Bone Response to Dietary Co-Enrichment with Powdered Whole Grape and Probiotics
Abstract
:1. Introduction
2. Methods
2.1. Experimental Design
2.2. Diets
2.3. Micro-Computed Tomography (µCT) Scanning of Bones
2.4. Data Analysis
3. Results
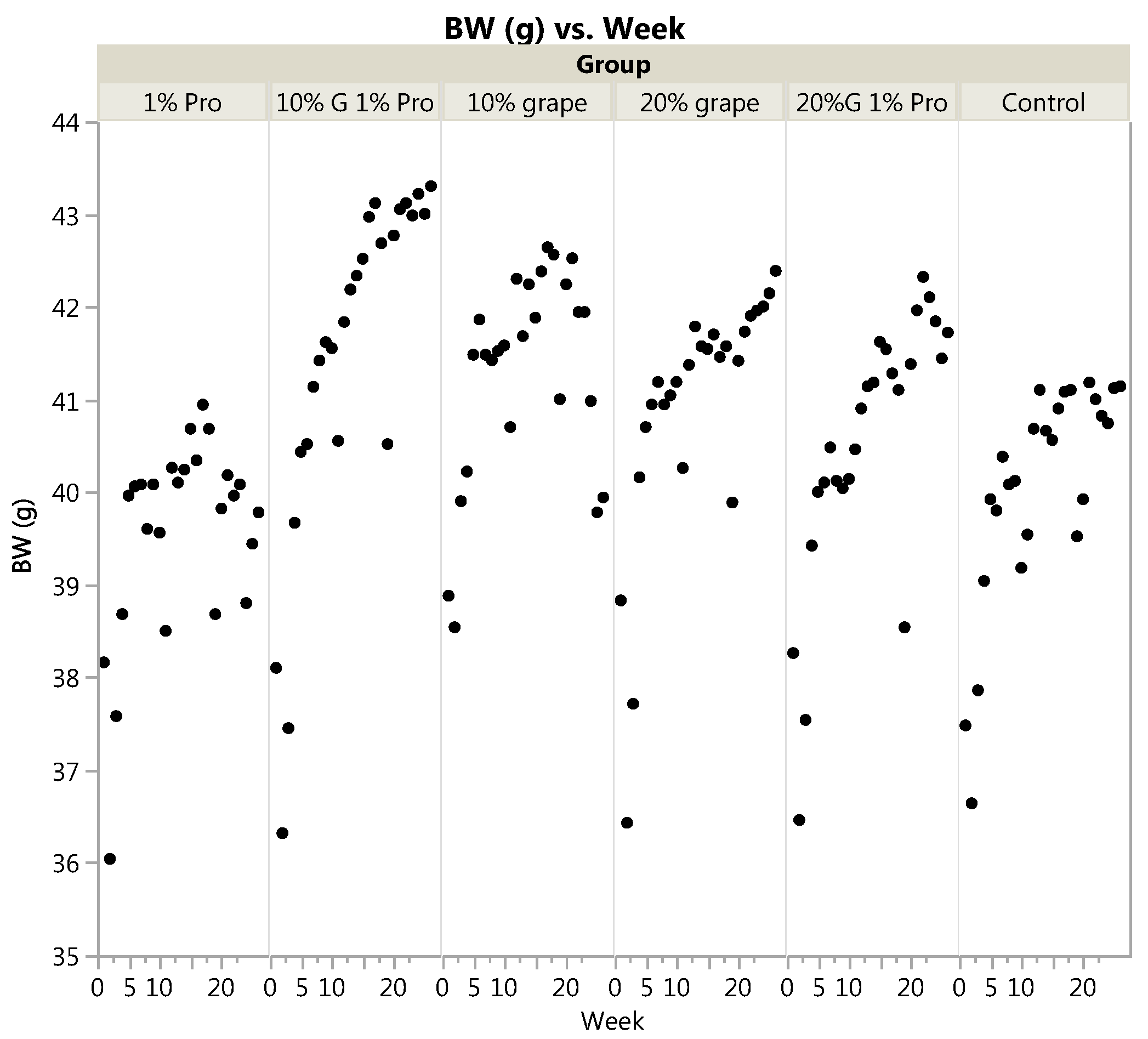
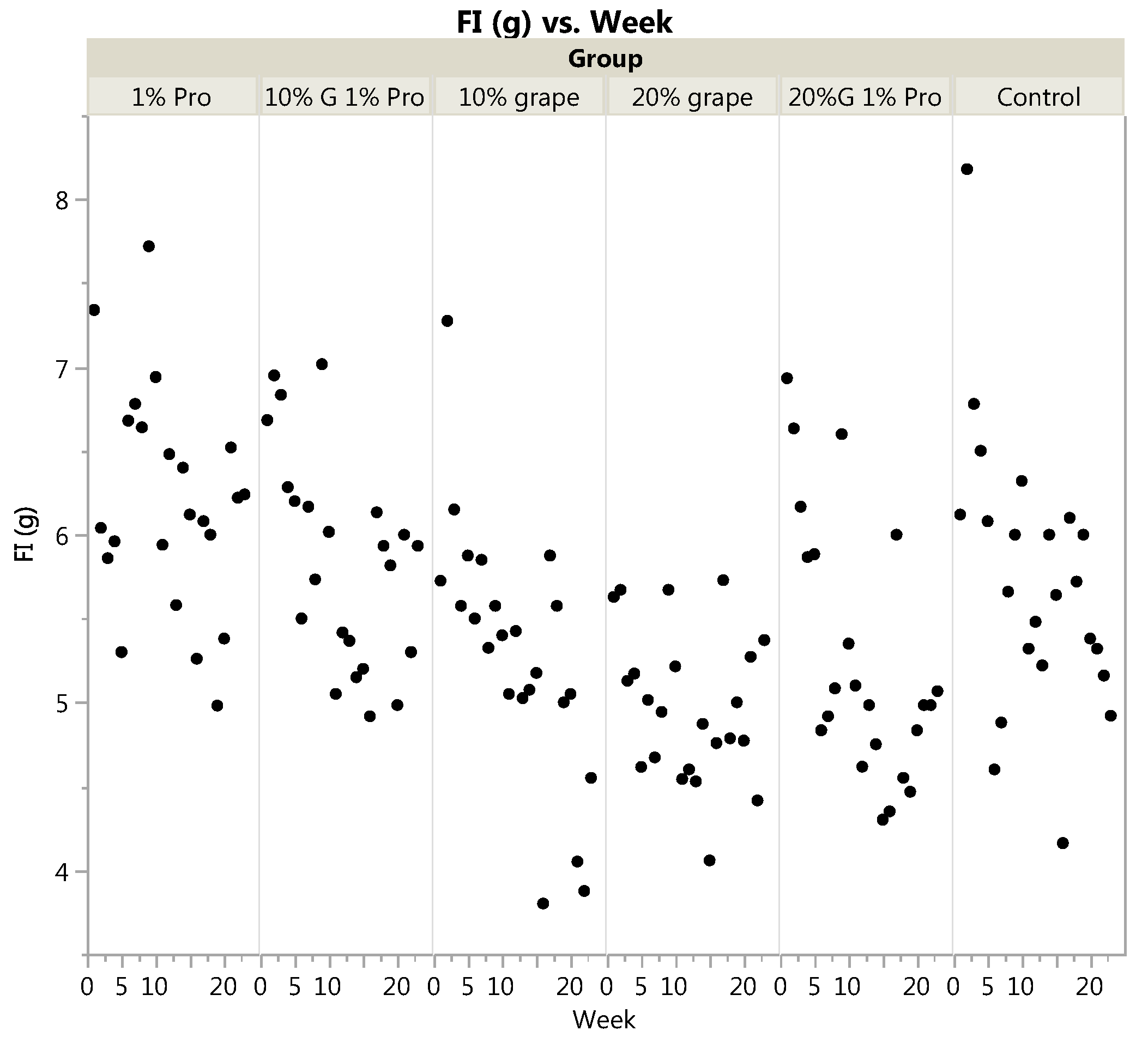
3.1. Body Weight and Food Intake
3.2. Bone Microarchitecture
3.2.1. Tibia
3.2.2. Femur
3.2.3. Vertebrae
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Looker, A.C.; Frenk, S.M. Percentage of Adults Aged 65 and over with Osteoporosis or Low Bone Mass at the Femur Neck or Lumbar Spine: United States, 2005–2010; U.S. Centers for Disease Control and Prevention Health E-Stat; National Center for Health Statistics: Hyattsville, MD, USA, 2015.
- Balasubramanian, A.; Tosi, L.L.; Lane, J.M.; Dirschl, D.R.; Ho, P.R.; O’Malley, C.D. Declining rates of osteoporosis management following fragility fractures in the U.S., 2000 through 2009. J. Bone Jt. Surg. Am. 2014, 96, e52. [Google Scholar] [CrossRef] [PubMed]
- Gehlbach, S.H.; Fournier, M.; Bigelow, C. Recognition of osteoporosis by primary care physicians. Am. J. Public Health 2002, 92, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Weaver, J.; de Papp, A.; Li, Z.; Martin, J.; Allen, K.; Hui, S.; Imel, E.A. Disparities in osteoporosis treatments. Osteoporos. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kroth, P.J.; Murray, M.D.; McDonald, C.J. Undertreatment of osteoporosis in women, based on detection of vertebral compression fractures on chest radiography. Am. J. Geriatr. Pharmacother. 2004, 2, 112–118. [Google Scholar] [CrossRef]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Huang, Z.W.; Wang, R.Q.; Ma, X.M.; Zhang, Z.Q.; Liu, Z.; Chen, Y.M.; Su, Y.X. Fruit and vegetable intake and bone mass in Chinese adolescents, young and postmenopausal women. Public Health Nutr. 2013, 16, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Cao, W.T.; Tian, H.Y.; He, J.; Chen, G.D.; Chen, Y.M. Greater Intake of Fruit and Vegetables is Associated with Greater Bone Mineral Density and Lower Osteoporosis Risk in Middle-Aged and Elderly Adults. PLoS ONE 2017, 12, e0168906. [Google Scholar] [CrossRef] [PubMed]
- Benetou, V.; Orfanos, P.; Feskanich, D.; Michaelsson, K.; Pettersson-Kymmer, U.; Eriksson, S.; Grodstein, F.; Wolk, A.; Bellavia, A.; Ahmed, L.A.; et al. Fruit and Vegetable Intake and Hip Fracture Incidence in Older Men and Women: The CHANCES Project. J. Bone Miner. Res. 2016, 31, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Byberg, L.; Bellavia, A.; Orsini, N.; Wolk, A.; Michaelsson, K. Fruit and vegetable intake and risk of hip fracture: A cohort study of Swedish men and women. J. Bone Miner. Res. 2015, 30, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Ho, S.C.; Woo, J.L.F. Greater fruit and vegetable intake is associated with increased bone mass among postmenopausal Chinese women. Br. J. Nutr. 2006, 96, 745–751. [Google Scholar] [PubMed]
- Lanham-New, S.A. Fruit and vegetables: The unexpected natural answer to the question of osteoporosis prevention? Am. J. Clin. Nutr. 2006, 83, 1254–1255. [Google Scholar] [PubMed]
- Liu, Z.M.; Leung, J.; Wong, S.Y.S.; Wong, C.K.M.; Chan, R.; Woo, J. Greater Fruit Intake was Associated with Better Bone Mineral Status among Chinese Elderly Men and Women: Results of Hong Kong Mr. Os and Ms. Os Studies. J. Am. Med. Dir. Assoc. 2015, 16, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Prynne, C.J.; Mishra, G.D.; O’Connell, M.A.; Muniz, G.; Laskey, M.A.; Yan, L.; Prentice, A.; Ginty, F. Fruit and vegetable intakes and bone mineral status: A cross sectional study in 5 age and sex cohorts. Am. J. Clin. Nutr. 2006, 83, 1420–1428. [Google Scholar] [PubMed]
- Tucker, K.L.; Chen, H.; Hannan, M.T.; Cupples, L.A.; Wilson, P.W.; Felson, D.; Kiel, D.P. Bone mineral density and dietary patterns in older adults: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2002, 76, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Hannan, M.T.; Chen, H.; Cupples, L.A.; Wilson, P.W.; Kiel, D.P. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am. J. Clin. Nutr. 1999, 69, 727–736. [Google Scholar] [PubMed]
- Welch, A.; MacGregor, A.; Jennings, A.; Fairweather-Tait, S.; Spector, T.; Cassidy, A. Habitual flavonoid intakes are positively associated with bone mineral density in women. J. Bone Miner. Res. 2012, 27, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; von Bergen, V.; Chyu, M.C.; Jenkins, M.R.; Mo, H.; Chen, C.H.; Kwun, I.S. Fruits and dietary phytochemicals in bone protection. Nutr. Res. 2012, 32, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Jung, J.W.; Ha, B.G.; Hong, J.M.; Park, E.K.; Kim, H.J.; Kim, S.Y. The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J. Nutr. Biochem. 2011, 22, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, S.; Suzuki, K.; Muramatsu, M.; Nomura, A.; Inoue, F.; Into, T.; Yoshiko, Y.; Niida, S. Delphinidin, one of the major anthocyanidins, prevents bone loss through the inhibition of excessive osteoclastogenesis in osteoporosis model mice. PLoS ONE 2014, 9, e97177. [Google Scholar] [CrossRef] [PubMed]
- Wattel, A.; Kamel, S.; Prouillet, C.; Petit, J.P.; Lorget, F.; Offord, E.; Brazier, M. Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NF kappa B and AP-1. J. Cell. Biochem. 2004, 92, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lazarenko, O.P.; Kang, J.; Blackburn, M.L.; Ronis, M.J.; Badger, T.M.; Chen, J.R. Feeding blueberry diets to young rats dose-dependently inhibits bone resorption through suppression of RANKL in stromal cells. PLoS ONE 2013, 8, e70438. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Lazarenko, O.P.; Wu, X.; Kang, J.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/beta-catenin canonical Wnt signaling. J. Bone Miner. Res. 2010, 25, 2399–2411. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Lazarenko, O.P.; Zhang, J.; Blackburn, M.L.; Ronis, M.J.; Badger, T.M. Diet-derived phenolic acids regulate osteoblast and adipocyte lineage commitment and differentiation in young mice. J. Bone Miner. Res. 2014, 29, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Chu, H.; Tang, Q.; Huang, H.; Hao, W.; Wei, X. Grape-seed proanthocyanidins inhibit the lipopolysaccharide-induced inflammatory mediator expression in RAW264.7 macrophages by suppressing MAPK and NF-kappab signal pathways. Environ. Toxicol. Pharmacol. 2015, 41, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Liang, H.L.; Hung, C.H.; Kuo, P.L. Syringetin, a flavonoid derivative in grape and wine, induces human osteoblast differentiation through bone morphogenetic protein-2/extracellular signal-regulated kinase 1/2 pathway. Mol. Nutr. Food Res. 2009, 53, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, L.; Zhao, Z.; Shi, J.; Wang, X.; Huang, J. Grape seed proanthocyanidins inhibit H2O2-induced osteoblastic MC3T3-E1 cell apoptosis via ameliorating H2O2-induced mitochondrial dysfunction. J. Toxicol. Sci. 2014, 39, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S. Pathogenesis of age-related bone loss in humans. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Lee, E.K.; Choi, Y.J.; Kim, J.M.; Kim, D.H.; Zou, Y.; Kim, C.H.; Lee, J.; Kim, H.S.; Kim, N.D.; et al. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J. Dent. Res. 2011, 90, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Schett, G. Pathways for bone loss in inflammatory disease. Curr. Osteoporos. Rep. 2012, 10, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ornstrup, M.J.; Harslof, T.; Kjaer, T.N.; Langdahl, B.L.; Pedersen, S.B. Resveratrol increases bone mineral density and bone alkaline phosphatase in obese men: A randomized placebo-controlled trial. J. Clin. Endocrinol. Metab. 2014, 99, 4720–4729. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; Wang, Z.; Xu, Z.; Zhang, Q.; Yin, M. Effects of dietary resveratrol on excess-iron-induced bone loss via antioxidative character. J. Nutr. Biochem. 2015, 26, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, K.; Ikeda, K.; Kawai, Y.; Yamori, Y. Resveratrol attenuates ovariectomy-induced hypertension and bone loss in stroke-prone spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2000, 46, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, G.; Poudel, S.B.; Kook, S.H.; Lee, J.C. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta Biomater. 2016, 29, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Habold, C.; Momken, I.; Ouadi, A.; Bekaert, V.; Brasse, D. Effect of prior treatment with resveratrol on density and structure of rat long bones under tail-suspension. J. Bone Miner. Metab. 2011, 29, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Hohman, E.E.; Weaver, C.M. A grape-enriched diet increases bone calcium retention and cortical bone properties in ovariectomized rats. J. Nutr. 2015, 145, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; Van de Wiele, T.; Possemiers, S. Gut metabotypes govern health effects of dietary polyphenols. Curr. Opin. Biotechnol. 2013, 24, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Dore, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4531–4538. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104 (Suppl. 3), S48–S66. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Cooney, J.C. Probiotic bacteria influence the composition and function of the intestinal microbiota. Interdiscip. Perspect. Infect. Dis. 2008, 2008, 175285. [Google Scholar] [CrossRef] [PubMed]
- Axling, U.; Olsson, C.; Xu, J.; Fernandez, C.; Larsson, S.; Strom, K.; Ahrne, S.; Holm, C.; Molin, G.; Berger, K. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr. Metab. 2012, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Jakesevic, M.; Aaby, K.; Borge, G.I.; Jeppsson, B.; Ahrne, S.; Molin, G. Antioxidative protection of dietary bilberry, chokeberry and Lactobacillus plantarum HEAL19 in mice subjected to intestinal oxidative stress by ischemia-reperfusion. BMC Complement. Altern. Med. 2011, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.L.; Lila, M.A.; Mawson, J.; De, S. In vitro and in vivo evaluation of the prebiotic activity of water-soluble blueberry extracts. World J. Microbiol. Biotechnol. 2009, 25, 1243–1249. [Google Scholar] [CrossRef]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef] [PubMed]
- Hakansson, A.; Branning, C.; Adawi, D.; Molin, G.; Nyman, M.; Jeppsson, B.; Ahrne, S. Blueberry husks, rye bran and multi-strain probiotics affect the severity of colitis induced by dextran sulphate sodium. Scand. J. Gastroenterol. 2009, 44, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Hakansson, A.; Stene, C.; Mihaescu, A.; Molin, G.; Ahrne, S.; Thorlacius, H.; Jeppsson, B. Rose hip and Lactobacillus plantarum DSM 9843 reduce ischemia/reperfusion injury in the mouse colon. Dig. Dis. Sci. 2006, 51, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Halloran, B.P.; Wronski, T.J.; VonHerzen, D.C.; Chu, V.; Xia, X.; Pingel, J.E.; Williams, A.A.; Smith, B.J. Dietary dried plum increases bone mass in adult and aged male mice. J. Nutr. 2010, 140, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Rendina, E.; Hembree, K.D.; Davis, M.R.; Marlow, D.; Clarke, S.L.; Halloran, B.P.; Lucas, E.A.; Smith, B.J. Dried plum’s unique capacity to reverse bone loss and alter bone metabolism in postmenopausal osteoporosis model. PLoS ONE 2013, 8, e60569. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Jakesevic, M.; Xu, J.; Aaby, K.; Jeppsson, B.; Ahrne, S.; Molin, G. Effects of bilberry (Vaccinium myrtillus) in combination with lactic acid bacteria on intestinal oxidative stress induced by ischemia-reperfusion in mouse. J. Agric. Food Chem. 2013, 61, 3468–3478. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Romanelli, L.; Palmery, M. Interactions between prebiotics, probiotics, polyunsaturated fatty acids and polyphenols: Diet or supplementation for metabolic syndrome prevention? Int. J. Food Sci. Nutr. 2014, 65, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Choman, M.; Blanton, C.; Gabaldón, A. The Effect of a Synbiotic Diet on Bone Structure in Aging Male Mice. FASEB J. 2015, 29, 738. [Google Scholar]
- Blanton, C.; He, Z.; Gottschall-Pass, K.T.; Sweeney, M.I. Probiotics blunt the anti-hypertensive effect of blueberry feeding in hypertensive rats without altering hippuric acid production. PLoS ONE 2015, 10, e0142036. [Google Scholar] [CrossRef] [PubMed]
- Blanton, C.A.; Gabaldon, A.M. Effect of dietary synbiotics on bone in mature male rats following recovery from hindlimb unloading. Int. J. Probiotics Prebiotics 2012, 7, 99–108. [Google Scholar]
- Smith, B.J.; Graef, J.L.; Wronski, T.J.; Rendina, E.; Williams, A.A.; Clark, K.A.; Clarke, S.L.; Lucas, E.A.; Halloran, B.P. Effects of dried plum supplementation on bone metabolism in adult C57BL/6 male mice. Calcif. Tissue Int. 2014, 94, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Bu, S.Y.; Wang, Y.; Rendina, E.; Lim, Y.F.; Marlow, D.; Clarke, S.L.; Cullen, D.M.; Lucas, E.A. A comparative study of the bone metabolic response to dried plum supplementation and PTH treatment in adult, osteopenic ovariectomized rat. Bone 2014, 58, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Glatt, V.; Canalis, E.; Stadmeyer, L.; Bouxsein, M.L. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J. Bone Miner. Res. 2007, 22, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Patan, F.; Barroso, E.; van de Wiele, T.; Jimenez-Giron, A.; Martin-Alvarez, P.J.; Moreno-Arribas, M.V.; Martinez-Cuesta, M.C.; Pelaez, C.; Requena, T.; Bartolome, B. Comparative in vitro fermentations of cranberry and grape seed polyphenols with colonic microbiota. Food Chem. 2015, 183, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Lala, G.; Malik, M.; Zhao, C.; He, J.; Kwon, Y.; Giusti, M.M.; Magnuson, B.A. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr. Cancer 2006, 54, 84–93. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Magnuson, B.A.; Lala, G.; Tian, Q.; Schwartz, S.J.; Giusti, M.M. Intact anthocyanins and metabolites in rat urine and plasma after 3 months of anthocyanin supplementation. Nutr. Cancer 2006, 54, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Waterhouse, A.L. Identification of Cabernet Sauvignon anthocyanin gut microflora metabolites. J. Agric. Food Chem. 2008, 56, 9299–9304. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between anthocyanins and gut microbiota. J. Agric. Food Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Patan, F.; Cueva, C.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Quintanilla-Lopez, J.E.; Lebron-Aguilar, R.; Martin-Alvarez, P.J.; Moreno-Arribas, M.V.; Bartolome, B. In vitro fermentation of a red wine extract by human gut microbiota: Changes in microbial groups and formation of phenolic metabolites. J. Agric. Food Chem. 2012, 60, 2136–2147. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W.; Chang, J.L. Interindividual differences in phytochemical metabolism and disposition. Semin. Cancer Biol. 2007, 17, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andres-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [PubMed]
- Zhang, Y.B.; Zhong, Z.M.; Hou, G.; Jiang, H.; Chen, J.T. Involvement of oxidative stress in age-related bone loss. J. Surg. Res. 2011, 169, e37–e42. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R. Osteoporosis and inflammation. Nutr. Rev. 2007, 65, S147–S151. [Google Scholar] [CrossRef] [PubMed]
- Ozgocmen, S.; Kaya, H.; Fadillioglu, E.; Yilmaz, Z. Effects of calcitonin, risedronate, and raloxifene on erythrocyte antioxidant enzyme activity, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Arch. Med. Res. 2007, 38, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Ozgocmen, S.; Kaya, H.; Fadillioglu, E.; Aydogan, R.; Yilmaz, Z. Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol. Cell. Biochem. 2007, 295, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Sendur, O.F.; Turan, Y.; Tastaban, E.; Serter, M. Antioxidant status in patients with osteoporosis: A controlled study. Jt. Bone Spine 2009, 76, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Dudonne, S.; Varin, T.V.; Anhe, F.F.; Dube, P.; Roy, D.; Pilon, G.; Marette, A.; Levy, E. Modulatory effects of a cranberry extract co-supplementation with Bacillus subtilis CU1 probiotic on phenoli compounds bioavailability and gut microbiota composition in high-fat diet-fed mice. Pharmanutrition 2015, 3, 89–100. [Google Scholar] [CrossRef]
- Barthelmebs, L.; Divies, C.; Cavin, J.F. Knockout of the p-coumarate decarboxylase gene from Lactobacillus plantarum reveals the existence of two other inducible enzymatic activities involved in phenolic acid metabolism. Appl. Environ. Microbiol. 2000, 66, 3368–3375. [Google Scholar] [CrossRef] [PubMed]
- Osawa, R.; Kuroiso, K.; Goto, S.; Shimizu, A. Isolation of tannin-degrading lactobacilli from humans and fermented foods. Appl. Environ. Microbiol. 2000, 66, 3093–3097. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M. A role for the gut microbiota in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Sanchez-Patan, F.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Martin-Alvarez, P.J.; Bartolome, B.; Moreno-Arribas, M.V. In vitro fermentation of grape seed flavan-3-ol fractions by human faecal microbiota: Changes in microbial groups and phenolic metabolites. FEMS Microbiol. Ecol. 2013, 83, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Duenas, M.; Munoz-Gonzalez, I.; Cueva, C.; Jimenez-Giron, A.; Sanchez-Patan, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolome, B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed. Res. Int. 2015, 2015, 850902. [Google Scholar]
- Hervert-Hernandez, D.; Pintado, C.; Rotger, R.; Goni, I. Stimulatory role of grape pomace polyphenols on Lactobacillus acidophilus growth. Int. J. Food Microbiol. 2009, 136, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Dolara, P.; Luceri, C.; De Filippo, C.; Femia, A.P.; Giovannelli, L.; Caderni, G.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Cresci, A. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mutat. Res. 2005, 591, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Sun, J.; Chen, P.; Lakshman, S.; Molokin, A.; Harnly, J.M.; Vinyard, B.T.; Urban, J.F., Jr.; Davis, C.D.; Solano-Aguilar, G. Flavanol-Enriched Cocoa Powder Alters the Intestinal Microbiota, Tissue and Fluid Metabolite Profiles, and Intestinal Gene Expression in Pigs. J. Nutr. 2016, 146, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Macia, A.; Motilva, M.J. Metabolic and Microbial Modulation of the Large Intestine Ecosystem by Non-Absorbed Diet Phenolic Compounds: A Review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; Wyns, C.; Possemiers, S.; Depypere, H.; De Keukeleire, D.; Bracke, M.; Verstraete, W.; Heyerick, A. Cosupplementation of isoflavones, prenylflavonoids, and lignans alters human exposure to phytoestrogen-derived 17beta-estradiol equivalents. J. Nutr. 2009, 139, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.C.; Hodgson, J.M.; Croft, K.D.; Burke, V.; Beilin, L.J.; Puddey, I.B. The combination of vitamin C and grape-seed polyphenols increases blood pressure: A randomized, double-blind, placebo-controlled trial. J. Hypertens. 2005, 23, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhang, Y.; Lu, N.; Felson, D.; Kiel, D.P.; Sahni, S. Association between dietary fiber intake and bone loss in the Framingham Offspring Study. J. Bone Miner. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, T.; Wang, S.E.; Wang, W.; Wang, Q.; Yu, H.X. Fructo-oligosaccharides enhance the mineral absorption and counteract the adverse effects of phytic acid in mice. Nutrition 2010, 26, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.R.; Braun, M.M.; Wigertz, K.; Bryant, R.; Zhao, Y.; Lee, W.; Kempa-Steczko, A.; Weaver, C.M. Fructo-oligosaccharides and calcium absorption and retention in adolescent girls. J. Am. Coll. Nutr. 2010, 29, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.A.; Griffin, I.J.; Hawthorne, K.M.; Liang, L.; Gunn, S.K.; Darlington, G.; Ellis, K.J. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am. J. Clin. Nutr. 2005, 82, 471–476. [Google Scholar] [PubMed]
- Lambert, M.N.T.; Thorup, A.C.; Hansen, E.S.S.; Jeppesen, P.B. Combined Red Clover isoflavones and probiotics potently reduce menopausal vasomotor symptoms. PLoS ONE 2017, 12, e0176590. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Zhao, X.; Shoaf, S.E.; Ragland, K. The pharmacokinetics of S-(-)equol administered as SE5-OH tablets to healthy postmenopausal women. J. Nutr. 2009, 139, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- McCabe, L.R.; Irwin, R.; Schaefer, L.; Britton, R.A. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J. Cell. Physiol. 2013, 228, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Parameswaran, N.; McCabe, L.R. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Physiol. 2014, 229, 1822–1830. [Google Scholar]
- Zhang, J.; Motyl, K.J.; Irwin, R.; MacDougald, O.A.; Britton, R.A.; McCabe, L.R. Loss of Bone and Wnt10b Expression in Male Type 1 Diabetic Mice is Blocked by the Probiotic Lactobacillus reuteri. Endocrinology 2015, 156, 3169–3182. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, C.; Engdahl, C.; Fak, F.; Andersson, A.; Windahl, S.H.; Farman, H.H.; Moverare-Skrtic, S.; Islander, U.; Sjogren, K. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS ONE 2014, 9, e92368. [Google Scholar] [CrossRef] [PubMed]
- Kiel, D.P.; Rosen, C.J.; Dempster, D. Age-Related Bone Loss. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism; Rosen, C.J., Ed.; American Society of Bone and Mineral Research: Washington, DC, USA, 2008; Volume 98–101. [Google Scholar]
- Jee, W.S.; Yao, W. Overview: Animal models of osteopenia and osteoporosis. J. Musculoskelet. Neuronal Interact. 2001, 1, 193–207. [Google Scholar] [PubMed]
- Halloran, B.P.; Ferguson, V.L.; Simske, S.J.; Burghardt, A.; Venton, L.L.; Majumdar, S. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J. Bone Miner. Res. 2002, 17, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Stoica, A.; Cardemil, C.; Palmquist, A. Multiscale characterization of cortical bone composition, microstructure, and nanomechanical properties in experimentally induced osteoporosis. J. Biomed. Mater. Res. A 2017. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.M.; Bab, I.; Fish, S.; Muller, R.; Uchiyama, T.; Gronowicz, G.; Nahounou, M.; Zhao, Q.; White, D.W.; Chorev, M.; et al. Human parathyroid hormone 1-34 reverses bone loss in ovariectomized mice. J. Bone Miner. Res. 2001, 16, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Kern, M.; Metti, D.; Shamloufard, P.; Chai, S.C.; Johnson, S.A.; Payton, M.E.; Arjmandi, B.H. The effect of two doses of dried plum on bone density and bone biomarkers in osteopenic postmenopausal women: A randomized, controlled trial. Osteoporos. Int. 2016, 27, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, C.L.; Molin, G.; Fak, F.; Johansson Hagslatt, M.L.; Jakesevic, M.; Hakansson, A.; Jeppsson, B.; Westrom, B.; Ahrne, S. Effects on weight gain and gut microbiota in rats given bacterial supplements and a high-energy-dense diet from fetal life through to 6 months of age. Br. J. Nutr. 2011, 106, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Angelakis, E.; Paul, M.; Armougom, F.; Leibovici, L.; Raoult, D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb. Pathog. 2012, 53, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef]
- Yirga, H. The use of probiotics in animal nutrition. J. Prob. Health 2015, 3, 132. [Google Scholar] [CrossRef]
| Ingredient (g) | 10% Grape | 20% Grape | 10% Grape + 1% Probiotic | 20% Grape + 1% Probiotic | 1% Probiotic | Control |
|---|---|---|---|---|---|---|
| Casein | 140.0 | 140.0 | 140.0 | 140.0 | 140.0 | 140.0 |
| Cornstarch | 520.7 | 520.7 | 510.7 | 510.7 | 510.7 | 520.7 |
| Dextrose | 50.0 | 0.0 | 50.0 | 0.0 | 100.0 | 100.0 |
| Fructose | 50.0 | 0.0 | 50.0 | 0.0 | 100.0 | 100.0 |
| Cellulose | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Grape Powder | 100.0 | 200.0 | 100.0 | 200.0 | 0.0 | 0.0 |
| Probiotic | 0.0 | 0.0 | 10.0 | 10.0 | 10.0 | 0.0 |
| Soybean Oil | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
| Mineral Salt Mix | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 |
| Vitamin Mix | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| l-Cystine | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 |
| Choline Bitartrate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Total g | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 |
| Total kcals | 3762.8 | 3722.8 | 3722.8 | 3682.8 | 3762.8 | 3802.8 |
| Kcals/g | 3.76 | 3.72 | 3.72 | 3.68 | 3.76 | 3.80 |
| 10% Grape | 20% Grape | 10% Grape + 1% Probiotic | 20% Grape + 1% Probiotic | 1% Probiotic | Control | Main Effect Diet, p-Value (One-Way ANOVA) | Main Effect Diet, p-Value (ANOVA Repeated Measures *) | |
|---|---|---|---|---|---|---|---|---|
| Initial Body Weight (g) | 37.55 | 38.82 | 38.10 | 38.65 | 38.16 | 37.48 | 0.99 | - |
| (3.62) | (4.36) | (3.68) | (4.59) | (4.75) | (3.56) | |||
| Final Body Weight (g) | 42.20 | 42.38 | 43.30 | 42.16 | 39.78 | 41.14 | 0.51 | <0.0001 |
| (1.97) | (3.87) | (2.06) | (3.48) | (2.55) | (2.82) | |||
| Body Weight Gain (% gain of initial) | 12.86 | 9.59 | 14.39 | 9.59 | 5.10 | 10.32 | 0.66 | - |
| (7.11) | (8.21) | (10.60) | (6.92) | (10.45) | (10.14) | |||
| Food Intake (g/day) | 5.29 | 4.97 | 5.85 | 5.27 | 6.19 | 5.72 | <0.0001 | <0.0001 * |
| (0.76) a,b | (0.44) b | (0.63) b,c | (0.77) a,b | (0.66) c | (0.82) b,c | |||
| Feed Efficiency (body weight/kcal/day) | 2.13 | 2.22 | 1.92 | 2.12 | 1.72 | 1.87 | <0.0001 | <0.0001 |
| (0.36) a,b | (0.20) a | (0.26) b,c | (0.33) a,b | (0.19) c | (0.30) c |
| 10-Month-Old Baseline | 10% Grape | 20% Grape | 10% Grape + 1% Probiotic | 20% Grape + 1% Probiotic | 1% Probiotic | Control | Main Effect Group, p-Value | |
|---|---|---|---|---|---|---|---|---|
| Tibia proximal metaphysis | ||||||||
| BV/TV, % | 12.0 (2.1) a | 5.9 (3.7) b,c | 6.4 (3.3) b,c | 6.1 (2.2) b | 3.67 (1.8) c | 6.1 (1.8) b,c | 6.4 (2.7) b,c | 0.0007 |
| SA/BV, mm −1 | 47.047 (3.356) | 52.343 (9.674) | 48.691 (8.348) | 52.942 (3.672) | 49.445 (6.944) | 48.969 (5.534) | 57.136 (6.211) | 0.2190 |
| Tb. Th, mm | 0.043 (0.003) | 0.0392 (0.007) | 0.042 (0.007) | 0.038 (0.003) | 0.041 (0.006) | 0.041 (0.004) | 0.035 (0.004) | 0.2173 |
| Tb. N, mm−1 | 2.817 (0.450) a | 1.399 (0.774) b,c | 1.482 (0.661) b,c | 1.608 (0.512) b | 0.862 (0.314) c | 1.458 (0.318) b | 1.776 (0.558) b | 0.0002 |
| Tb. Sp, mm | 0.319 (0.050) a | 1.345 (1.672) b,c | 0.793 (0.424) b,c | 0.626 (0.161) b | 1.260 (0.497) c | 0.676 (0.187) b | 0.575 (0.195) b | 0.0006 |
| Tb. Patt. Fact, mm−1 | 9.481 (3.098) | 17.980 (7.979) | 16.242 (8.052) | 16.413 (5.642) | 18.852 (4.531) | 14.671 (5.378) | 14.895 (2.887) | 0.1611 |
| Tibia mid- diaphysis | ||||||||
| Cortical wall thickness, mm | 0.263 (0.021) | 0.256 (0.015) | 0.256 (0.011) | 0.258 (0.012) | 0.258 (0.017) | 0.258 (0.008) | 0.260 (0.007) | 0.9735 |
| Medullary area, mm2 | 3.585 (0.332) | 3.918 (0.288) | 3.790 (0.293) | 3.630 (0.399) | 3.724 (0.278) | 4.165 (0.245) | 3.919 (0.308) | 0.0659 |
| 10-Month-Old Baseline | 10% Grape | 20% Grape | 10% Grape + 1% Probiotic | 20% Grape + 1% Probiotic | 1% Probiotic | Control | Main Effect Group, p-Value | |
|---|---|---|---|---|---|---|---|---|
| Femur distal metaphysis | ||||||||
| BV/TV, % | 11.9 (2.0) a | 8.4 (3.4) a,b | 8.5 (3.8) a,b | 7.6 (3.5) a,b | 5.6 (1.7) b | 7.4 (2.6) a,b | 7.2 (3.7) a,b | 0.049 |
| SA/BV, mm−1 | 44.183 (2.633) | 40.412 (4.900) | 43.594 (3.171) | 43.613 (1.917) | 45.437 (4.084) | 43.105 (7.204) | 45.709 (2.784) | 0.523 |
| Tb. Th, mm | 0.045 (0.003) | 0.050 (0.006) | 0.046 (0.003) | 0.046 (0.002) | 0.044 (0.004) | 0.048 (0.009) | 0.044 (0.003) | 0.473 |
| Tb. N, mm−1 | 2.622 (0.412) a | 1.641 (0.593) a,b | 1.821 (0.720) a,b | 1.645 (0.735) a,b | 1.266 (0.429) b | 1.572 (0.424) a,b | 1.605 (0.735) a,b | 0.018 |
| Tb. Sp, mm | 0.344 (0.061) a | 0.678 (0.431) b | 0.614 (0.342) a,b | 1.072 (0.864) b | 0.802 (0.201) b | 0.628 (0.188) b | 0.667 (0.299) a,b | 0.052 |
| Tb. Patt. Fact, mm−1 | 12.439 (3.132) a | 12.981 (3.031) a,b | 14.433 (3.293) a,b | 15.562 (1.944) a,b | 17.863 (1.650) b | 15.143 (3.135) a,b | 16.261 (2.360) a,b | 0.0373 |
| Femur mid- diaphysis | ||||||||
| Cortical wall thickness, mm | 0.254 (0.027) | 0.264 (0.031) | 0.264 (0.015) | 0.252 (0.019) | 0.252 (0.017) | 0.279 (0.034) | 0.258 (0.010) | 0.4509 |
| Medullary area, mm2 | 6.081 (0.514) | 6.807 (0.534) | 6.387 (0.583) | 6.653 (0.319) | 6.641 (0.429) | 6.807 (0.551) | 6.478 (0.097) | 0.1659 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanton, C. Bone Response to Dietary Co-Enrichment with Powdered Whole Grape and Probiotics. Nutrients 2018, 10, 146. https://doi.org/10.3390/nu10020146
Blanton C. Bone Response to Dietary Co-Enrichment with Powdered Whole Grape and Probiotics. Nutrients. 2018; 10(2):146. https://doi.org/10.3390/nu10020146
Chicago/Turabian StyleBlanton, Cynthia. 2018. "Bone Response to Dietary Co-Enrichment with Powdered Whole Grape and Probiotics" Nutrients 10, no. 2: 146. https://doi.org/10.3390/nu10020146
APA StyleBlanton, C. (2018). Bone Response to Dietary Co-Enrichment with Powdered Whole Grape and Probiotics. Nutrients, 10(2), 146. https://doi.org/10.3390/nu10020146





