Standardized Parenteral Nutrition for the Transition Phase in Preterm Infants: A Bag That Fits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Nutrition Database Design and Construction
2.3. Nutrition Database Modeling for the TN Phase
2.3.1. TN Phase Classification
2.3.2. Fluid and Nutritional Constraints
2.3.3. Nutrient Modeling Steps
2.4. Statistics
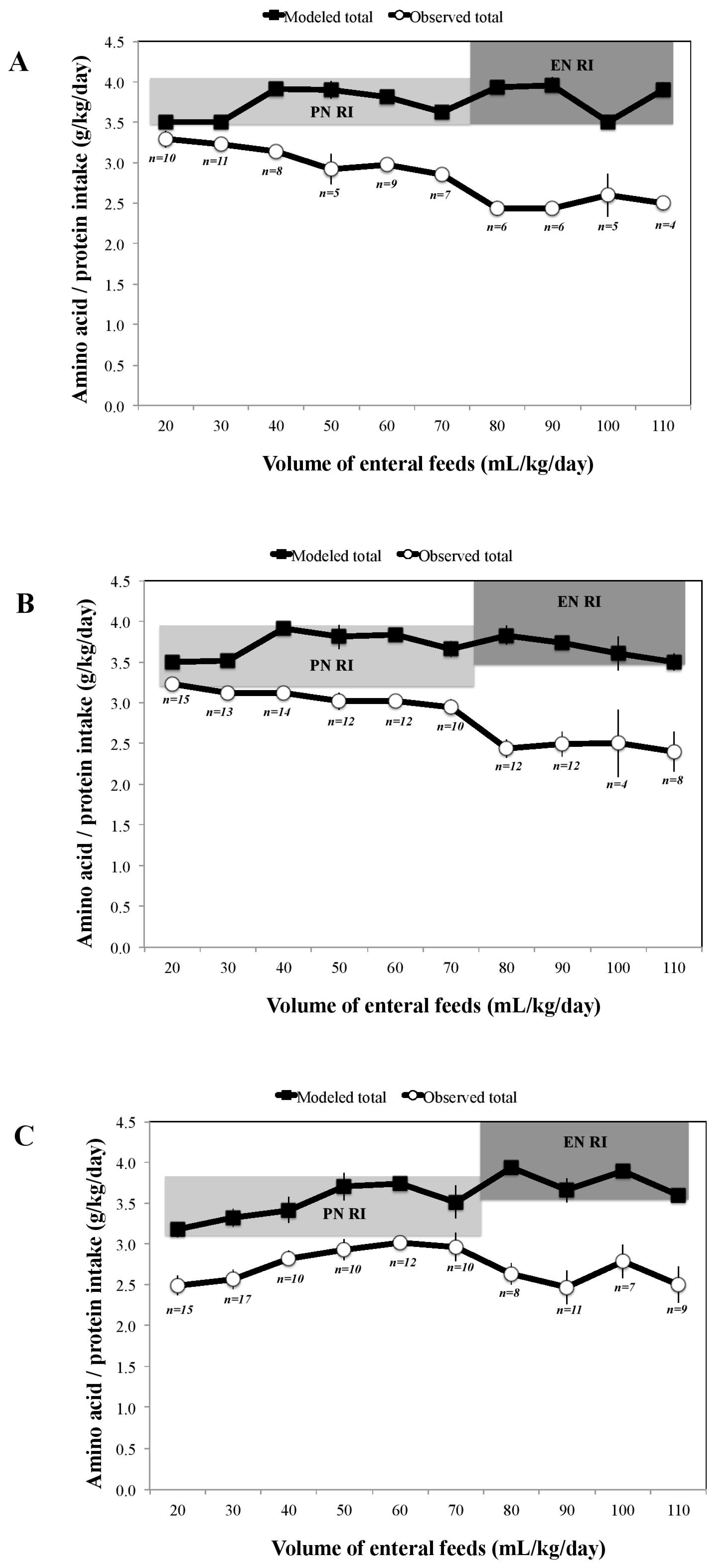
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brennan, A.M.; Fenton, S.; Murphy, B.P.; Kiely, M.E. Transition Phase Nutrition Recommendations: A Missing Link in the Nutrition Management of Preterm Infants. J. Parenter. Enter. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Donda, K.; Bhutada, A.; Rastogi, D.; Rastogi, S. Transitioning Preterm Infants From Parenteral Nutrition: A Comparison of 2 Protocols. J. Parenter. Enter. Nutr. 2017, 41, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Vaidya, R.; Rastogi, D.; Bhutada, A.; Rastogi, S. From parenteral to enteral nutrition: A nutrition-based approach for evaluating postnatal growth failure in preterm infants. J. Parenter. Enter. Nutr. 2014, 38, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Senterre, T.T.; Terrin, G.; De Curtis, M.; Rigo, J. Parenteral Nutrition in Premature Infants. In Textbook of Pediatric Gastroenterology, Hepatology and Nutrition: A Comprehensive Guide to Practice; Guandalini, S., Dhawan, A., Branski, D., Eds.; Springer International Publishing: Basel, Switzerland, 2016. [Google Scholar]
- Koletzko, B.; Poindexter, B.; Uauy, R. Nutritional Care of Preterm Infants: Scientific Basis and Practical Guidelines; Karger: Basel, Switzerland, 2014. [Google Scholar]
- Tsang, R.C.; Uauy, R.; Koletzko, B.; Zlotkin, S. Appendix 3-summary of reasonable nutrient intakes (mass units) for preterm infants. In Nutrition of the Preterm Infant: Scientific Basis and Practical Guidelines, 2nd ed.; Tsang, R.C., Uauy, R., Koletzko, B., Zlotkin, S., Eds.; Digital Educational Publishing, Inc.: Cincinnati, OH, USA, 2005; pp. 415–416. [Google Scholar]
- Koletzko, B.; Goulet, O.; Hunt, J.; Krohn, K.; Shamir, R. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), Supported by the European Society of Paediatric Research (ESPR). J. Pediatr. Gastroenterol. Nutr. 2005, 41, S1–S87. [Google Scholar] [PubMed]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellof, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; McGowan, P.; Herwitker, S.; Hart, A.E.; Turner, M.A. Postnatal head growth in preterm infants: A randomized controlled parenteral nutrition study. Pediatrics 2014, 133, e120–e128. [Google Scholar] [CrossRef] [PubMed]
- Cormack, B.E.; Bloomfield, F.H. Increased protein intake decreases postnatal growth faltering in ELBW babies. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F399–F404. [Google Scholar] [CrossRef] [PubMed]
- Moltu, S.J.; Strommen, K.; Blakstad, E.W.; Almaas, A.N.; Westerberg, A.C.; Braekke, K.; Ronnestad, A.; Nakstad, B.; Berg, J.P.; Veierod, M.B.; et al. Enhanced feeding in very-low-birth-weight infants may cause electrolyte disturbances and septicemia—A randomized, controlled trial. Clin. Nutr. 2013, 32, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.L.; Gong, A.K.; Schoolfield, J.; Green, B.K.; Daniels, W.; Liechty, E.A.; Ramamurthy, R. Impact of early and high amino acid supplementation on ELBW infants at 2 years. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Bonsante, F.; Iacobelli, S.; Chantegret, C.; Martin, D.; Gouyon, J.B. The effect of parenteral nitrogen and energy intake on electrolyte balance in the preterm infant. Eur. J. Clin. Nutr. 2011, 65, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Brener Dik, P.H.; Galletti, M.F.; Fernandez Jonusas, S.A.; Alonso, G.; Mariani, G.L.; Fustinana, C.A. Early hypophosphatemia in preterm infants receiving aggressive parenteral nutrition. J. Perinat. 2015, 35, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Guellec, I.; Gascoin, G.; Beuchee, A.; Boubred, F.; Tourneux, P.; Ramful, D.; Zana-Taieb, E.; Baud, O. Biological impact of recent guidelines on parenteral nutrition in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Probst, Y.; Morrison, E.; Sullivan, E.; Dam, H.K. First-stage development and validation of a web-based automated dietary modeling tool: Using constraint optimization techniques to streamline food group and macronutrient focused dietary prescriptions for clinical trials. J. Med. Internet Res. 2016, 18, e190. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Sasaki, S.; Murakami, K.; Yokoyama, T.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Designing optimal food intake patterns to achieve nutritional goals for Japanese adults through the use of linear programming optimization models. Nutr. J. 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Masset, G.; Monsivais, P.; Maillot, M.; Darmon, N.; Drewnowski, A. Diet optimization methods can help translate dietary guidelines into a cancer prevention food plan. J. Nutr. 2009, 139, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Soden, P.M.; Fletcher, L.R. Modifying diets to satisfy nutritional requirements using linear programming. Br. J. Nutr. 1992, 68, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Briend, A.; Darmon, N.; Ferguson, E.; Erhardt, J.G. Linear programming: A mathematical tool for analyzing and optimizing children’s diets during the complementary feeding period. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.M.; Murphy, B.P.; Kiely, M. Nutritional management and assessment of preterm infants: The BabyGrow longitudinal nutrition and growth study. Top. Clin. Nutr. 2015, 30, 80–93. [Google Scholar] [CrossRef]
- Tudehope, D.; Fewtrell, M.; Kashyap, S.; Udaeta, E. Nutritional needs of the micropreterm infant. J. Pediatr. 2013, 162, S72–S80. [Google Scholar] [CrossRef] [PubMed]
- Cormack, B.E.; Embleton, N.D.; van Goudoever, J.B.; Hay, W.W., Jr.; Bloomfield, F.H. Comparing apples with apples: It is time for standardized reporting of neonatal nutrition and growth studies. Pediatr. Res. 2016, 79, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Food Standard Agency (FSA). McCance and Widdowson’s The Composition of Foods, 6th ed.; Royal Society of Chemistry: Cambridge, UK, 2002.
- Food and Agriculture Organization of the United Nations (FAO). Energy and Protein Requirements: Report of a Joint FAO/WHO Ad Hoc Expert Committee; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 1971; pp. 1–118. [Google Scholar]
- Atwater, W.O.; Bryant, A.P. The Availability and Fuel Value of Food Materials; 12th Annual Report 73-110; US Government Printing Office, (Agriculture Experiment Station): Washington, DC, USA, 1900.
- Ball, P.A.; Booth, I.W.; Holden, C.E.; Puntis, J.W. Paediatric Parenteral Nutrition, 3rd ed.; Pharmacia Ltd.: Milton Keynes, UK, 1998. [Google Scholar]
- Iacobelli, S.; Viaud, M.; Lapillonne, A.; Robillard, P.Y.; Gouyon, J.B.; Bonsante, F. Nutrition practice, compliance to guidelines and postnatal growth in moderately premature babies: The NUTRIQUAL French survey. BMC Pediatr. 2015, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.; Khashu, M.; Mukherjee, A.; Kairamkonda, V. Iatrogenic malnutrition in neonatal intensive care units: Urgent need to modify practice. J. Parenter. Enter. Nutr. 2008, 32, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Herwitker, S.; Badhawi, I.; Hart, A.; Tan, M.; Mayes, K.; Newland, P.; Turner, M.A. SCAMP: Standardised, concentrated, additional macronutrients, parenteral nutrition in very preterm infants: A phase IV randomised, controlled exploratory study of macronutrient intake, growth and other aspects of neonatal care. BMC Pediatr. 2011, 11, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, W.W.; Ziegler, E.E. Growth failure among preterm infants due to insufficient protein is not innocuous and must be prevented. J. Perinat. 2016, 36, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.V.; Brennan-Donnan, J.; Unger, S.; Bando, N.; Gibbins, S.; Nash, A.; Kiss, A.; O’Connor, D.L. How close are we to achieving energy and nutrient goals for very low birth weight infants in the first week? J. Parenter. Enter. Nutr. 2017, 41, 500–506. [Google Scholar] [CrossRef] [PubMed]
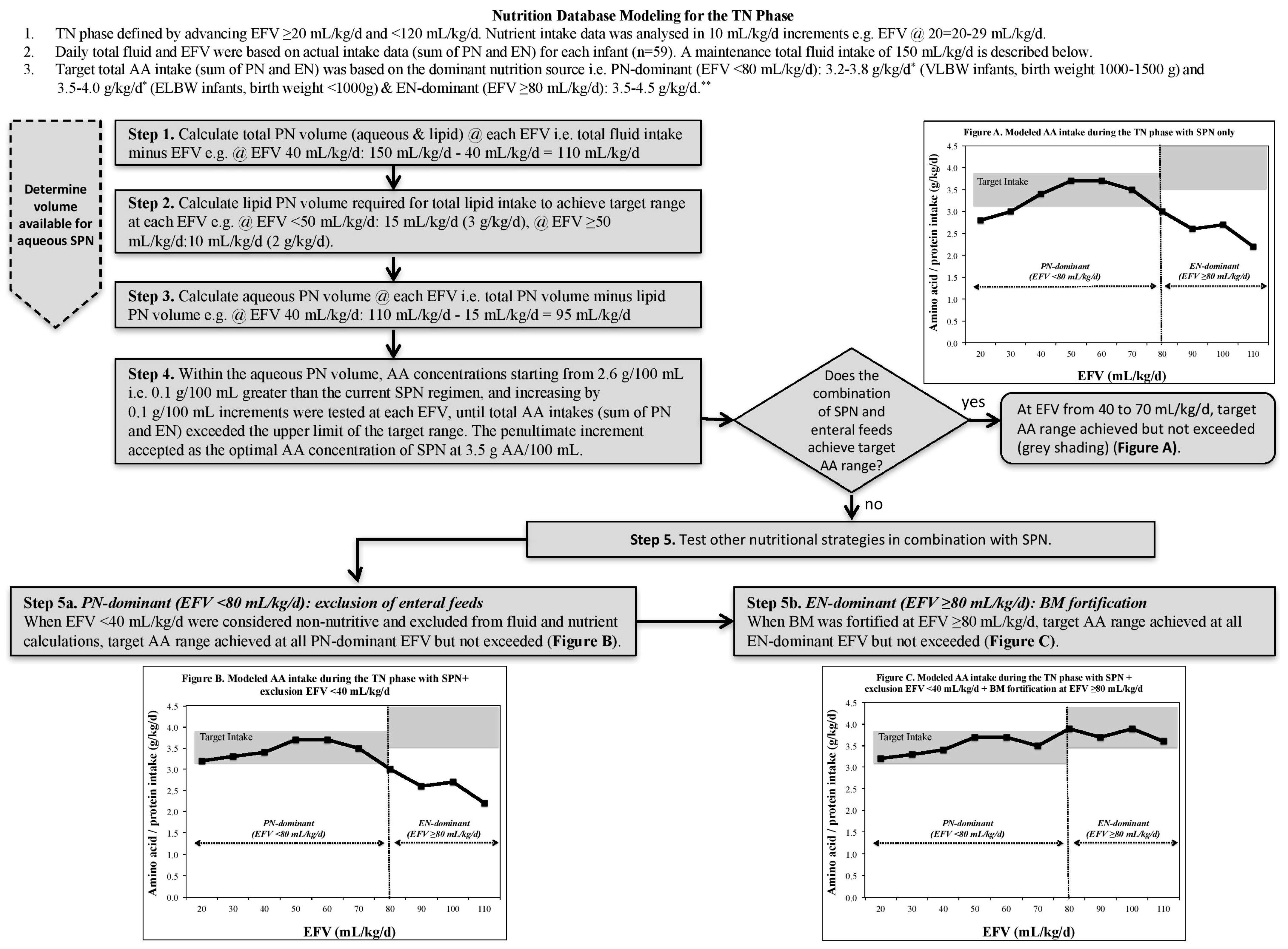
| Nutritional * | Amino acid, g/kg/day | PN-dominant TN phase: 3.5–4.0 (ELBW), 3.2–3.8 (VLBW)
EN-dominant TN phase: 3.5–4.5 ** |
| Lipid, g/kg/day | PN-dominant TN phase: 3.0–4.0 ***
EN-dominant TN phase: 4.8–6.6 | |
| Glucose, g/kg/day | PN-dominant TN phase: 13.0–17.0 (ELBW), 9.7–15.0 (VLBW)
EN-dominant TN phase: 11.6–13.2 | |
| Energy, kcal/kg/day | PN-dominant TN phase: 90–115
EN-dominant TN phase: 110–130 | |
| Fluid | Total daily fluid intake | Actual total daily fluid intakes were not altered |
| Enteral feed intake | Actual daily enteral feed intakes were not altered | |
| Parenteral lipid | Each g lipid is delivered in 5 mL (20% concentration) | |
| IV fluid concentration | PN regimens and IV fluids set at a maximum 12.5% dextrose to allow flexibility for peripheral and central access |
| ELBW (n = 12) | VLBW < 30 Weeks (n = 23) | VLBW ≥ 30 Weeks (n = 24) | P * | |
|---|---|---|---|---|
| Perinatal and postnatal data | ||||
| Male | 5 (42%) | 9 (39%) | 10 (42%) | 0.98 |
| Gestational age, weeks | 26.9 ± 1.8 a | 28.0 ± 0.8 b | 31.3 ± 1.1 c | <0.001 |
| Birth weight, g | 834 ± 113 a | 1220 ± 120 b | 1330 ± 140 c | <0.001 |
| SGA at birth | 4 (33%) | 1 (4%) | 11 (46%) | 0.005 |
| Maternal hypertension | 3 (25%) | 1 (4%) | 7 (29%) | 0.08 |
| Cesarean section | 7 (58%) | 14 (61%) | 21 (88%) | 0.07 |
| Antenatal steroids | 11 (92%) | 20 (87%) | 22 (92%) | 0.84 |
| Multiple births | 6 (50%) | 11 (48%) | 16 (67%) | 0.39 |
| Nasal CPAP | 12 (100%) | 22 (96%) | 17 (71%) | 0.01 |
| Conventional ventilation after birth | 9 (75%) | 13 (57%) | 4 (17%) | 0.001 |
| Chronic lung disease | 3 (25%) | 1 (4%) | 0 | 0.02 |
| Patent ductus arteriosus | 9 (75%) | 12 (52%) | 6 (25%) | 0.01 |
| Late onset sepsis | 2 (17%) | 5 (22%) | 2 (8%) | 0.44 |
| Nutrition data | ||||
| Age PN commenced, day | 1.0 ± 0.0 | 1.1 ± 0.3 | 1.2 ± 0.4 | 0.24 |
| Age lipid commenced, day | 1.5 ± 0.5 a,b | 2.2 ± 1.1 a | 1.6 ± 1.0 b | 0.04 |
| Individualized PN | 12 (100%) | 16 (70%) | 3 (13%) | <0.001 |
| Duration of PN phase, day | 6.3 ± 2.8 a | 4.5 ± 1.7 b | 2.6 ± 1.1 c | <0.001 |
| Duration of TN phase, day | 9.0 ± 2.2 a | 6.0 ± 3.0 b | 5.9 ± 3.0 b | 0.005 |
| Days receiving PN | 15.3 ± 3.5 a | 10.5 ± 3.7 b | 8.5 ± 3.1 b | <0.001 |
| Age EN commenced, day | 2.9 ± 2.0 a | 2.9 ± 0.6 a | 1.9 ± 0.7 b | 0.003 |
| Age when feeds ≥ 150 mL/kg/day achieved, day | 17.7 ± 4.5 a | 13.0 ± 4.0 b | 10.7 ± 2.7 b | <0.001 |
| Fortification of BM at EN volume, mL/kg/day | 117 ± 22 | 121 ± 20 | 125 ± 20 | 0.61 |
| BM, any ** | 12 (100%) | 23 (100%) | 21 (88%) | 0.10 |
| BM, >80% of total enteral feeds | 12 (100%) | 21 (91%) | 19 (79%) | 0.16 |
| Enteral Nutrition | Parenteral Nutrition | Nutritional Strategy | ||
|---|---|---|---|---|
| Enteral Feed Volume mL/kg/day | Target Aqueous Volume mL/kg/day | Target Lipid Volume mL/kg/day | Target Total PN Volume mL/kg/day | |
| 40 | 95 | 15 (3 g/kg/day) | 110 | |
| 50 | 85 | 15 (3 g/kg/day) | 100 | |
| 60 | 80 | 10 (2 g/kg/day) | 90 | Reduce lipid from 3 to 2 g/kg/day |
| 70 | 70 | 10 (2 g/kg/day) | 80 | |
| 80 | 60 | 10 (2 g/kg/day) | 70 | Commence breastmilk fortifier |
| 90 | 50 | 10 (2 g/kg/day) | 60 | |
| 100 | 40 | 10 (2 g/kg/day) | 50 | |
| 110 | 30 | 10 (2 g/kg/day) | 40 | |
| 120 | Consider stopping PN | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brennan, A.-M.; Kiely, M.E.; Fenton, S.; Murphy, B.P. Standardized Parenteral Nutrition for the Transition Phase in Preterm Infants: A Bag That Fits. Nutrients 2018, 10, 170. https://doi.org/10.3390/nu10020170
Brennan A-M, Kiely ME, Fenton S, Murphy BP. Standardized Parenteral Nutrition for the Transition Phase in Preterm Infants: A Bag That Fits. Nutrients. 2018; 10(2):170. https://doi.org/10.3390/nu10020170
Chicago/Turabian StyleBrennan, Ann-Marie, Mairead E. Kiely, Sarah Fenton, and Brendan P. Murphy. 2018. "Standardized Parenteral Nutrition for the Transition Phase in Preterm Infants: A Bag That Fits" Nutrients 10, no. 2: 170. https://doi.org/10.3390/nu10020170
APA StyleBrennan, A.-M., Kiely, M. E., Fenton, S., & Murphy, B. P. (2018). Standardized Parenteral Nutrition for the Transition Phase in Preterm Infants: A Bag That Fits. Nutrients, 10(2), 170. https://doi.org/10.3390/nu10020170





