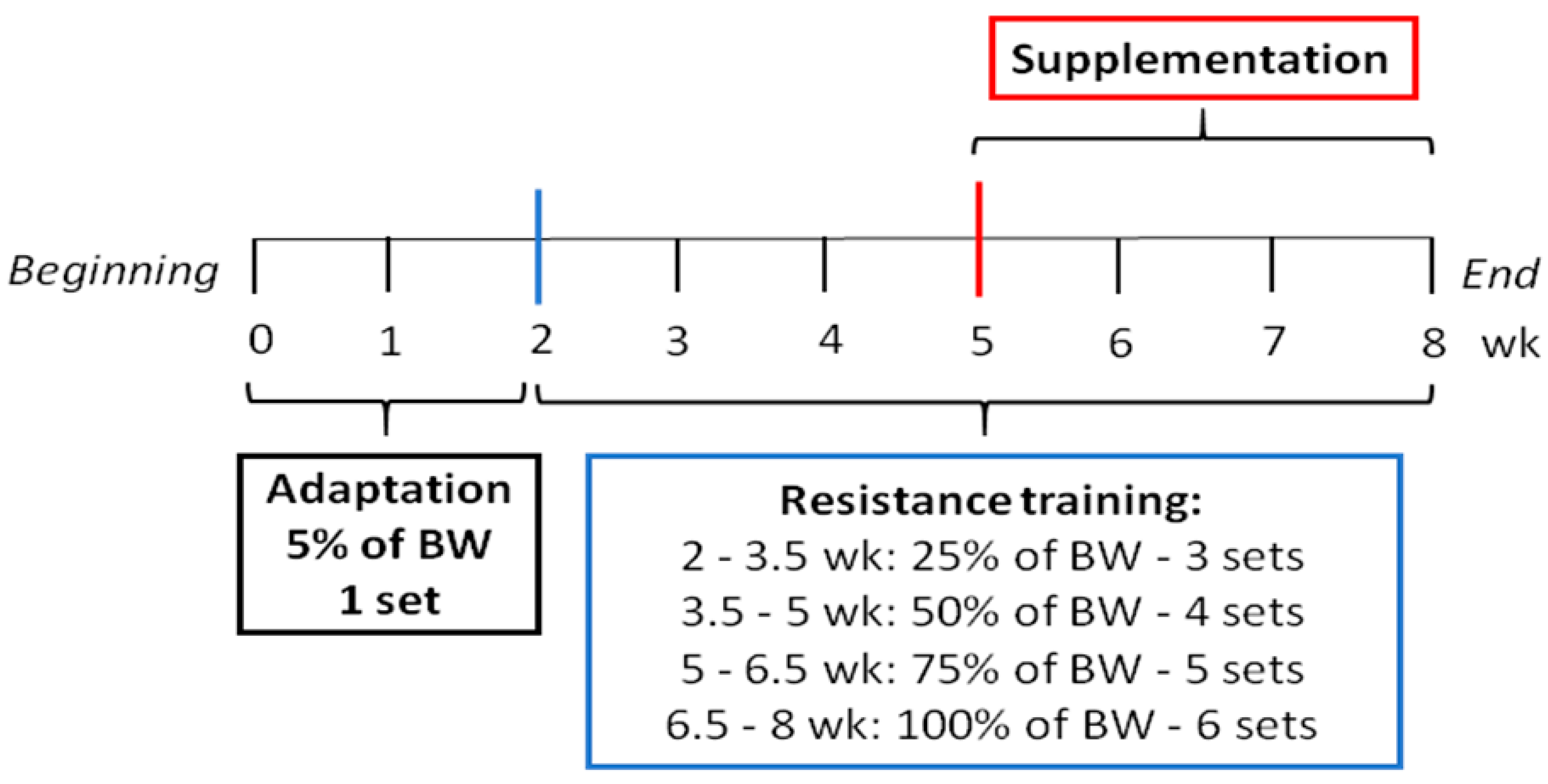
2.2. Resistance Training
2.3. Maximum Carrying Capacity (MCC) Tests
2.4. Supplementation
2.5. Plasma Parameters
2.6. Tissue Measurements
2.7. Statistical Analysis
3. Results
3.1. Food Consumption, Fluid Intake, and Body Weight Gain
3.2. Plasma Amino Acids
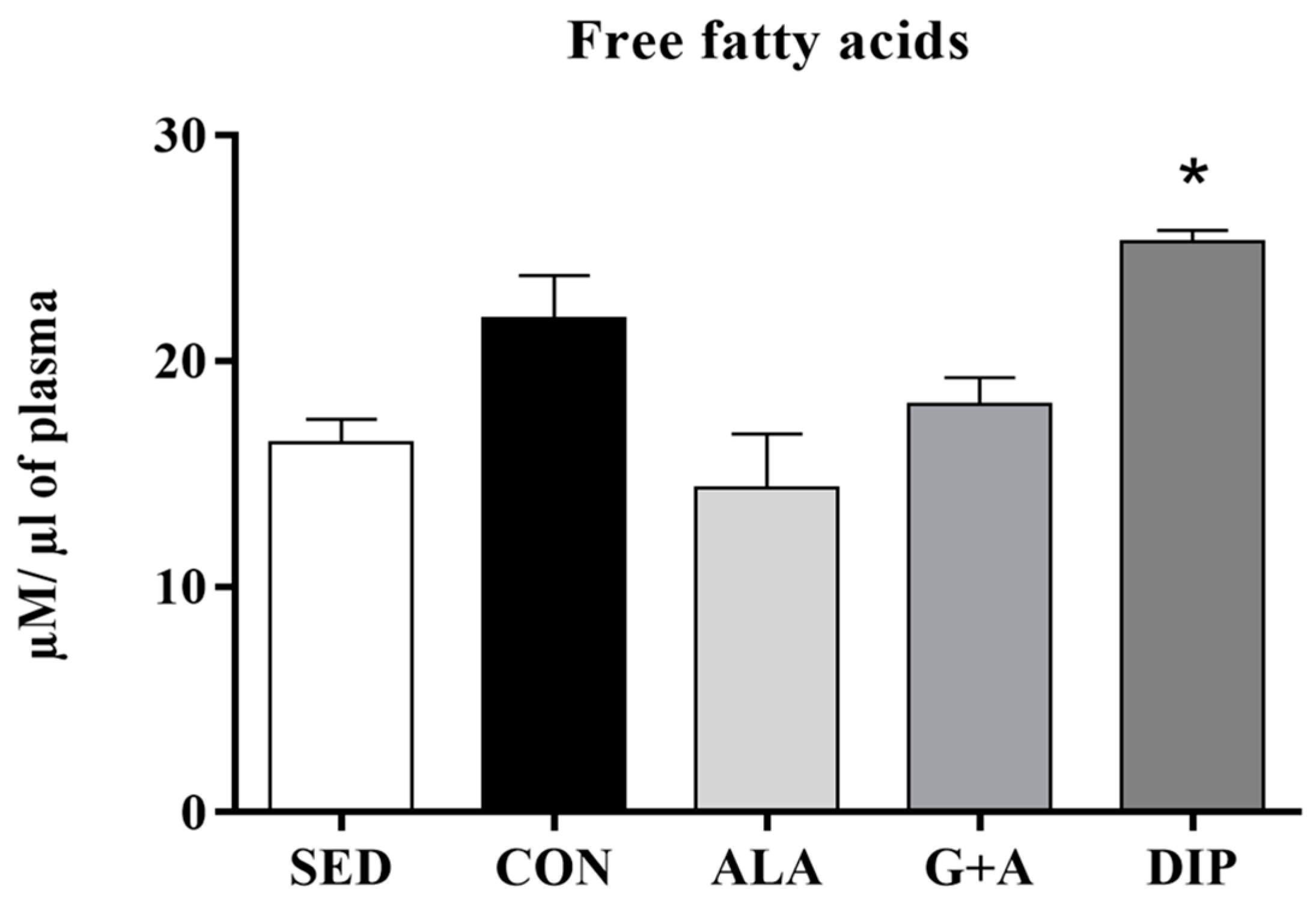
3.3. Glycemia, Glycogen, and Free Fatty Acids (FFA)
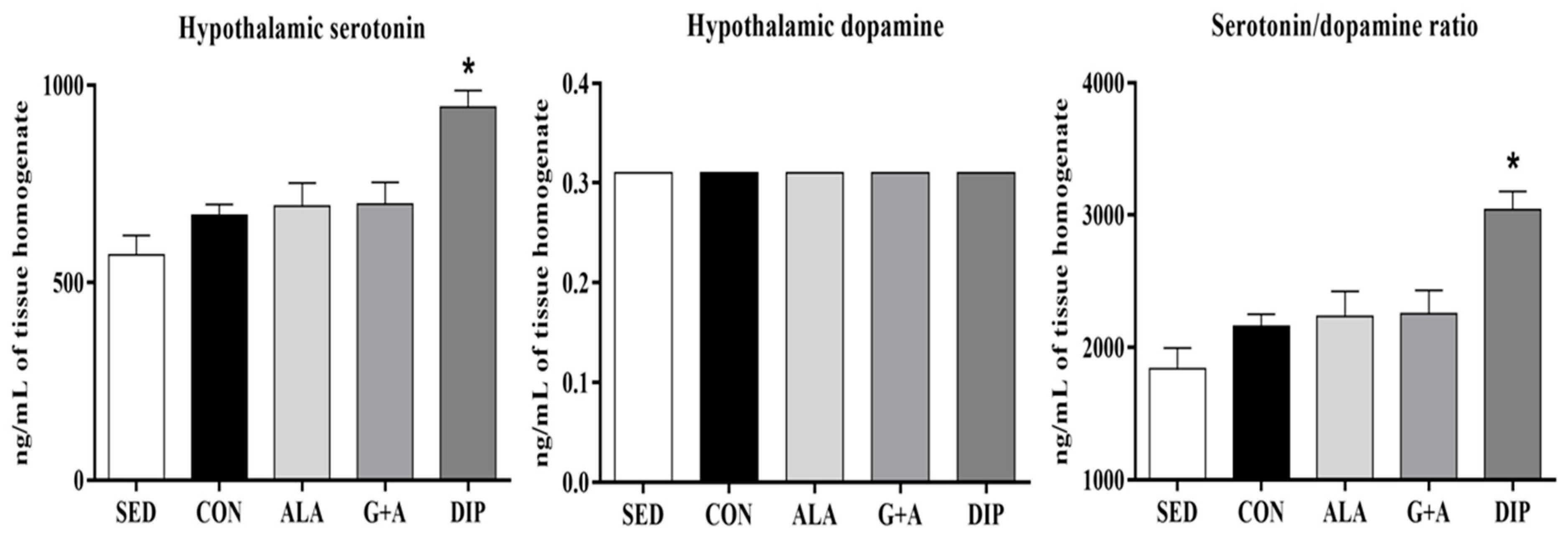
3.4. Hypothalamic Serotonin, and Dopamine
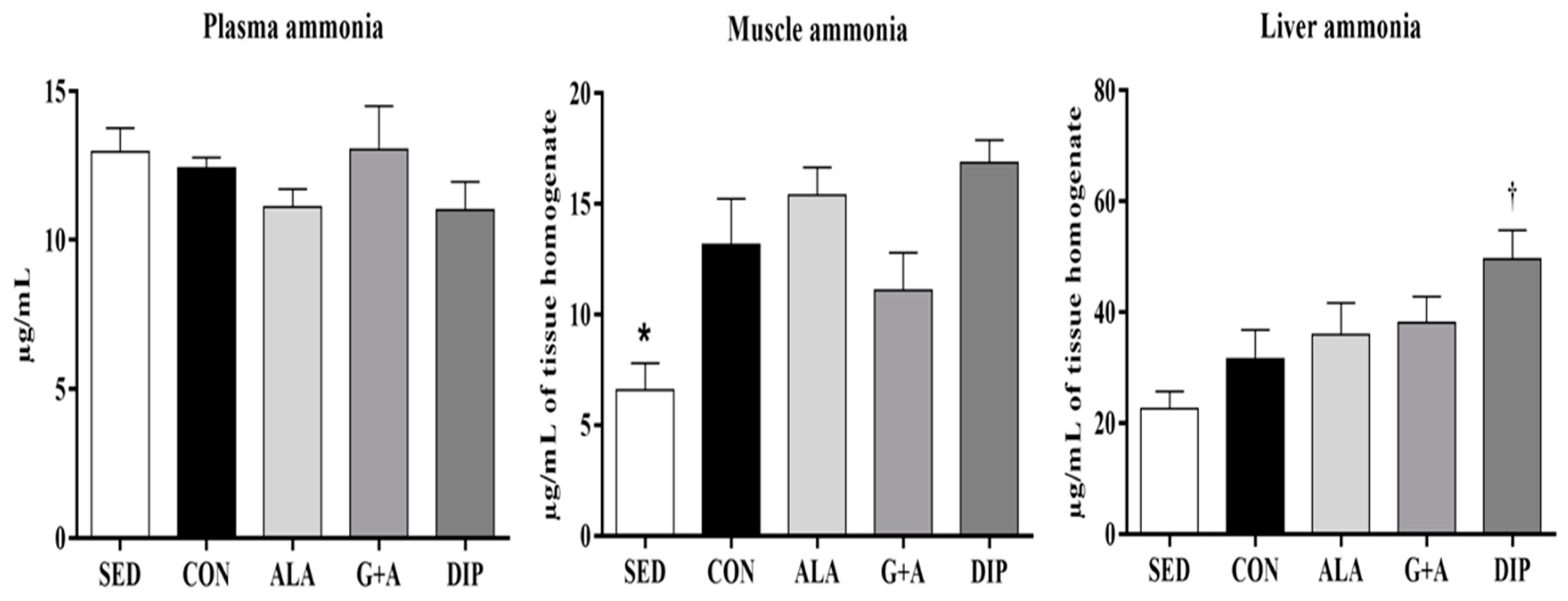
3.5. Plasma, Muscle, and Liver Ammonia
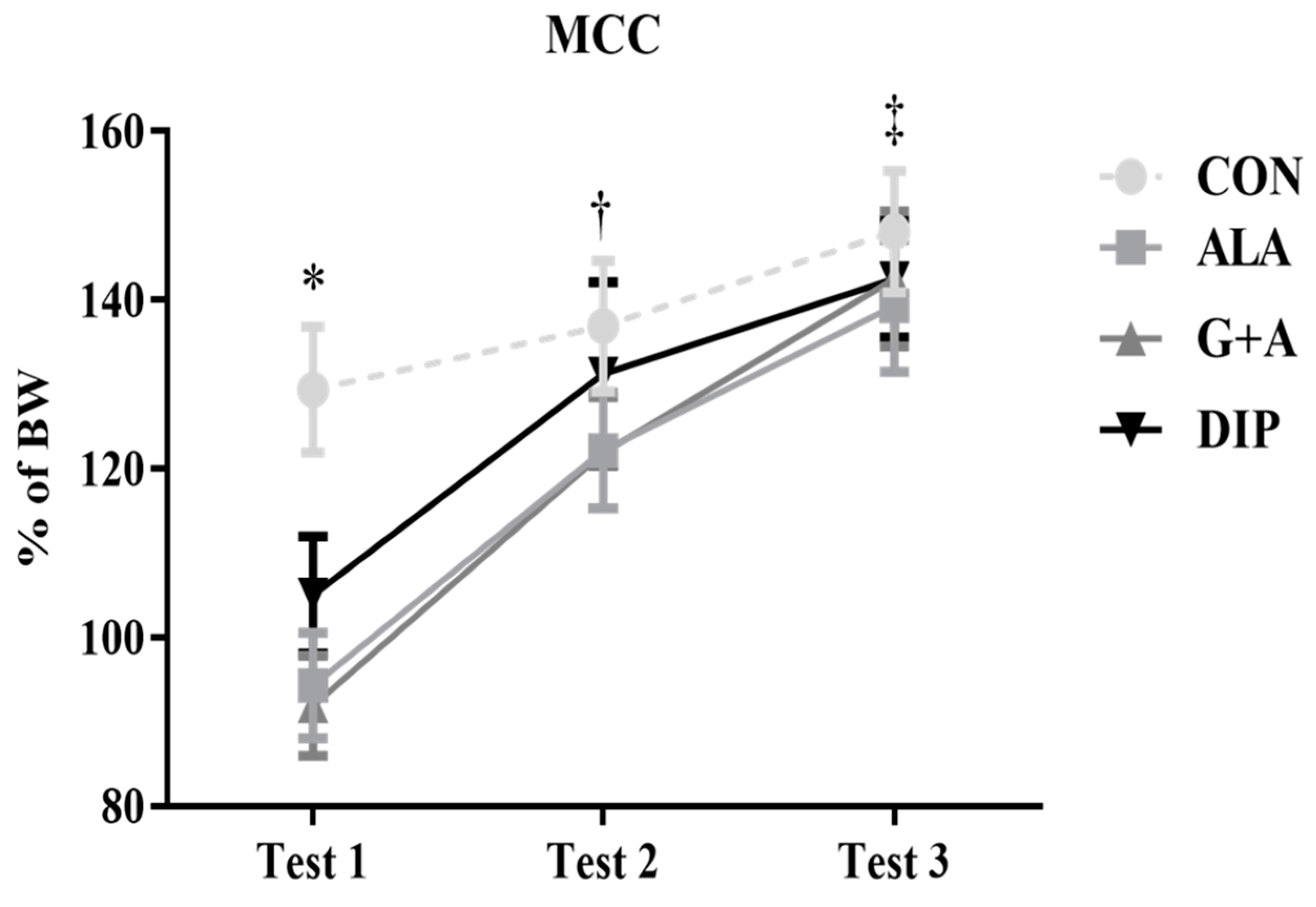
3.6. Maximum Carrying Capacity (MCC) Tests
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Béquet, F.; Gomez-Merino, D.; Berthelot, M.; Guezennec, C.Y. Evidence that brain glucose availability influences exercise-enhanced extracellular 5-HT level in hippocampus: A microdialysis study in exercising rats. Acta Physiol. Scand. 2002, 176, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet. Disord. 2012, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, E.A.; Blomstrand, E. Branched-Chain Amino Acids and Central Fatigue. J. Nutr. 2006, 136, 274S–276S. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Smeeton, N.J.; Watt, P.W. Ammonia metabolism, the brain and fatigue; Revisiting the link. Prog. Neurobiol. 2010, 91, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Chaouloff, F.; Laude, D.; Guezennec, Y.; Elghozi, J.L. Motor Activity Increases Tryptophan, 5-Hydroxyindoleacetic Acid, and Homovanillic Acid in Ventricular Cerebrospinal Fluid of the Conscious Rat. J. Neurochem. 1986, 46, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, E.; Celsing, F.; Newsholme, E.A. Changes in plasma concentrations of aromatic and branched—Chain amino acids during sustained exercise in man and their possible role in fatigue. Acta Physiol. Scand. 1988, 133, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Smriga, M.; Kameishi, M.; Tanaka, T.; Kondoh, T.; Torii, K. Preference for a solution of branched-chain amino acids plus glutamine and arginine correlates with free running activity in rats: Involvement of serotonergic-dependent processes of lateral hypothalamus. Nutr. Neurosci. 2002, 5, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; Watson, P.; Hasegawa, H.; Roelands, B.; Piacentini, M.F. Central fatigue: The serotonin hypothesis and beyond. Sports Med. 2006, 36, 881–909. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.M.S.; Guimarães, J.B.; Wanner, S.P.; La Guardia, R.B.; Miranda, R.M.; Marubayashi, U.; Soares, D.D. Inhibition of tryptophan hydroxylase abolishes fatigue induced by central tryptophan in exercising rats. Scand. J. Med. Sci. Sports 2014, 24, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, E.; Perrett, D.; Parry-Billings, M.; Newsholme, E.A. Effect of sustained exercise on plasma amino acid concentrations and on 5-hydroxytryptamine metabolism in six different brain regions in the rat. Acta Physiol. Scand. 1989, 136, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Weicker, H.; Struder, H.K. Influence of exercis on serotonergic neuromeodulation in the brain. J. Amin. Acids 2001, 20, 35. [Google Scholar] [CrossRef]
- Skeie, B.; Kvetan, V.; Gil, K.M.; Rothkopf, M.M.; Newsholme, E.A.; Askanazi, J. Branch-chain amino acids: Their metabolism and clinical utility. Crit. Care Med. 1990, 18, 549–571. [Google Scholar] [CrossRef] [PubMed]
- Verger, P.; Aymard, P.; Cynobert, L.; Anton, G.; Luigi, R. Effects of administration of branched-chain amino acids vs. glucose during acute exercise in the rat. Physiol. Behav. 1994, 55, 523–526. [Google Scholar] [CrossRef]
- Blomstrand, E. Amino acids and central fatigue. Amino Acids 2001, 20, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, E.; Møller, K.; Secher, N.H.; Nybo, L. Effect of carbohydrate ingestion on brain exchange of amino acids during sustained exercise in human subjects. Acta Physiol. Scand. 2005, 185, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D. Branched-Chain Amino Acids and Brain Function. In Proceedings of the 4th Amino Acid Assessment Workshop, Kobe, Japan, 28–29 October 2004; American Society for Nutrition: Rockville, MD, USA, 2005; pp. 1539S–1546S. [Google Scholar]
- Blomstrand, E.; Hassmén, P.; Ek, S.; Ekblom, B.; Newsholme, E.A. Influence of ingesting a solution of branched-chain amino acids on perceveid exertion during exercise. Acta Physiol. Scand. 1997, 159, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.K.; Chien, K.M.C.; Chang, J.H.; Huang, M.H.; Liang, Y.C.; Liu, T.H. Branched-chain amino acids and arginine improve performance in two consecutive days of simulated handball games in male and female athletes: A randomized trial. PLoS ONE 2015, 10, e0121866. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-F.; Wu, H.-J.; Chen, C.-Y.; Chou, K.-M.; Chang, C.-K. Branched-chain amino acids, arginine, citrulline alleviate central fatigue after 3 simulated matches in taekwondo athletes: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2016, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Perriello, G.; Meyer, C.; Gerich, J. Role of glutamine in human carbohydrate metabolism in kidney and other tissues. Kidney Int. 1999, 55, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.J.; Bowtell, J.L.; Bruce, M.; Khogali, S.E.O. Glutamine Metabolism: Nutritional and Clinical Significance Interaction between Glutamine Availability and Metabolism of Glycogen, Tricarboxylic Acid Cycle Intermediates and Glutathione 1, 2. J. Nutr. 2001, 131, 2488–2490. [Google Scholar] [CrossRef]
- Curi, R.; Lagranha, C.J.; Doi, S.Q.; Sellitti, D.F.; Procopio, J.; Pithon-Curi, T.C.; Corless, M.; Newsholme, P. Molecular mechanisms of glutamine action. J. Cell. Physiol. 2005, 204, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Bassini-Cameron, A.; Monteiro, A.; Gomes, A.; Werneck-de-Castro, J.P.S.; Cameron, L. Glutamine protects against increases in blood ammonia in football players in an exercise intensity-dependent way. Br. J. Sports Med. 2008, 42, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.; Oehler, R.; Manhart, N.; Exner, R.; Wessner, B.; Strasser, E.; Spittler, A. Regulative potential of glutamine—Relation to glutathione metabolism. Nutrition 2002, 18, 217–221. [Google Scholar] [CrossRef]
- Newsholme, P.; Procopio, J.; Ramos Lima, M.M.; Pithon-Curi, T.C.; Curi, R. Glutamine and glutamate—Their central role in cell metabolism and function. Cell Biochem. Funct. 2003, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guezennec, C.Y.; Abdelmalki, A.; Merino, D.; Bigord, X.; Berthelot, M.; Pierard, C.; Peres, M. Effects of prolonged exercise on brain ammonia and amino acids. Int. J. Sports Med. 1998, 19, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Raizel, R.; Leite, J.S.M.; Hypólito, T.M.; Coqueiro, A.Y.; Newsholme, P.; Cruzat, V.F.; Tirapegui, J. Determination of the anti-inflammatory and cytoprotective effects of l-glutamine and l-alanine, or dipeptide, supplementation in rats submitted to resistance exercise. Br. J. Nutr. 2016, 116, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.S.M.; Raizel, R.; Hypólito, T.M.; Rosa, T.D.S.; Cruzat, V.F.; Tirapegui, J. l-glutamine and l-alanine supplementation increase glutamine-glutathione axis and muscle HSP-27 in rats trained using a progressive high-intensity resistance exercise. Appl. Physiol. Nutr. Metab. 2016, 41, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Coqueiro, A.Y.; Raizel, R.; Hypólito, T.M.; Tirapegui, J. Effects of supplementation with l-glutamine and L-alanine in the body composition of rats submitted to resistance exercise. Rev. Bras. Cienc. Esporte 2017, 39, 417–423. [Google Scholar] [CrossRef]
- Curi, T.C.; De Melo, M.P.; De Azevedo, R.B.; Zorn, T.M.; Curi, R. Glutamine utilization by rat neutrophils: Presence of phosphate-dependent glutaminase. Am. J. Physiol. 1997, 273, C1124–C1129. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Tirapegui, J.; Pedrosa, R.G.; de Castro, I.A.; de Oliveira Pires, I.S. Effect of alanyl-glutamine supplementation on plasma and tissue glutamine concentrations in rats submitted to exhaustive exercise. Nutrition 2006, 22, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M. Dosing and efficacy of glutamine supplementation in human exercise and sport training. J. Nutr. 2008, 138, 2045S–2049S. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Tirapegui, J.; Pedrosa, R.G.; De Oliveira Pires, I.S.; De Castro, I.A. Plasma and tissue glutamine response to acute and chronic supplementation with l-glutamine and l-alanyl-l-glutamine in rats. Nutr. Res. 2004, 24, 261–270. [Google Scholar] [CrossRef]
- Cruzat, V.F.; Tirapegui, J. Effects of oral supplementation with glutamine and alanyl-glutamine on glutamine, glutamate, and glutathione status in trained rats and subjected to long-duration exercise. Nutrition 2009, 25, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, D.L.; Silva, L.A.; Tromm, C.B.; da Rosa, G.L.; Silveira, P.C.L.; de Souza, C.T.; Latini, A.; Pinho, R.A. Impact of different resistance training protocols on muscular oxidative stress parameters. Appl. Physiol. Nutr. Metab. 2012, 37, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, T.A., Jr.; Farrar, R.P. Physiological Hypertrophy of the FHL Muscle Following 8 Weeks of Progressive Resistance Exercise in the Rat. Can. J. Appl. Physiol. 2004, 29, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Sanches, I.C.; Conti, F.F.; Sartori, M.; Irigoyen, M.C.; De Angelis, K. Standardization of resistance exercise training: Effects in diabetic ovariectomized rats. Int. J. Sports Med. 2014, 35, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Petry, É.R.; Cruzat, V.F.; Heck, T.G.; Leite, J.S.M.; De Bittencourt, P.I.H.; Tirapegui, J. Alanyl-glutamine and glutamine plus alanine supplements improve skeletal redox status in trained rats: Involvement of heat shock protein pathways. Life Sci. 2014, 94, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F.; Rogero, M.M.; Tirapegui, J. Effects of supplementation with free glutamine and the dipeptide alanyl-glutamine on parameters of muscle damage and inflammation in rats submitted to prolonged exercise. Cell Biochem. Funct. 2010, 28, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Prada, P.O.; Hirabara, S.M.; De Souza, C.T.; Schenka, A.A.; Zecchin, H.G.; Vassallo, J.; Velloso, L.A.; Carneiro, E.; Carvalheira, J.B.C.; Curi, R.; et al. l-glutamine supplementation induces insulin resistance in adipose tissue and improves insulin signalling in liver and muscle of rats with diet-induced obesity. Diabetologia 2007, 50, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Hassid, W.; Albrahams, S. Chemical procedures for analyses of polisacchardies methods enzimol. Methods Enzim. 1957, 3, 34–51. [Google Scholar]
- MacLean, D.A.; Graham, T.E.; Saltin, B. Stimulation of muscle ammonia production during exercise following branched-chain amino acid supplementation in humans. J. Physiol. 1996, 493 Pt 3, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Strüder, H.; Hollmann, W.; Platen, P.; Donike, M.; Gotzmann, A.; Weber, K. Influence of Paroxetine, Branched-Chain Amino Acids and Tyrosine on Neuroendocrine System Responses and Fatigue in Humans. Horm. Metab. Res. 1998, 30, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Welsh, R.S.; De Volve, K.L.; Alderson, N.A. Effects of branched-chain amino acids and carbohydrate on fatigue during intermittent, high-intensity running. Int. J. Sports Med. 1999, 20, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; Shirreffs, S.M.; Maughan, R.J. The effect of acute branched-chain amino acid supplementation on prolonged exercise capacity in a warm environment. Eur. J. Appl. Physiol. 2004, 93, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Kim, S.-H.; Jeong, W.-S.; Lee, H.-Y. Effect of BCAA intake during endurance exercises on fatigue substances, muscle damage substances, and energy metabolism substances. J. Exerc. Nutr. Biochem. 2013, 1721, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Bowtell, J.L.; Bruce, M. Glutamine: An Anaplerotic Precursor. Nutrition 2002, 18, 222–224. [Google Scholar] [CrossRef]
- Iwashita, S.; Williams, P.; Jabbour, K.; Ueda, T.; Kobayashi, H.; Baier, S.; Flakoll, P.J. Impact of glutamine supplementation on glucose homeostasis during and after exercise. J. Appl. Physiol. 2005, 99, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Bode, B.P. Recent Molecular Advances in Mammalian Glutamine Transport. J. Nutr. 2001, 131, 2475–2485. [Google Scholar] [CrossRef]
- Melis, G.C.; ter Wengel, N.; Boelens, P.G.; van Leeuwen, P.A.M. Glutamine: Recent developments in research on the clinical significance of glutamine. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.; Piquard, F.; Geny, B.; Doutreleau, S.; Lampert, E.; Mettauer, B.; Lonsdorfer, J. L-arginine reduces exercise-induced increase in plasma lactate and ammonia. Int. J. Sports Med. 2002, 23, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Kowalchuk, J.M.; Curi, R.; Newsholme, E.A. Glutamine metabolism in isolated incubated adipocytes of the rat. Biochem. J. 1988, 249, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Bailey, S.P.; Woods, J.A.; Galiano, F.J.; Hamilton, M.T.; Bartoli, W.P. Applied Physiology on plasma free tryptophan and branched, chain amino acids during prolonged cycling. Eur. J. Appl. Physiol. 1992, 65, 513–519. [Google Scholar] [CrossRef]
- Jang, T.-R.; Wu, C.-L.; Chang, C.-M.; Hung, W.; Fang, S.-H.; Chang, C.-K. Effects of carbohydrate, branched-chain amino acids, and arginine in recovery period on the subsequent performance in wrestlers. J. Int. Soc. Sports Nutr. 2011, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Thamotharan, M.; Bawani, S.Z.; Zhou, X.; Adibi, S.A. Functional and molecular expression of intestinal oligopeptide transporter (Pept-1) after a brief fast. Metabolism 1999, 48, 681–684. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Dietary amino acids and brain function. J. Am. Diet. Assoc. 1994, 94, 71–77. [Google Scholar] [CrossRef]
- Meeusen, R.; Thorré, K.; Chaouloff, F.; Sarre, S.; De Meirleir, K.; Ebinger, G.; Michotte, Y. Effects of tryptophan and/or acute running on extracellular 5-HT and 5-HIAA levels in the hippocampus of food-deprived rats. Brain Res. 1996, 740, 245–252. [Google Scholar] [CrossRef]
- Bailey, S.P.; Davis, J.M.; Ahlborn, E.N. Endurance Performance in the Rat. Int. J. Sports Med. 1993, 14, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Marvin, G.; Sharma, A.; Aston, W.; Field, C.; Kendall, M.J.; Jones, D.A. The effects of buspirone on perceived exertion and time to fatigue in man. Exp. Physiol. 1997, 82, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Van Hall, G.; Raaymakers, J.S.H.; Saris, W.H.M.; Wagenmakers, A.J.M. Ingestion of branched-chain amino acids and tryptophan during sustained exercise in man: failure to affect performance. J. Physiol. 1995, 486, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Pannier, J.L.; Bouckaert, J.J.; Lefebvre, R.A. The antiserotonin agent pizotifen does not increase endurance performance in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 72, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, M.F.; Meeusen, R.; Buyse, L.; de Schutter, G.; de Meirleir, K. No effect of a selective serotonergic/noradrenergic reuptake inhibitor on endurance performance. Eur. J. Sport Sci. 2002, 2, 37–41. [Google Scholar] [CrossRef]
- Hobson, R.M.; Watson, P.; Maughan, R.J. Acute tryptophan depletion does not improve endurance cycling capacity in a warm environment. Amino Acids 2013, 44, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, A.; Obeid, O.A.; Powell-Tuck, J. A placebo controlled investigation of the effects of tryptophan or placebo on subjective and objective measures of fatigue. Eur. J. Clin. Nutr. 1998, 52, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, M.F. Hormonal responses during prolonged exercise are influenced by a selective DA/NA reuptake inhibitor. Br. J. Sports Med. 2004, 38, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Gerald, M.C. Effects of (+)-amphetamine on the treadmill endurance performance of rats. Neuropharmacology 1978, 17, 703–704. [Google Scholar] [CrossRef]
- Heyes, M.P.; Garnett, E.S.; Coates, G. Central dopaminergic activity influences rats ability to exercise. Life Sci. 1985, 36, 671–677. [Google Scholar] [CrossRef]
- Hattori, S.; Naoi, M.; Nishino, H. Striatal dopamine turnover during treadmill running in the rat: Releation to the speed of running. Brain Res. Bull. 1994, 35, 41–49. [Google Scholar] [CrossRef]
- Watson, P.; Hasegawa, H.; Roelands, B.; Piacentini, M.F.; Looverie, R.; Meeusen, R. Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. J. Physiol. 2005, 565, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Roelands, B.; Hasegawa, H.; Watson, P.; Piacentini, M.F.; Buyse, L.; De Schutter, G.; Meeusen, R.R. The effects of acute dopamine reuptake inhibition on performance. Med. Sci. Sports Exerc. 2008, 40, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; Roelands, B. Central fatigue and neurotransmitters, can thermoregulation be manipulated? Scand. J. Med. Sci. Sports 2010, 20, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Bliss, E.L.; Ailion, J. Relationship of stress and activity to brain dopamine and homovanillic acid. Life Sci. 1971, 10, 1161–1169. [Google Scholar] [CrossRef]

| Parameter | SED | CON | ALA | G + A | DIP | p |
|---|---|---|---|---|---|---|
| Food consumption (g/week) | 189.5 ± 18.0 | 173.0 ± 7.9 | 170.6 ± 12.6 | 173.2 ± 11.3 | 170.1 ± 9.8 | 0.082 |
| Fluid intake (mL/week) | 270.2 ± 28.1 | 295.2 ± 25.7 | 326.4 ± 23.5 | 355.8 ± 25.7 * | 310.2 ± 14.6 | <0.001 |
| Baseline body weight (g) | 325.5 ± 19.9 | 298.8 ± 38.4 | 290.7 ± 25.7 | 302.6 ± 19.6 | 290.8 ± 17.3 | 0.056 |
| Body weight gain (g/week) | 16.5 ± 4.1 | 11.9 ± 3.5 † | 14.9 ± 2.5 | 14.7 ± 1.9 | 14.5 ± 2.8 | 0.044 |
| Amino Acids (mmol/L) | SED | CON | ALA | G + A | DIP | p |
|---|---|---|---|---|---|---|
| Alanine | 0.81 ± 0.15 | 0.75 ± 0.09 | 0.79 ± 0.11 | 0.82 ± 0.09 | 0.82 ± 0.13 | 0.724 |
| Arginine | 0.68 ± 0.08 | 0.67 ± 0.11 | 0.62 ± 0.08 | 0.62 ± 0.06 | 0.63 ± 0.05 | 0.468 |
| Asparagine | 0.21 ± 0.02 | 0.19 ± 0.02 | 0.19 ± 0.03 | 0.19 ± 0.02 | 0.19 ± 0.03 | 0.590 |
| Aspartate | 0.17 ± 0.04 | 0.13 ± 0.02 | 0.14 ± 0.02 | 0.15 ± 0.02 | 0.15 ± 0.03 | 0.214 |
| Cysteine | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.718 |
| Glutamate | 1.60 ± 0.21 | 1.44 ± 0.18 | 1.47 ± 0.22 | 1.61 ± 0.14 | 1.68 ± 016 | 0.073 |
| Glutamine | 0.84 ± 0.28 | 1.04 ± 0.26 | 0.98 ± 0.16 | 0.85 ± 0.22 | 0.88 ± 0.19 | 0.327 |
| Glycine | 0.52 ± 0.07 | 0.47 ± 0.04 | 0.48 ± 0.11 | 0.48 ± 0.04 | 0.53 ± 0.04 | 0.346 |
| Histidine | 0.23 ± 0.02 | 0.23 ± 0.02 | 0.21 ± 0.03 | 0.20 ± 0.02 | 0.20 ± 0.02 | 0.051 |
| Isoleucine | 0.30 ± 0.05 | 0.27 ± 0.04 | 0.28 ± 0.05 | 0.24 ± 0.04 | 0.26 ± 0.03 | 0.097 |
| Leucine | 0.55 ± 0.09 | 0.49 ± 0.06 | 0.54 ± 0.08 | 0.47 ± 0.06 | 0.50 ± 0.07 | 0.273 |
| Lysine | 1.15 ± 0.09 | 1.11 ± 0.12 | 1.04 ± 0.08 | 1.14 ± 0.12 | 1.07 ± 0.11 | 0.205 |
| Methionine | 0.19 ± 0.02 | 0.19 ± 0.02 | 0.20 ± 0.02 | 0.20 ± 0.02 | 0.19 ± 0.01 | 0.976 |
| Phenylalanine | 0.30 ± 0.03 | 0.29 ± 0.03 | 0.30 ± 0.01 | 0.28 ± 0.03 | 0.26 ± 0.03 | 0.089 |
| Proline | 0.48 ± 0.04 | 0.43 ± 0.04 | 0.48 ± 0.09 | 0.48 ± 0.06 | 0.44 ± 0.02 | 0.165 |
| Serine | 0.49 ± 0.06 | 0.46 ± 0.05 | 0.46 ± 0.05 | 0.45 ± 0.04 | 0.48 ± 0.07 | 0.603 |
| Taurine | 0.83 ± 0.11 | 0.77 ± 0.13 | 0.73 ± 0.13 | 0.79 ± 0.10 | 0.83 ± 0.06 | 0.452 |
| Threonine | 0.42 ± 0.09 | 0.37 ± 0.05 | 0.38 ± 0.08 | 0.36 ± 0.08 | 0.34 ± 0.05 | 0.250 |
| Tyrosine | 0.29 ± 0.06 | 0.26 ± 0.05 | 0.27 ± 0.06 | 0.27 ± 0.05 | 0.26 ± 0.04 | 0.938 |
| Valine | 0.62 ± 0.08 | 0.55 ± 0.04 | 0.57 ± 0.06 | 0.53 ± 0.05 | 0.54 ± 0.07 | 0.078 |
| Total tryptophan | 0.009 ± 0.002 | 0.010 ± 0.003 | 0.013 ± 0.005 | 0.007 ± 0.005 | 0.006 ± 0.005 * | 0.022 |
| Free tryptophan | 0.003 ± 0.002 | 0.005 ± 0.003 | 0.002 ± 0.001 | 0.003 ± 0.001 | 0.004 ± 0.002 | 0.097 |
| Free tryptophan/total tryptophan ratio | 0.51 ± 0.32 | 0.65 ± 0.14 | 0.32 ± 0.22 | 0.30 ± 0.14 | 1.64 ± 1.38 † | 0.017 |
| Free tryptophan/BCAA ratio | 0.001 ± 0.001 | 0.003 ± 0.002 | 0.001 ± 0.001 | 0.002 ± 0.001 | 0.003 ± 0.002 | 0.147 |
| Glycemia (mg/dL) | CON | ALA | G + A | DIP | p |
|---|---|---|---|---|---|
| Pre-training 25% of BW | 112.0 ± 11.68 | 111.9 ± 6.52 | 118.1 ± 9.00 | 113.0 ± 18.86 | 0.715 |
| Post-training 25% of BW | 120.4 ± 15.84 | 122.9 ± 9.52 | 130.0 ± 14.97 | 128.8 ± 13.52 | 0.473 |
| Pre-training 50% of BW | 83.0 ± 5.68 | 80.4 ± 3.19 | 85.8 ± 4.35 | 85.9 ± 5.06 | 0.097 |
| Post-training 50% of BW | 97.7 ± 9.26 * | 94.7 ± 4.51 * | 99.8 ± 14.05 * | 97.5 ± 7.87 * | 0.791 |
| Pre-training 75% of BW | 95.8 ± 5.58 | 93.3 ± 12.94 | 97.5 ± 5.91 | 94.1 ± 5.14 | 0.729 |
| Post-training 75% of BW | 115.6 ± 16.24 * | 106.6 ± 20.34 * | 118.2 ± 11.44 * | 118.2 ± 8.83 * | 0.416 |
| Pre-training 100% of BW | 89.2 ± 11.70 | 89.7 ± 2.67 | 97.7 ± 4.51 | 91.0 ± 8.07 | 0.132 |
| Post-training 100% of BW | 109.9 ± 12.70 * | 109.4 ± 9.97 * | 117.3 ± 12.99 * | 115.7 ± 9.92 * | 0.448 |
| One hour after the last training | 146.4 ± 25.13 | 145.5 ± 13.11 | 152.7 ± 18.43 | 155.6 ± 16.55 | 0.791 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coqueiro, A.Y.; Raizel, R.; Bonvini, A.; Hypólito, T.; Godois, A.D.M.; Pereira, J.R.R.; Garcia, A.B.d.O.; Lara, R.D.S.B.; Rogero, M.M.; Tirapegui, J. Effects of Glutamine and Alanine Supplementation on Central Fatigue Markers in Rats Submitted to Resistance Training. Nutrients 2018, 10, 119. https://doi.org/10.3390/nu10020119
Coqueiro AY, Raizel R, Bonvini A, Hypólito T, Godois ADM, Pereira JRR, Garcia ABdO, Lara RDSB, Rogero MM, Tirapegui J. Effects of Glutamine and Alanine Supplementation on Central Fatigue Markers in Rats Submitted to Resistance Training. Nutrients. 2018; 10(2):119. https://doi.org/10.3390/nu10020119
Chicago/Turabian StyleCoqueiro, Audrey Yule, Raquel Raizel, Andrea Bonvini, Thaís Hypólito, Allan Da Mata Godois, Jéssica Ramos Rocha Pereira, Amanda Beatriz de Oliveira Garcia, Rafael De Souza Bittencourt Lara, Marcelo Macedo Rogero, and Julio Tirapegui. 2018. "Effects of Glutamine and Alanine Supplementation on Central Fatigue Markers in Rats Submitted to Resistance Training" Nutrients 10, no. 2: 119. https://doi.org/10.3390/nu10020119





