A Randomized Double-Blinded, Placebo-Controlled Trial Investigating the Effect of Fish Oil Supplementation on Gene Expression Related to Insulin Action, Blood Lipids, and Inflammation in Gestational Diabetes Mellitus-Fish Oil Supplementation and Gestational Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Procedures
2.3. Assessment of Anthropometric Measures
2.4. Primary and Secondary Outcomes
2.5. Laboratory Procedures
Isolation of PBMCs
2.6. RNA Extraction and Real-Time PCR (RT-PCR)
2.7. Biochemical Assessment
2.8. Clinical Assessment
2.9. Statistical Analysis
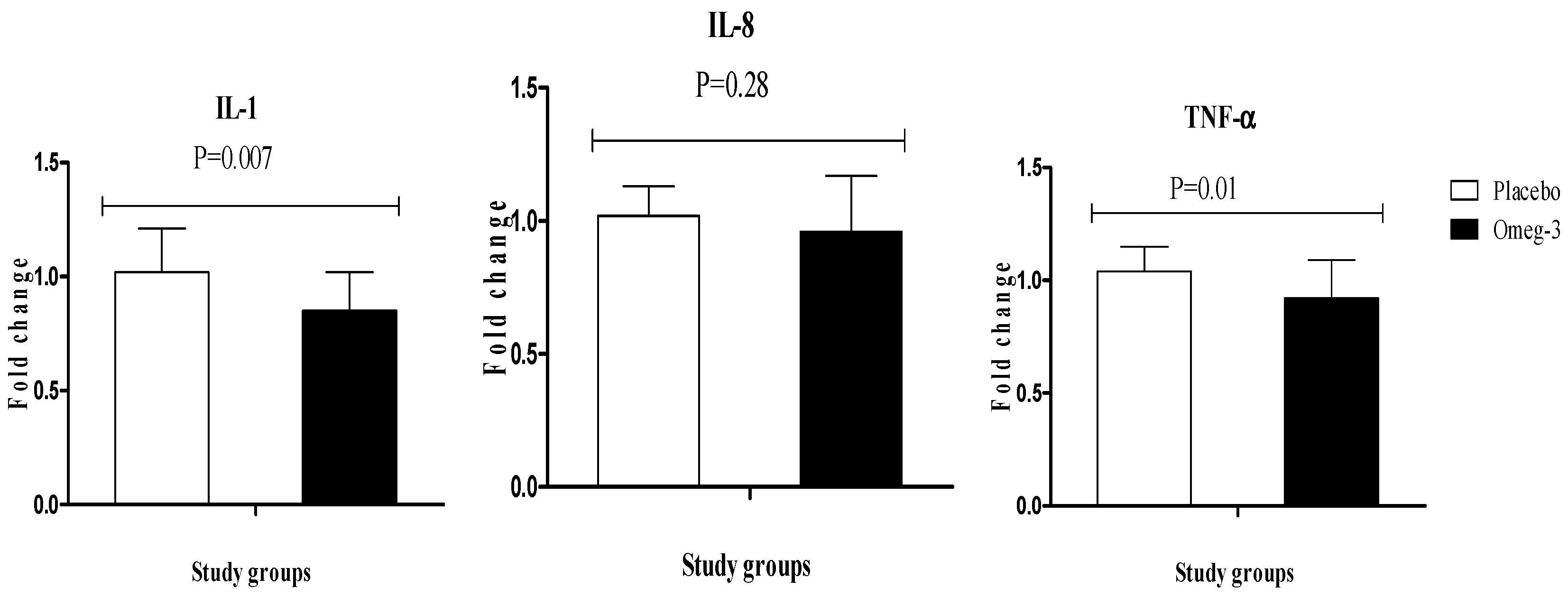
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spaight, C.; Gross, J.; Horsch, A.; Puder, J.J. Gestational diabetes mellitus. Endocr. Dev. 2016, 31, 163–178. [Google Scholar] [PubMed]
- Zhou, S.J.; Yelland, L.; McPhee, A.J.; Quinlivan, J.; Gibson, R.A.; Makrides, M. Fish-oil supplementation in pregnancy does not reduce the risk of gestational diabetes or preeclampsia. Am. J. Clin. Nutr. 2012, 95, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, M.; Putoto, G.; Laterza, F.; Pizzol, D. Gestational diabetes: An overview with attention for developing countries. Endocr. Regul. 2016, 50, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Shah, B.R. Role of type 2 diabetes in determining retinal, renal, and cardiovascular outcomes in women with previous gestational diabetes mellitus. Diabetes Care 2017, 40, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lambrinoudaki, I.; Vlachou, S.A.; Creatsas, G. Genetics in gestational diabetes mellitus: Association with incidence, severity, pregnancy outcome and response to treatment. Curr. Diabetes Rev. 2010, 6, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Salmi, A.A.; Zaki, N.M.; Zakaria, R.; Nor Aliza, A.G.; Rasool, A.H. Arterial stiffness, inflammatory and pro-atherogenic markers in gestational diabetes mellitus. Vasa 2012, 41, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Tinoco, C.; Roca, M.; Fernandez-Deudero, A.; Garcia-Valero, A.; Bugatto, F.; Aguilar-Diosdado, M.; Bartha, J.L. Cytokine profile, metabolic syndrome and cardiovascular disease risk in women with late-onset gestational diabetes mellitus. Cytokine 2012, 58, 14–19. [Google Scholar] [CrossRef] [PubMed]
- King, G.L. The role of inflammatory cytokines in diabetes and its complications. J. Periodontol. 2008, 79, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, L.; Belkacemi, L. Altered cytokine network in gestational diabetes mellitus affects maternal insulin and placental-fetal development. J. Diabetes Complicat. 2016, 30, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.H.; Lu, J.H.; Zheng, S.Y.; Long, T.; Li, Y.T.; Wu, W.Z.; Wang, F. Changes in serum adipocyte fatty acid-binding protein in women with gestational diabetes mellitus and normal pregnant women during mid- and late pregnancy. J. Diabetes Investig. 2016, 7, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Radesky, J.S.; Oken, E.; Rifas-Shiman, S.L.; Kleinman, K.P.; Rich-Edwards, J.W.; Gillman, M.W. Diet during early pregnancy and development of gestational diabetes. Paediatr. Perinat. Epidemiol. 2008, 22, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Storlien, L.H.; Jenkins, A.B.; Tapsell, L.C.; Jin, Y.; Pan, J.F.; Shao, Y.F.; Calvert, G.D.; Moses, R.G.; Shi, H.L.; et al. Dietary variables and glucose tolerance in pregnancy. Diabetes Care 2000, 23, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Samimi, M.; Kolahdooz, F.; Khalaji, F.; Razavi, M.; Asemi, Z. Omega-3 fatty acid supplementation affects pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. J. Matern. Fetal Neonatal Med. 2016, 29, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Samimi, M.; Jamilian, M.; Asemi, Z.; Esmaillzadeh, A. Effects of omega-3 fatty acid supplementation on insulin metabolism and lipid profiles in gestational diabetes: Randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2015, 34, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Bassaganya-Riera, J.; Hontecillas, R. CLA and n-3 PUFA differentially modulate clinical activity and colonic PPAR-responsive gene expression in a pig model of experimental IBD. Clin. Nutr. 2006, 25, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Joshi-Barve, S.; Barve, S.; Chen, L.H. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J. Am. Coll. Nutr. 2004, 23, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, A.; Sotoudeh, G.; Djalali, M.; Eshraghian, M.R.; Keramatipour, M.; Nasli-Esfahani, E.; Shidfar, F.; Alvandi, E.; Toupchian, O.; Koohdani, F. Docosahexaenoic acid-rich fish oil supplementation improves body composition without influence of the PPARgamma Pro12Ala polymorphism in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. J. Nutrigenet. Nutrigenom. 2015, 8, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, D.; Chen, J.; Roberts, G.J.; Saldeen, T.; Mehta, J.L. EPA and DHA attenuate ox-LDL-induced expression of adhesion molecules in human coronary artery endothelial cells via protein kinase B pathway. J. Mol. Cell. Cardiol. 2003, 35, 769–775. [Google Scholar] [CrossRef]
- Zaree, M.; Shahnazi, V.; Fayezi, S.; Darabi, M.; Mehrzad-Sadaghiani, M.; Khani, S.; Nouri, M. Expression levels of PPARgamma and CYP-19 in polycystic ovarian syndrome primary granulosa cells: Influence of omega-3 fatty acid. Int. J. Fertil. Steril. 2015, 9, 197–204. [Google Scholar] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. 1), S81–S90. [Google Scholar]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S504. [Google Scholar] [CrossRef] [PubMed]
- Mizuarai, S.; Irie, H.; Kotani, H. Gene expression-based pharmacodynamic biomarkers: The beginning of a new era in biomarker-driven anti-tumor drug development. Curr. Mol. Med. 2010, 10, 596–607. [Google Scholar] [PubMed]
- Xavier, D.J.; Takahashi, P.; Evangelista, A.F.; Foss-Freitas, M.C.; Foss, M.C.; Donadi, E.A.; Passos, G.A.; Sakamoto-Hojo, E.T. Assessment of DNA damage and mRNA/miRNA transcriptional expression profiles in hyperglycemic versus non-hyperglycemic patients with type 2 diabetes mellitus. Mutat. Res. 2015, 776, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, H.; Alizadeh, S.; Esfahani, E.N.; Razi, F.; Meshkani, R. Decreased expression of microRNA-21 is associated with increased cytokine production in peripheral blood mononuclear cells (PBMCs) of obese type 2 diabetic and non-diabetic subjects. Mol. Cell. Biochem. 2016, 419, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Dunkley, P.R.; Jarvie, P.E.; Robinson, P.J. A rapid Percoll gradient procedure for preparation of synaptosomes. Nat. Protoc. 2008, 3, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Pisprasert, V.; Ingram, K.H.; Lopez-Davila, M.F.; Munoz, A.J.; Garvey, W.T. Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: Impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care 2013, 36, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Nobile de Santis, M.S.; Radaelli, T.; Taricco, E.; Bertini, S.; Cetin, I. Excess of amniotic fluid: Pathophysiology, correlated diseases and clinical management. Acta Biomed. 2004, 75 (Suppl. 1), 53–55. [Google Scholar] [PubMed]
- Anderson, E.J.; Thayne, K.A.; Harris, M.; Shaikh, S.R.; Darden, T.M.; Lark, D.S.; Williams, J.M.; Chitwood, W.R.; Kypson, A.P.; Rodriguez, E. Do fish oil omega-3 fatty acids enhance antioxidant capacity and mitochondrial fatty acid oxidation in human atrial myocardium via PPARgamma activation? Antioxid. Redox Signal. 2014, 21, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Oster, R.T.; Tishinsky, J.M.; Yuan, Z.; Robinson, L.E. Docosahexaenoic acid increases cellular adiponectin mRNA and secreted adiponectin protein, as well as PPARgamma mRNA, in 3T3-L1 adipocytes. Appl. Physiol. Nutr. Metab. 2010, 35, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Tishinsky, J.M.; Ma, D.W.; Robinson, L.E. Eicosapentaenoic acid and rosiglitazone increase adiponectin in an additive and PPARgamma-dependent manner in human adipocytes. Obesity (Silver Spring) 2011, 19, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Fan, C.; Liu, X.; Xu, F.; Qi, K. Brain histological changes in young mice submitted to diets with different ratios of n-6/n-3 polyunsaturated fatty acids during maternal pregnancy and lactation. Clin. Nutr. 2011, 30, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Gajos, G.; Zalewski, J.; Mostowik, M.; Konduracka, E.; Nessler, J.; Undas, A. Polyunsaturated omega-3 fatty acids reduce lipoprotein-associated phospholipase A(2) in patients with stable angina. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, E.; Samimi, M.; Ebrahimi, F.A.; Foroozanfard, F.; Ahmadi, S.; Rahimi, M.; Jamilian, M.; Aghadavod, E.; Bahmani, F.; Taghizadeh, M.; et al. The effects of omega-3 fatty acids and vitamin E co-supplementation on gene expression of lipoprotein(a) and oxidized low-density lipoprotein, lipid profiles and biomarkers of oxidative stress in patients with polycystic ovary syndrome. Mol. Cell. Endocrinol. 2017, 439, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Kanazawa, S.; Fukuhara, S. Effects of eicosapentaenoic acid on platelet activation markers and cell adhesion molecules in hyperlipidemic patients with type 2 diabetes mellitus. J. Diabetes Complicat. 2003, 17, 153–159. [Google Scholar] [CrossRef]
- Helland, I.B.; Saugstad, O.D.; Saarem, K.; van Houwelingen, A.C.; Nylander, G.; Drevon, C.A. Supplementation of n-3 fatty acids during pregnancy and lactation reduces maternal plasma lipid levels and provides DHA to the infants. J. Matern. Fetal Neonatal Med. 2006, 19, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.E.; Dunstan, J.A.; Beilin, L.J.; Prescott, S.L.; Mori, T.A. n-3 fatty acid supplementation during pregnancy in women with allergic disease: Effects on blood pressure, and maternal and fetal lipids. Clin. Sci. (Lond.) 2006, 111, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Mejia-Barradas, C.M.; Del-Rio-Navarro, B.E.; Dominguez-Lopez, A.; Campos-Rodriguez, R.; Martinez-Godinez, M.; Rojas-Hernandez, S.; Lara-Padilla, E.; Abarca-Rojano, E.; Miliar-Garcia, A. The consumption of n-3 polyunsaturated fatty acids differentially modulates gene expression of peroxisome proliferator-activated receptor alpha and gamma and hypoxia-inducible factor 1 alpha in subcutaneous adipose tissue of obese adolescents. Endocrine 2014, 45, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.J.; Pomeroy, S.E.; Sasahara, T.; Yamashita, T.; Liang, Y.L.; Dart, A.M.; Jennings, G.L.; Abbey, M.; Cameron, J.D. Arterial compliance in obese subjects is improved with dietary plant n-3 fatty acid from flaxseed oil despite increased LDL oxidizability. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, F.A.; Pyarasani, R.D.; Delgado-Lopez, F.; Moore-Carrasco, R. Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Mediat. Inflamm. 2013, 2013, 549627. [Google Scholar] [CrossRef] [PubMed]
- Kuzmicki, M.; Telejko, B.; Wawrusiewicz-Kurylonek, N.; Lipinska, D.; Pliszka, J.; Wilk, J.; Zielinska, A.; Skibicka, J.; Szamatowicz, J.; Kretowski, A.; et al. The expression of genes involved in NF-kappaB activation in peripheral blood mononuclear cells of patients with gestational diabetes. Eur. J. Endocrinol. 2013, 168, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Chon, S.J.; Kim, S.Y.; Cho, N.R.; Min, D.L.; Hwang, Y.J.; Mamura, M. Association of variants in PPARgamma(2), IGF2BP2, and KCNQ1 with a susceptibility to gestational diabetes mellitus in a Korean population. Yonsei Med. J. 2013, 54, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Fairall, L.; Amin, K.; Inaba, Y.; Szanto, A.; Balint, B.L.; Nagy, L.; Yamamoto, K.; Schwabe, J.W. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat. Struct. Mol. Biol. 2008, 15, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Gani, O.A. Are fish oil omega-3 long-chain fatty acids and their derivatives peroxisome proliferator-activated receptor agonists? Cardiovasc. Diabetol. 2008, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.G.; Liang, C.; Han, S.F.; Wu, Z.G. Vitamin E ameliorates ox-LDL-induced foam cells formation through modulating the activities of oxidative stress-induced NF-kappaB pathway. Mol. Cell. Biochem. 2012, 363, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tayyebi-Khosroshahi, H.; Houshyar, J.; Dehgan-Hesari, R.; Alikhah, H.; Vatankhah, A.M.; Safaeian, A.R.; Zonouz, N.R. Effect of treatment with omega-3 fatty acids on C-reactive protein and tumor necrosis factor-alfa in hemodialysis patients. Saudi J. Kidney Dis. Transpl. 2012, 23, 500–506. [Google Scholar] [PubMed]
- Ellulu, M.S.; Khaza’ai, H.; Patimah, I.; Rahmat, A.; Abed, Y. Effect of long chain omega-3 polyunsaturated fatty acids on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: A randomized controlled trial. Food Nutr. Res. 2016, 60, 29268. [Google Scholar] [CrossRef] [PubMed]
- Haghiac, M.; Yang, X.H.; Presley, L.; Smith, S.; Dettelback, S.; Minium, J.; Belury, M.A.; Catalano, P.M.; Hauguel-de Mouzon, S. Dietary omega-3 fatty acid supplementation reduces inflammation in obese pregnant women: A randomized double-blind controlled clinical trial. PLoS ONE 2015, 10, e0137309. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.L.; Hughes, C.E.; Flannery, C.R.; Little, C.B.; Harwood, J.L.; Caterson, B. n-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J. Biol. Chem. 2000, 275, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Lalia, A.Z.; Lanza, I.R. Insulin-Sensitizing Effects of omega-3 fatty acids: Lost in translation? Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Naini, A.E.; Asiabi, R.E.; Keivandarian, N.; Moeinzadeh, F. Effect of omega-3 supplementation on inflammatory parameters in patients on chronic ambulatory peritoneal dialysis. Adv. Biomed. Res. 2015, 4, 167. [Google Scholar] [PubMed]
- Cormier, H.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M.C. Expression and sequence variants of inflammatory genes; Effects on plasma inflammation biomarkers following a 6-week supplementation with fish oil. Int. J. Mol. Sci. 2016, 17, 375. [Google Scholar] [CrossRef] [PubMed]
- Ategbo, J.M.; Grissa, O.; Yessoufou, A.; Hichami, A.; Dramane, K.L.; Moutairou, K.; Miled, A.; Grissa, A.; Jerbi, M.; Tabka, Z.; et al. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J. Clin. Endocrinol. Metab. 2006, 91, 4137–4143. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, M.T.; Oliva, K.; Georgiou, H.M.; Permezel, J.M.; Rice, G.E. Glucose-induced release of tumour necrosis factor-alpha from human placental and adipose tissues in gestational diabetes mellitus. Diabet. Med. 2001, 18, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.P.; Hauguel-De Mouzon, S.; Lepercq, J.; Challier, J.C.; Huston-Presley, L.; Friedman, J.E.; Kalhan, S.C.; Catalano, P.M. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes 2002, 51, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ruan, X.Z.; Powis, S.H.; Fernando, R.; Mon, W.Y.; Wheeler, D.C.; Moorhead, J.F.; Varghese, Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: Evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005, 67, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Kapahi, P.; Natoli, G.; Takahashi, T.; Chen, Y.; Karin, M.; Santoro, M.G. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature 2000, 403, 103–108. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer | Product Size (bp) | Annealing Temperature (C) |
|---|---|---|---|
| GAPDH | F: AAGCTCATTTCCTGGTATGACAACG | 126 | 61.3 |
| R: TCTTCCTCTTGTGCTCTTGCTGG | |||
| PPAR-γ | F: ATGACAGACCTCAGACAGATTG | 210 | 54 |
| R: AATGTTGGCAGTGGCTCAG | |||
| LDLR | F: ACTTACGGACAGACAGACAG | 223 | 57 |
| R: GGCCACACATCCCATGATTC | |||
| IL-1 | F: GCTTCTCTCTGGTCCTTGG | 174 | 56 |
| R: AGGGCAGGGTAGAGAAGAG | |||
| IL-8 | F: GCAGAGGGTTGTGGAGAAGT | 150 | 56 |
| R: ACCCTACAACAGACCCACAC | |||
| TNF-α | F: GTCAACCTCCTCTCTGCCAT | 188 | 52 |
| R: CCAAAGTAGACCTGCCCAGA |
| Placebo Group (n = 20) | Omega-3 Group (n = 20) | P 1 | |
|---|---|---|---|
| Gestational age before intervention (weeks) | 25.3 ± 1.1 | 25.4 ± 1.2 | 0.89 |
| Age (y) | 30.8 ± 2.4 | 30.5 ± 3.8 | 0.80 |
| Height (cm) | 162.1 ± 5.8 | 161.6 ± 3.4 | 0.71 |
| Weight at study baseline (kg) | 70.6 ± 5.7 | 73.1 ± 6.7 | 0.22 |
| Weight at end-of-trial (kg) | 72.5 ± 5.7 | 75.1 ± 6.6 | 0.19 |
| Weight change (kg) | 1.9 ± 0.6 | 2.0 ± 0.6 | 0.51 |
| BMI at study baseline (kg/m2) | 27.0 ± 3.1 | 28.0 ± 2.6 | 0.27 |
| BMI at end-of-trial (kg/m2) | 27.7 ± 3.2 | 28.8 ± 2.6 | 0.25 |
| BMI change (kg/m2) | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.54 |
| MET-h/day at study baseline | 27.6 ± 2.0 | 27.1 ± 2.0 | 0.40 |
| MET-h/day at end-of-trial | 27.3 ± 2.1 | 26.9 ± 2.2 | 0.46 |
| MET-h/day change | −0.3 ± 0.5 | −0.2 ± 0.4 | 0.73 |
| Placebo Group (n = 20) | Omega-3 Group (n = 20) | P 1 | |||||
|---|---|---|---|---|---|---|---|
| Wk0 | Wk6 | Change | Wk0 | Wk6 | Change | ||
| FPG (mg/dL) | 95.5 ± 10.1 | 98.3 ± 10.0 | 2.9 ± 14.3 | 95.6 ± 4.3 | 91.1 ± 3.8 | −4.4 ± 2.3 | 0.02 |
| Insulin (μIU/mL) | 12.6 ± 4.7 | 13.0 ± 6.1 | 0.4 ± 5.5 | 13.0 ± 5.7 | 12.0 ± 4.6 | −1.0 ± 8.0 | 0.51 |
| HOMA-IR | 3.0 ± 1.2 | 3.2 ± 1.6 | 0.2 ± 1.6 | 3.1 ± 1.4 | 2.7 ± 1.0 | −0.4 ± 1.9 | 0.29 |
| Triglycerides (mg/dL) | 200.1 ± 56.0 | 215.8 ± 56.1 | 15.7 ± 29.9 | 221.3 ± 80.7 | 213.1 ± 71.9 | −8.3 ± 28.3 | 0.01 |
| Total cholesterol (mg/dL) | 209.5 ± 43.6 | 213.6 ± 41.5 | 4.1 ± 19.5 | 221.7 ± 43.4 | 234.1 ± 39.2 | 12.3 ± 15.5 | 0.14 |
| LDL-cholesterol (mg/dL) | 110.3 ± 32.9 | 1191.9 ± 35.4 | 1.6 ± 15.2 | 119.9 ± 34.2 | 131.1 ± 33.6 | 11.2 ± 13.5 | 0.04 |
| HDL-cholesterol (mg/dL) | 59.2 ± 15.6 | 58.5 ± 13.2 | −0.7 ± 5.8 | 57.6 ± 9.1 | 60.4 ± 9.6 | 2.9 ± 3.9 | 0.02 |
| hs-CRP (ng/mL) | 6592.2 ± 4476.75 | 6675.1 ± 4354.4 | 82.8 ± 3149.7 | 6911.2 ± 5249.3 | 3535.6 ± 4529.7 | −3375.7 ± 4836.8 | 0.01 |
| Placebo Group (n = 20) | Omega-3 Group (n = 20) | P 1 | |
|---|---|---|---|
| Preterm delivery (%) | 1 (5.0) | 0 (0.0) | 0.31 † |
| Pre-eclampsia (%) | 2 (10.0) | 1 (5.0) | >0.999 † |
| Polyhydramnios (%) | 2 (10.0) | 1 (5.0) | >0.999 † |
| Macrosomia > 4000 g (%) | 3 (15.0) | 1 (5.0) | 0.60 † |
| Gestational age (weeks) | 39.0 ± 1.2 | 38.3 ± 1.3 | 0.11 |
| Newborns’ weight (g) | 3337.0 ± 483.7 | 3467.5 ± 420.3 | 0.36 |
| Newborns’ length (cm) | 51.0 ± 1.7 | 51.7 ± 2.4 | 0.27 |
| Newborns’ head circumference (cm) | 36.0 ± 1.4 | 35.7 ± 0.7 | 0.28 |
| 1-min Apgar score | 8.7 ± 0.5 | 8.6 ± 0.5 | 0.75 |
| 5-min Apgar score | 9.7 ± 0.5 | 9.6 ± 0.5 | 0.75 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamilian, M.; Samimi, M.; Mirhosseini, N.; Afshar Ebrahimi, F.; Aghadavod, E.; Taghizadeh, M.; Asemi, Z. A Randomized Double-Blinded, Placebo-Controlled Trial Investigating the Effect of Fish Oil Supplementation on Gene Expression Related to Insulin Action, Blood Lipids, and Inflammation in Gestational Diabetes Mellitus-Fish Oil Supplementation and Gestational Diabetes. Nutrients 2018, 10, 163. https://doi.org/10.3390/nu10020163
Jamilian M, Samimi M, Mirhosseini N, Afshar Ebrahimi F, Aghadavod E, Taghizadeh M, Asemi Z. A Randomized Double-Blinded, Placebo-Controlled Trial Investigating the Effect of Fish Oil Supplementation on Gene Expression Related to Insulin Action, Blood Lipids, and Inflammation in Gestational Diabetes Mellitus-Fish Oil Supplementation and Gestational Diabetes. Nutrients. 2018; 10(2):163. https://doi.org/10.3390/nu10020163
Chicago/Turabian StyleJamilian, Mehri, Mansooreh Samimi, Naghmeh Mirhosseini, Faraneh Afshar Ebrahimi, Esmat Aghadavod, Mohsen Taghizadeh, and Zatollah Asemi. 2018. "A Randomized Double-Blinded, Placebo-Controlled Trial Investigating the Effect of Fish Oil Supplementation on Gene Expression Related to Insulin Action, Blood Lipids, and Inflammation in Gestational Diabetes Mellitus-Fish Oil Supplementation and Gestational Diabetes" Nutrients 10, no. 2: 163. https://doi.org/10.3390/nu10020163
APA StyleJamilian, M., Samimi, M., Mirhosseini, N., Afshar Ebrahimi, F., Aghadavod, E., Taghizadeh, M., & Asemi, Z. (2018). A Randomized Double-Blinded, Placebo-Controlled Trial Investigating the Effect of Fish Oil Supplementation on Gene Expression Related to Insulin Action, Blood Lipids, and Inflammation in Gestational Diabetes Mellitus-Fish Oil Supplementation and Gestational Diabetes. Nutrients, 10(2), 163. https://doi.org/10.3390/nu10020163





