Suppression of Fatty Acid and Triglyceride Synthesis by the Flavonoid Orientin through Decrease of C/EBPδ Expression and Inhibition of PI3K/Akt-FOXO1 Signaling in Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Purification of Orientin from C. communis
2.3. Cell Culture
2.4. Cytotoxicity Assay
2.5. Measurement of Intracellular TG Level
2.6. Quantification of mRNA Level by Quantitative PCR
2.7. Western Blot Analysis
2.8. Lipolysis Assay
2.9. Cell Proliferation Assay
2.10. Chromatin Immunoprecipitation Assay
2.11. Statistical Analysis
3. Results
3.1. Reduction of Intracellular TG Levels by Orientin
3.2. Change in mRNA Level of Adipogenic, Lipogenic, and Lipolytic Genes by Orientin
3.3. Change in Expression Level of TG Synthetic Enzyme Genes by Orientin
3.4. Decrease in Expression Level of C/ebpδ Gene by Orientin in Early Stage of Adipogenesis
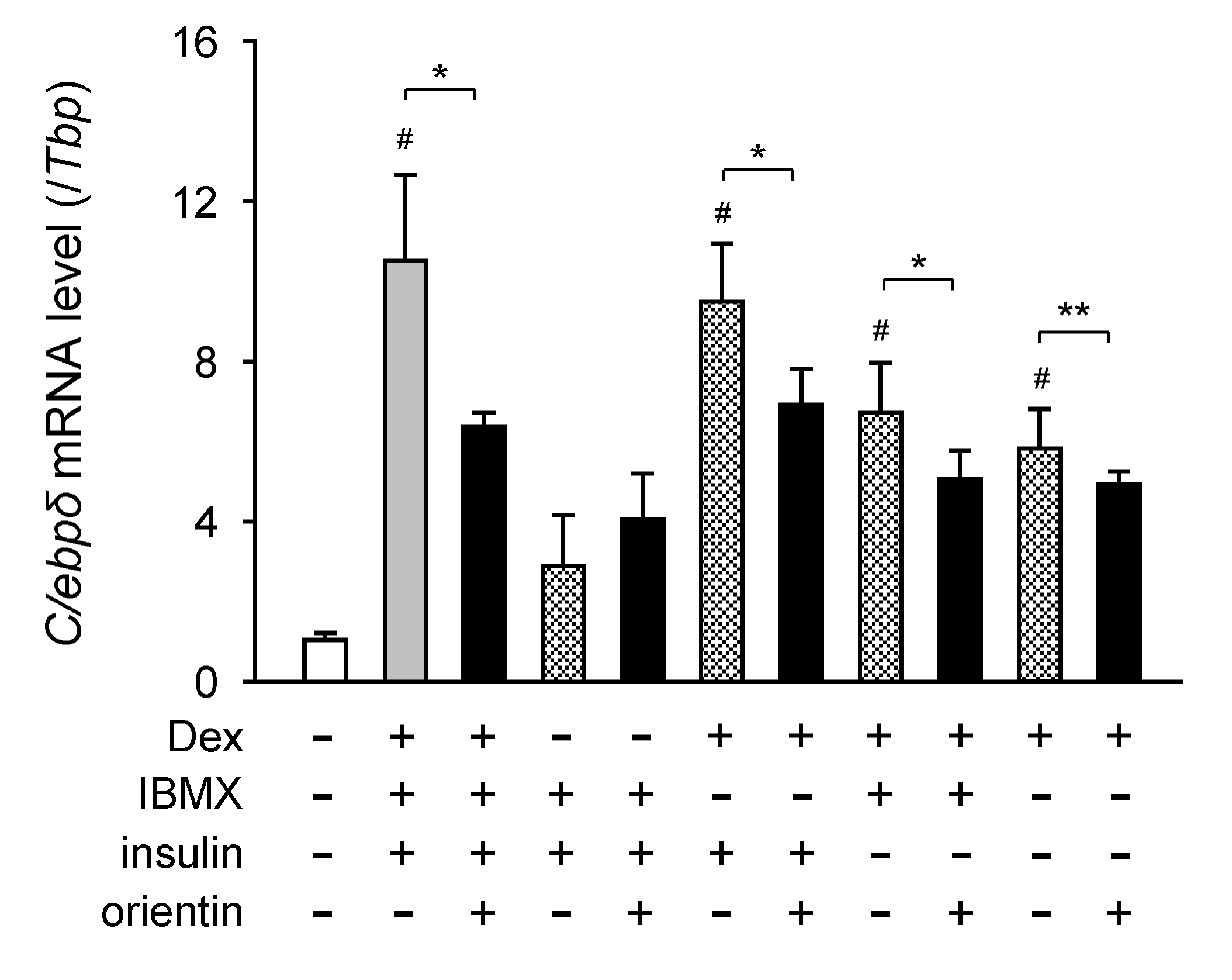
3.5. Repression of Dex-Mediated Activation of C/ebpδ Expression by Orientin
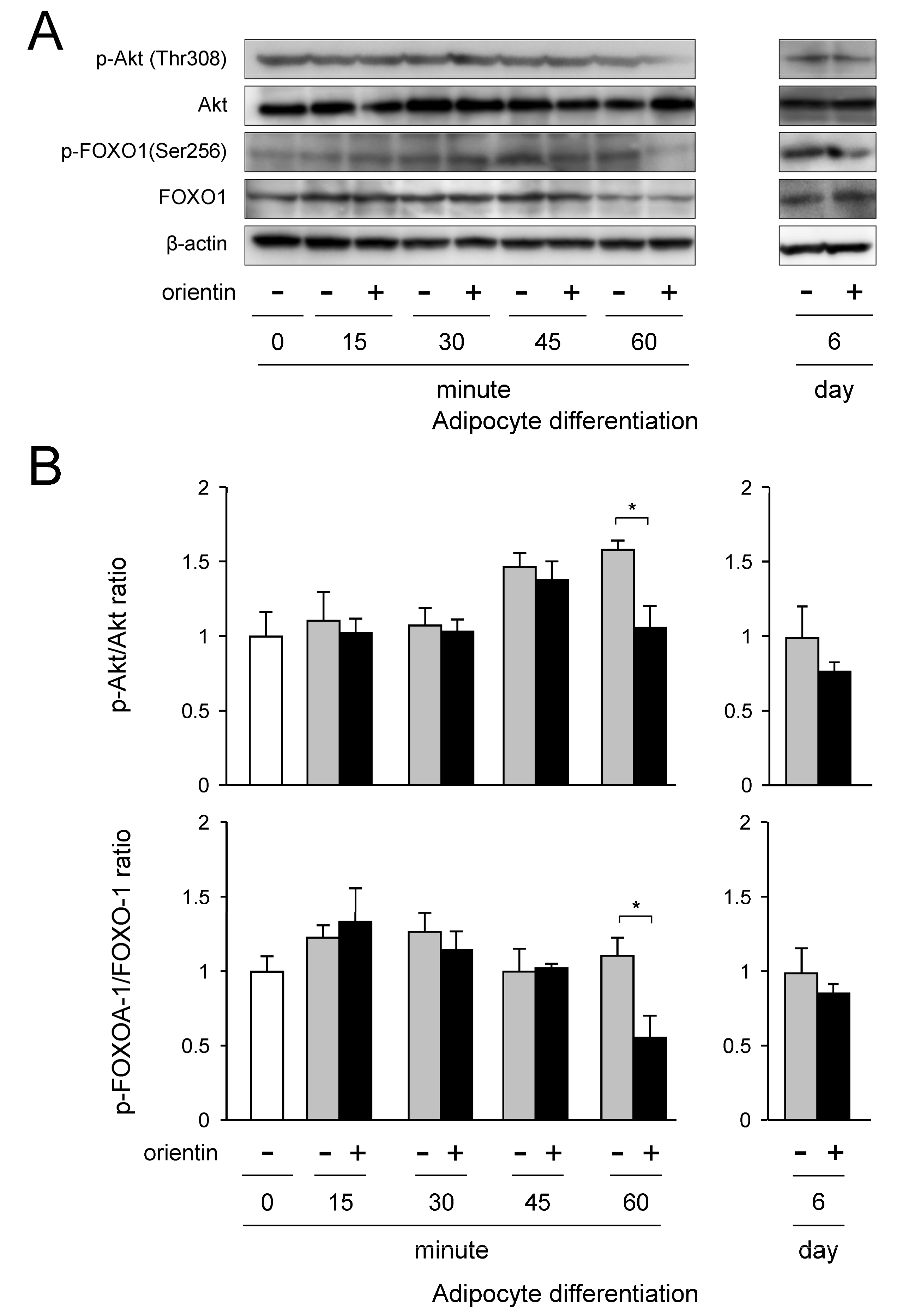
3.6. Repression of Activation of PI3K/Akt-FOXO1 Signaling by Orientin
3.7. Stage-Specific Repression of Adipogenesis by Orientin
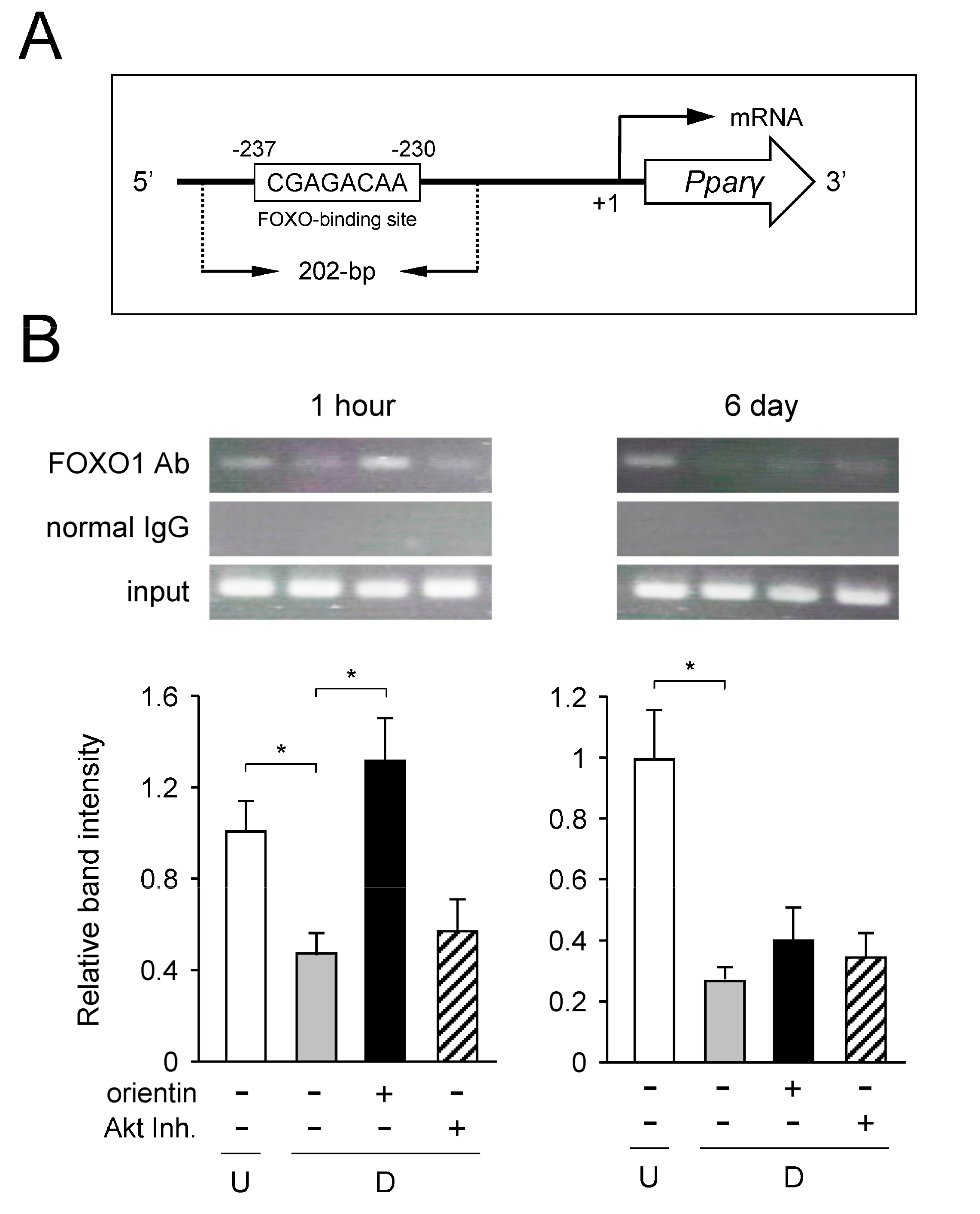
3.8. Decrease of Binding of FOXO1 to Pparγ Promoter by Orientin
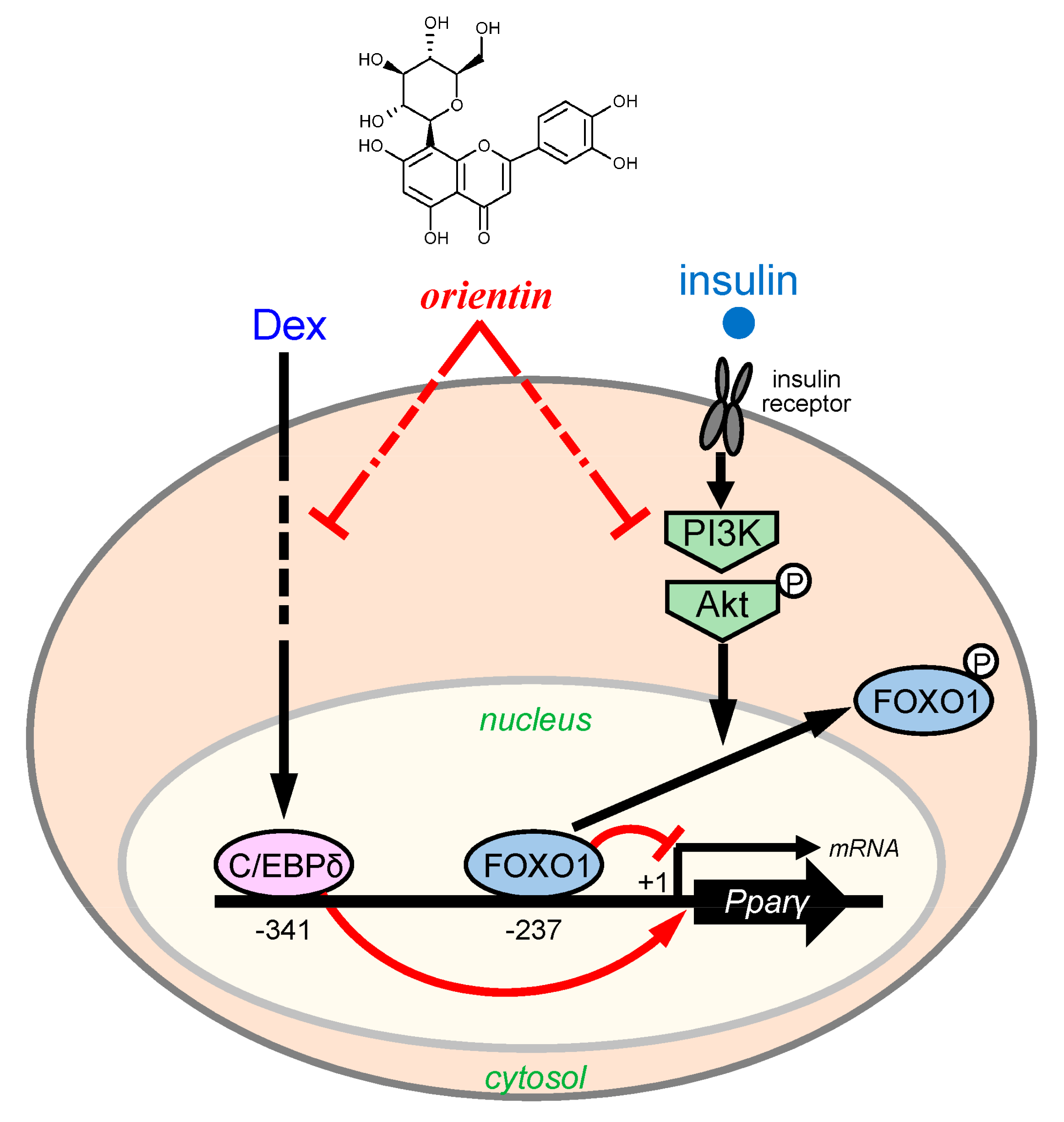
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef]
- Attie, A.D.; Scherer, P.E. Adipocyte metabolism and obesity. J. Lipid Res. 2009, 50, S395–S399. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y. The metabolic syndrome and adipocytokines. FEBS Lett. 2006, 580, 2917–2921. [Google Scholar] [CrossRef] [PubMed]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPα induces adipogenesis through PPARγ: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar] [CrossRef]
- Shibano, M.; Kakutani, K.; Taniguchi, M.; Yasuda, M.; Baba, K. Antioxidant constituents in the dayflower (Commelina communis L.) and their α-glucosidase-inhibitory activity. J. Nat. Med. 2008, 62, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.B.; Tian, L.Q.; Li, Y.M.; Liao, Y.F.; Li, J.; Bing, F.H. Protective effect of homonojirimycin from Commelina communis (dayflower) on influenza virus infection in mice. Phytomedicine 2013, 20, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Wakai, E.; Shibano, M.; Fujimori, K. Anti-obesity effects of Asian dayflower, Commelina communis, in mice with high-fat diet-induced obesity and in 3T3-L1 cells. J. Funct. Foods 2016, 22, 490–503. [Google Scholar] [CrossRef]
- Lam, K.Y.; Ling, A.P.; Koh, R.Y.; Wong, Y.P.; Say, Y.H. A review on medicinal properties of orientin. Adv. Pharmacol. Sci. 2016, 2016, 4104595. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, I.; Seo, J.; Jung, M.; Kim, Y.; Yim, N.; Bae, K. Vitexin, orientin and other flavonoids from Spirodela polyrhiza inhibit adipogenesis in 3T3-L1 cells. Phytother. Res. 2010, 24, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Ueno, Y.; Kikuchi, T.; Tanaka, R.; Fujimori, K. A limonoid Kihadanin B from immature Citrus unshiu peels suppresses adipogenesis through repression of the Akt-FOXO1-PPARγ axis in adipocytes. J. Agric. Food Chem. 2016, 64, 9607–9615. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Wang, Y.; Sul, H.S. Lipolysis in adipocytes. Int. J. Biochem. Cell. Biol. 2010, 42, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Fujimori, K. Very long-chain-fatty acids enhance adipogenesis through coregulation of Elovl3 and PPARγ in 3T3-L1 cells. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1461–E1471. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Reue, K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1195–E1209. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Umek, R.M.; McKnight, S.L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991, 5, 1538–1552. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, R.W.; Elliott, B.T. Akt/PKB activation and insulin signaling: A novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab. Syndr. Obes. 2014, 7, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Y.; Graves, D.T. FOXO transcription factors: Their clinical significance and regulation. Biomed. Res. Int. 2014, 2014, 925350. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer 2009, 9, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.W.; Wiviott, S.D. Modern obesity pharmacotherapy: Weighing cardiovascular risk and benefit. Clin. Cardiol. 2014, 37, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.O.; Horvath, T.L. Limitations in anti-obesity drug development: The critical role of hunger-promoting neurons. Nat. Rev. Drug Discov. 2012, 11, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [PubMed]
- Nakachi, Y.; Yagi, K.; Nikaido, I.; Bono, H.; Tonouchi, M.; Schonbach, C.; Okazaki, Y. Identification of novel PPARgamma target genes by integrated analysis of ChIP-on-chip and microarray expression data during adipocyte differentiation. Biochem. Biophys. Res. Commun. 2008, 372, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.A.; Haas, J.T.; Streeper, R.S.; Stone, S.J.; Kumari, M.; Yang, K.; Han, X.; Brownell, N.; Gross, R.W.; Zechner, R.; et al. DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J. Lipid Res. 2011, 52, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.L.; Stone, S.J.; Koliwad, S.; Harris, C.; Farese, R.V., Jr. Thematic review series: Glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 2008, 49, 2283–2301. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, J.L.; Li, D.; Tobin, J.F.; Gimeno, R.E. Molecular identification of microsomal acyl-CoA:glycerol-3-phosphate acyltransferase, a key enzyme in de novo triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 19695–19700. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.L.; Robinson, C.E.; Gimble, J.M. CAAT/enhancer binding proteins directly modulate transcription from the peroxisome proliferator-activated receptor gamma 2 promoter. Biochem. Biophys. Res. Commun. 1997, 240, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Darlington, G.J.; Ross, S.E.; MacDougald, O.A. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 1998, 273, 30057–30060. [Google Scholar] [CrossRef] [PubMed]
- Hishida, T.; Nishizuka, M.; Osada, S.; Imagawa, M. The role of C/EBPδ in the early stages of adipogenesis. Biochimie 2009, 91, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Han, M.; Ting, H.L.; Liu, Z.; Zhang, D. Scutellarin from Scutellaria baicalensis suppresses adipogenesis by upregulating PPARα in 3T3-L1 cells. J. Nat. Prod. 2013, 76, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Pessin, J.E. An adipocentric view of signaling and intracellular trafficking. Diabetes Metab. Res. Rev. 2002, 18, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, I.; Ullrich, A. Endothelin-1 inhibits adipogenesis: Role of phosphorylation of Akt and ERK1/2. FEBS Lett. 2006, 580, 5765–5771. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.W.; Brosens, J.J.; Gomes, A.R.; Koo, C.Y. Forkhead box proteins: Tuning forks for transcriptional harmony. Nat. Rev. Cancer 2013, 13, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Nakae, J.; Barr, V.; Accili, D. Differential regulation of gene expression by insulin and IGF-1 receptors correlates with phosphorylation of a single amino acid residue in the forkhead transcription factor FKHR. EMBO J. 2000, 19, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Nakae, J.; Kitamura, T.; Kitamura, Y.; Biggs, W.H., 3rd; Arden, K.C.; Accili, D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev. Cell 2003, 4, 119–129. [Google Scholar] [CrossRef]
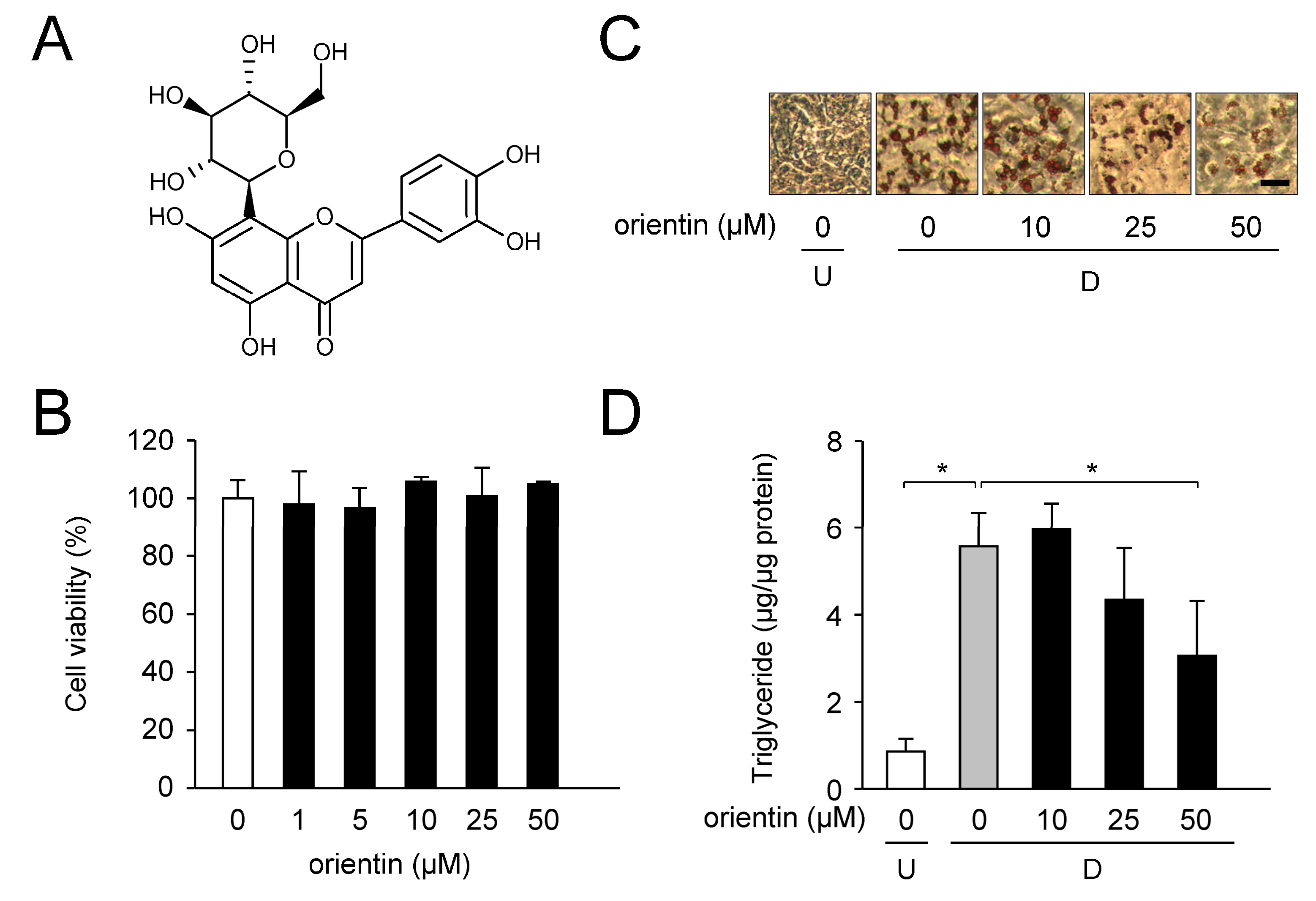
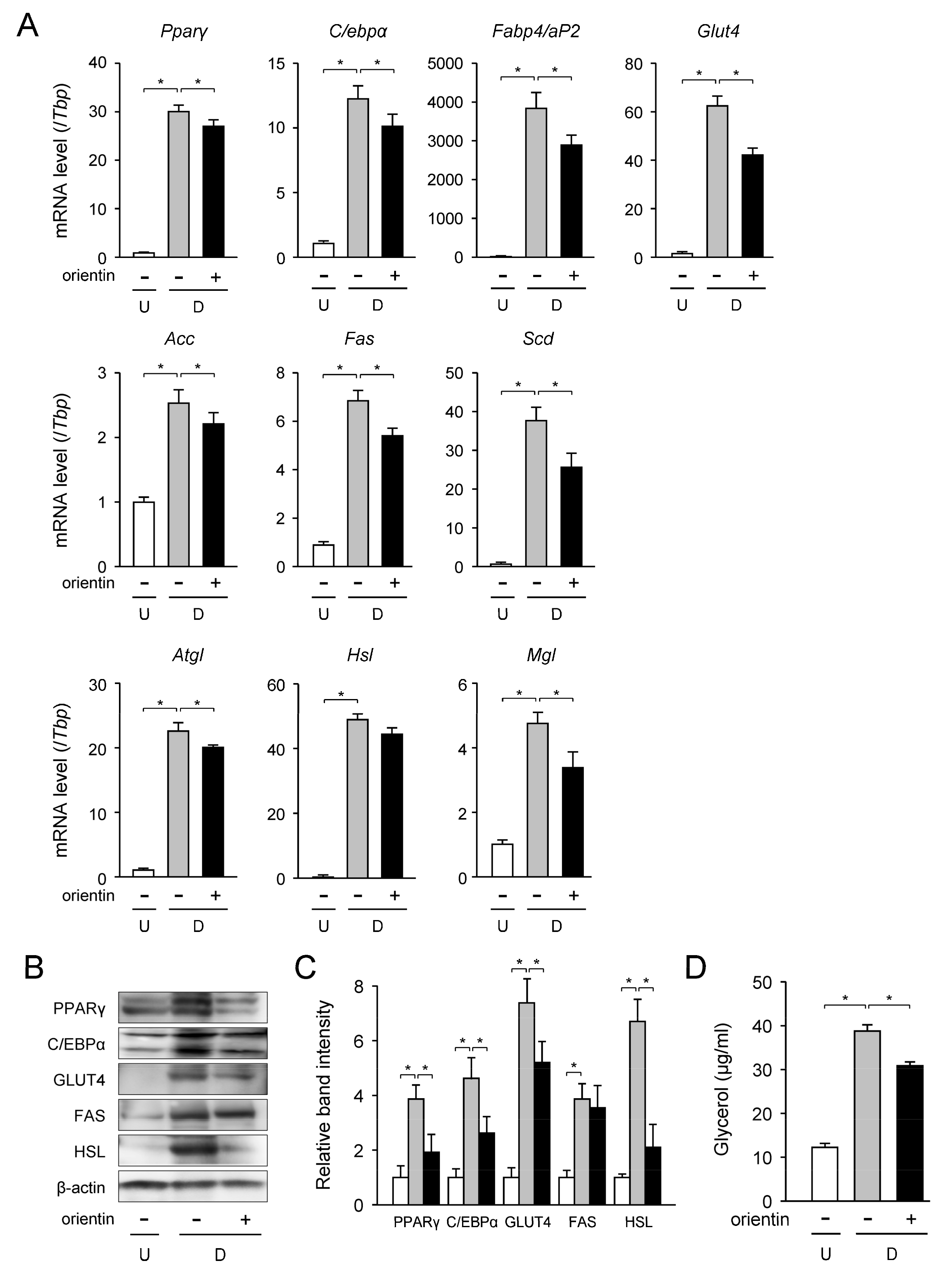
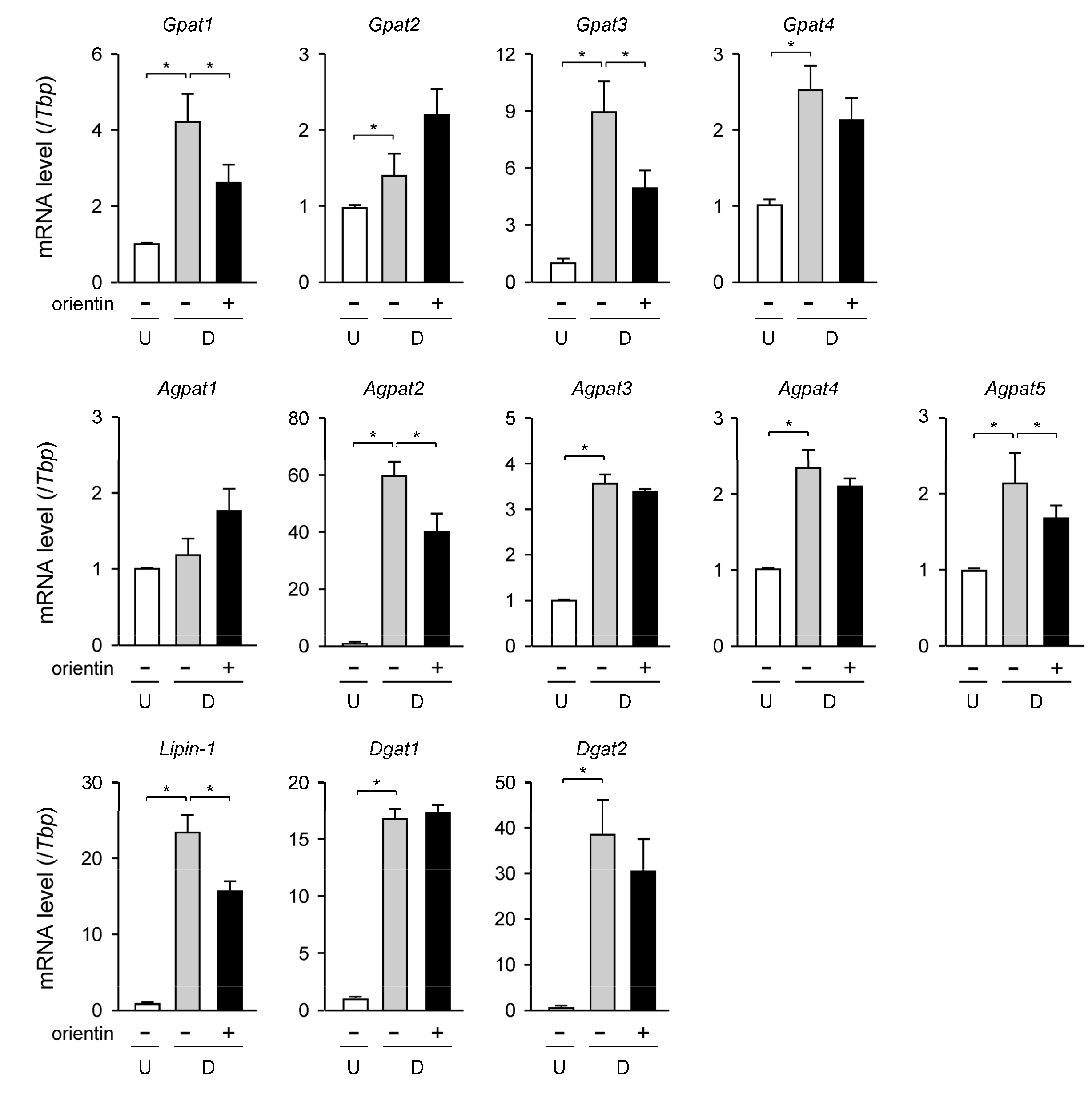
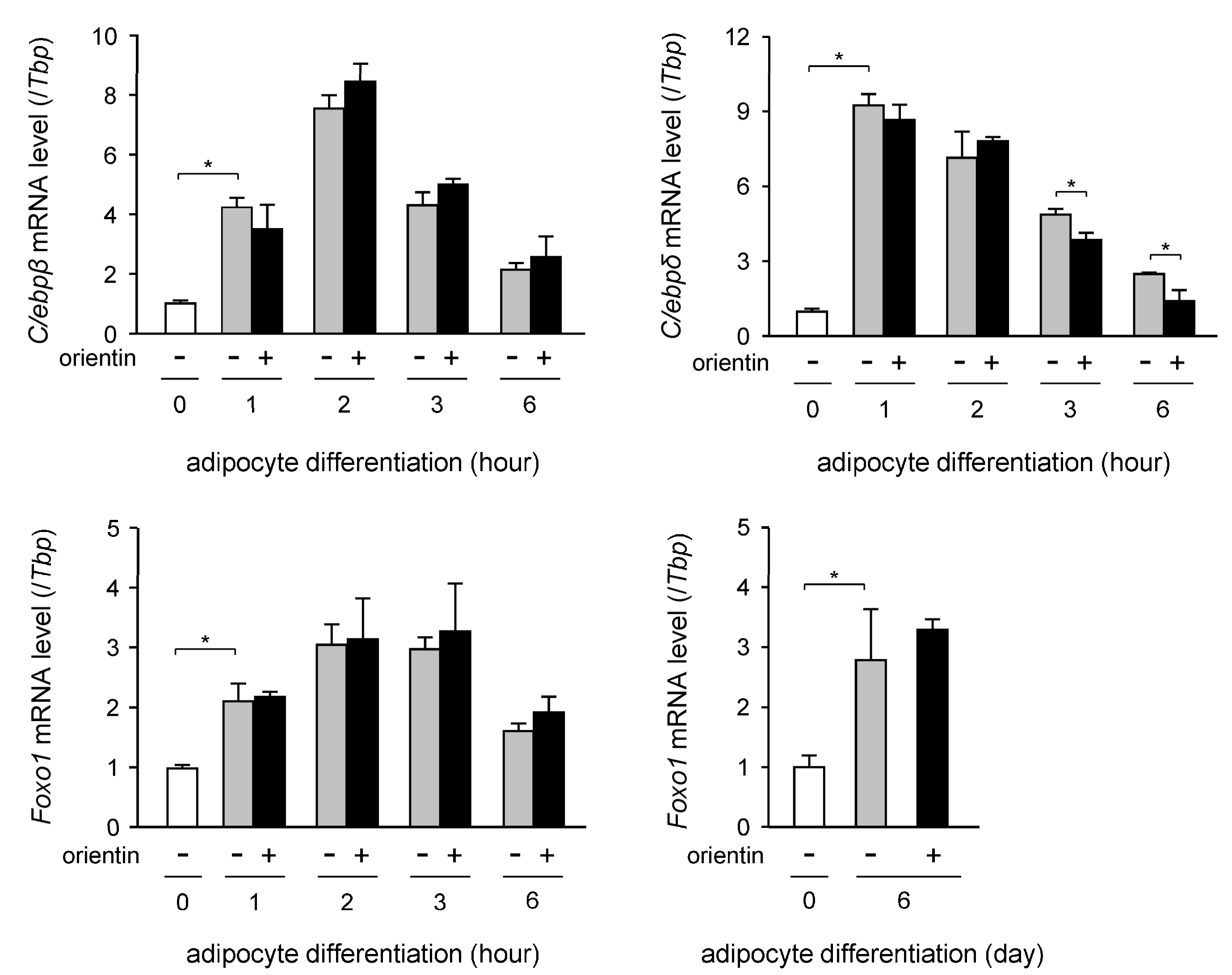

| Gene | Accession No. * | Forward Primer | Reverse Primer |
|---|---|---|---|
| Pparγ | NM_011146 | 5′-CAAGAATACCAAAGTGCGATCAA-3′ | 5′-GAGCTGGGTCTTTTCAGAATAATAAG-3′ |
| C/Eebpα | NM_007678 | 5′-CTGGAAAGAAGGCCACCTC-3′ | 5′-AAGAGAAGGAAGCGGTCCA-3′ |
| C/ebpβ | NM_009883 | 5′-TGATGCAATCCGGATCAA-3′ | 5′-CACGTGTGTTGCGTCAGTC-3′ |
| C/ebpδ | NM_007679 | 5′-GGGCAGTGGAGTAAGGTACAGA-3′ | 5′-GCACTGTCACCCATACAATGTT-3′ |
| Fabp4(aP2) | NM_024406 | 5′-CAGCCTTTCTCACCTGGAAG-3′ | 5′-TTGTGGCAAAGCCCACTC-3′ |
| Glut4 | NM_009204 | 5′-GACGGACACTCCATCTGTTG-3′ | 5′-GCCACGATGGAGACATAGC-3′ |
| Acc | NM_133360 | 5′-GCGTCGGGTAGATCCAGTT-3′ | 5′-CTCAGTGGGGCTTAGCTCTG-3′ |
| Fas | NM_007988 | 5′-GTTGGGGGTGTCTTCAACC-3′ | 5′-GAAGAGCTCTGGGGTCTGG-3′ |
| Scd | NM_009127 | 5′-CGTCTGGAGGAACATCATTCT-3′ | 5′-CAGAGCGCTGGTCATGTAGT-3′ |
| Atgl | NM_001163689 | 5′-TGACCATCTGCCTTCCAGA-3′ | 5′-TGTAGGTGGCGCAAGACA-3′ |
| Hsl | NM_010719 | 5′-GCACTGTGACCTGCTTGGT-3′ | 5′-CTGGCACCCTCACTCCATA-3′ |
| Mgl | NM_011844 | 5′-TCGGAACAAGTCGGAGGT-3′ | 5′-TCAGCAGCTGTATGCCAAAG-3′ |
| Gpat1 | NM_008149 | 5′-GGAAGGTGCTGCTATTCCTG-3′ | 5′-TGGGATACTGGGGTTGAAAA-3′ |
| Gpat2 | NM_001081089 | 5′-GCTGCCAGACCTGTACTCCT-3′ | 5′-AGCCCAGGTCCATTATGCTT-3′ |
| Gpat3 | NM_172715 | 5′-GTGCTGGGTGTCCTAGTGC-3′ | 5′-AAGCTGATCCCAATGAAAGC-3′ |
| Gpat4 | NM_018743 | 5′-GAGTGCTGATTCGGTATTGCT-3′ | 5′-CACTACCAAGAGGCCAATCC-3′ |
| Agpat1 | NM_018862 | 5′-CTGTCTGTGGAAGCACCTTG-3′ | 5′-GCAGAACCACAGGGTGGA-3′ |
| Agpat2 | NM_026212 | 5′-AAGACGAAGCTCTTCACCTCA-3′ | 5′-TCTGTCAGACCATTGGTAGGG-3′ |
| Agpat3 | NM_053014 | 5′-CTGCCCCCACTCAAGTACC-3′ | 5′-TCAGGGTCACGTCATAGATAGC-3′ |
| Agpat4 | NM_026644 | 5′-ACGCTGACTGCTACGTTCG-3′ | 5′-TGTGTAACCAGGCAGAGCAC-3′ |
| Agpat5 | NM_026792 | 5′-CTAGCGAATCATCAAAGCACA-3′ | 5′-TCTTTCAGTACGTAGCGCACA-3′ |
| lipin-1 | NM_015763 | 5′-TCCCAGTTCGGACAGAGAAT-3′ | 5′-GGGAGTCCTCTGGCAATCTA-3′ |
| Dgat1 | NM_010046 | 5′-GCCCCATGCGTGATTATT-3′ | 5′-TCTGTCAGGGCACCCACT-3′ |
| Dgat2 | NM_026384 | 5′-GGCGCTACTTCCGAGACTAC-3′ | 5′-TGGTCAGCAGGTTGTGTGTC-3′ |
| Foxo1 | NM_019739 | 5′-CTTCAAGGATAAGGGCGACA-3′ | 5′-GACAGATTGTGGCGAATTGA-3′ |
| Tbp | NM_013684 | 5′-GTGATGTGAAGTTCCCCATAAGG-3′ | 5′-CTACTGAACTGCTGGTGGGTCA-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, S.; Matsumoto, C.; Shibano, M.; Fujimori, K. Suppression of Fatty Acid and Triglyceride Synthesis by the Flavonoid Orientin through Decrease of C/EBPδ Expression and Inhibition of PI3K/Akt-FOXO1 Signaling in Adipocytes. Nutrients 2018, 10, 130. https://doi.org/10.3390/nu10020130
Nagai S, Matsumoto C, Shibano M, Fujimori K. Suppression of Fatty Acid and Triglyceride Synthesis by the Flavonoid Orientin through Decrease of C/EBPδ Expression and Inhibition of PI3K/Akt-FOXO1 Signaling in Adipocytes. Nutrients. 2018; 10(2):130. https://doi.org/10.3390/nu10020130
Chicago/Turabian StyleNagai, Shiori, Chihiro Matsumoto, Makio Shibano, and Ko Fujimori. 2018. "Suppression of Fatty Acid and Triglyceride Synthesis by the Flavonoid Orientin through Decrease of C/EBPδ Expression and Inhibition of PI3K/Akt-FOXO1 Signaling in Adipocytes" Nutrients 10, no. 2: 130. https://doi.org/10.3390/nu10020130
APA StyleNagai, S., Matsumoto, C., Shibano, M., & Fujimori, K. (2018). Suppression of Fatty Acid and Triglyceride Synthesis by the Flavonoid Orientin through Decrease of C/EBPδ Expression and Inhibition of PI3K/Akt-FOXO1 Signaling in Adipocytes. Nutrients, 10(2), 130. https://doi.org/10.3390/nu10020130





