Empirically Derived Dietary Patterns in UK Adults Are Associated with Sociodemographic Characteristics, Lifestyle, and Diet Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. Statistical Analyses
3. Results
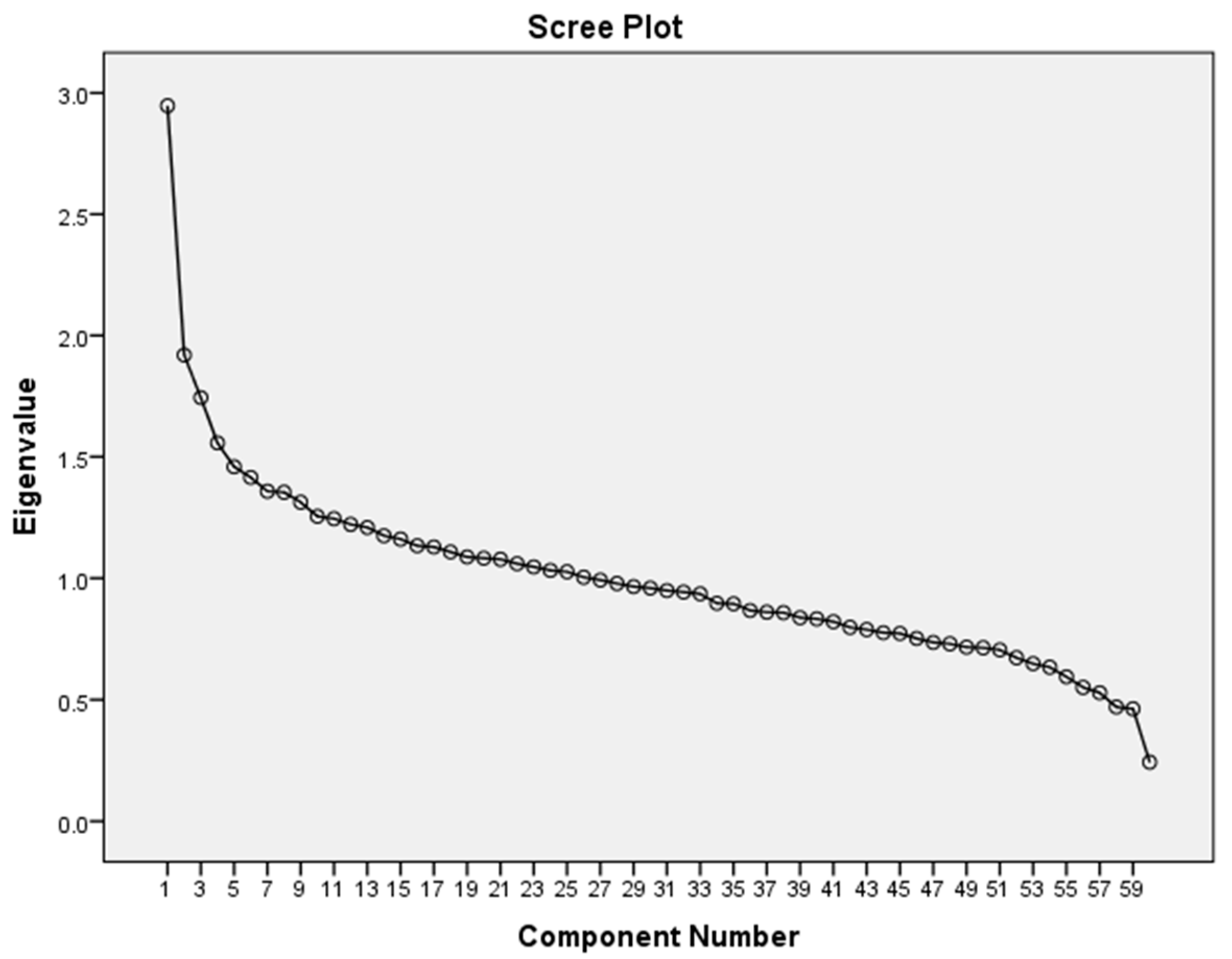
3.1. Principal Component Analysis of Dietary Patterns
3.2. Regression Analyses
3.2.1. Associations between Dietary Patterns and Sociodemographic Characteristics and Lifestyle Factors
3.2.2. Associations between Dietary Patterns and Nutrient Intake and Diet Quality
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Willett, W.C. Nutritional Epidemiology; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Phytochemicals: Guardians of our health. J. Am. Diet. Assoc. 1997, 97, S199–S204. [Google Scholar] [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed]
- Mertz, W. Foods and nutrients. J. Am. Diet. Assoc. 1984, 84, 769–770. [Google Scholar] [PubMed]
- Kant, A.K. Indexes of overall diet quality: A review. J. Am. Diet. Assoc. 1996, 96, 785–791. [Google Scholar] [CrossRef]
- Cunha, D.B.; Varnier, R.M.; de Almeda, R.; Pereira, R.A. A comparison of three statistical methods applied in the identification of eating patterns. Cadernos de Saúde Pública 2010, 26, 2138–2148. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; McCullough, M.L. Dietary pattern analysis for the evaluation of dietary guidelines. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 1), 75–78. [Google Scholar] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Maiorino, M.I.; Ceriello, A.; Giugliano, D. Prevention and control of type 2 diabetes by mediterranean diet: A systematic review. Diabetes Res. Clin. Pract. 2010, 89, 97–102. [Google Scholar] [CrossRef] [PubMed]
- De Lorgeril, M.; Salen, P. The mediterranean-style diet for the prevention of cardiovascular diseases. Public Health Nutr. 2006, 9, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.T.; Ohls, J.; Carlson, S.; Fleming, K. The healthy eating index: Design and applications. J. Am. Diet. Assoc. 1995, 95, 1103–1108. [Google Scholar] [CrossRef]
- Guo, X.; Warden, B.A.; Paeratakul, S.; Bray, G.A. Healthy eating index and obesity. Eur. J. Clin. Nutr. 2004, 58, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.J.; Vogt, T.M.; Gerrior, S.A. Healthy eating index scores are associated with blood nutrient concentrations in the third national health and nutrition examination survey. J. Am. Diet. Assoc. 2004, 104, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.S.; Freeland-Graves, J.H.; Cahill, J.M.; Lu, H.; Graves, G.R. Diet quality as measured by the healthy eating index and the association with lipid profile in low-income women in early postpartum. J. Am. Diet. Assoc. 2010, 110, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Aickin, M.; Champagne, C.; Craddick, S.; Sacks, F.M.; McCarron, P.; Most-Windhauser, M.M.; Rukenbrod, F.; Haworth, L.; Dash-Sodium Collaborative Research Group. Food group sources of nutrients in the dietary patterns of the Dash-Sodium trial. J. Am. Diet. Assoc. 2003, 103, 488–496. [Google Scholar] [PubMed]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., III; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. Dash-sodium collaborative research group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Reedy, J.; Wirfalt, E.; Flood, A.; Mitrou, P.N.; Krebs-Smith, S.M.; Kipnis, V.; Midthune, D.; Leitzmann, M.; Hollenbeck, A.; Schatzkin, A.; et al. Comparing 3 dietary pattern methods—Cluster analysis, factor analysis, and index analysis—With colorectal cancer risk: The NIH-AARP diet and health study. Am. J. Epidemiol. 2010, 171, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Hu, F.B. Dietary patterns and risk of hypertension, type 2 diabetes mellitus, and coronary heart disease. Curr. Atheroscler. Rep. 2002, 4, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Osler, M.; Helms Andreasen, A.; Heitmann, B.; Hoidrup, S.; Gerdes, U.; Morch Jorgensen, L.; Schroll, M. Food intake patterns and risk of coronary heart disease: A prospective cohort study examining the use of traditional scoring techniques. Eur. J. Clin. Nutr. 2002, 56, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.C.; Cade, J.E.; Draper, A.; Barrett, J.H.; Calvert, C.; Greenhalgh, A. Seven unique food consumption patterns identified among women in the UK women’s cohort study. Eur. J. Clin. Nutr. 2000, 54, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Bermudez, O.I.; Tucker, K.L. Dietary patterns of Hispanic elders are associated with acculturation and obesity. J. Nutr. 2003, 133, 3651–3657. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Mander, A.P.; Jones, L.R.; Emmett, P.M.; Jebb, S.A. Energy-dense, low-fiber, high-fat dietary pattern is associated with increased fatness in childhood. Am. J. Clin. Nutr. 2008, 87, 846–854. [Google Scholar] [PubMed]
- Ambrosini, G.L.; Emmett, P.M.; Northstone, K.; Howe, L.D.; Tilling, K.; Jebb, S.A. Identification of a dietary pattern prospectively associated with increased adiposity during childhood and adolescence. Int. J. Obes. 2012, 36, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Northstone, K.; Emmett, P.M. Dietary patterns of men in ALSPAC: Associations with socio-demographic and lifestyle characteristics, nutrient intake and comparison with women’s dietary patterns. Eur. J. Clin. Nutr. 2010, 64, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Northstone, K.; Joinson, C.; Emmett, P.; Ness, A.; Paus, T. Are dietary patterns in childhood associated with IQ at 8 years of age? A population-based cohort study. J. Epidemiol. Community Health 2012, 66, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, L.; Sabates, R.; Sorhaindo, A.; Rogers, I.; Herrick, D.; Northstone, K.; Emmett, P. Dietary patterns related to attainment in school: The importance of early eating patterns. J. Epidemiol. Community Health 2008, 62, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.D.; dos Santos Silva, I.; McNaughton, S.A.; Stephen, A.; Kuh, D. Energy intake and dietary patterns in childhood and throughout adulthood and mammographic density: Results from a British prospective cohort. Cancer Causes Control 2011, 22, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.O.; Jameson, K.A.; Syddall, H.E.; Aihie Sayer, A.; Dennison, E.M.; Cooper, C.; Robinson, S.M.; Hertfordshire Cohort Study Group. The relationship of dietary patterns with adult lung function and COPD. Eur. Respir. J. 2010, 36, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Poslusna, K.; Ruprich, J.; de Vries, J.H.; Jakubikova, M.; van’t Veer, P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br. J. Nutr. 2009, 101 (Suppl. 2), S73–S85. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Hand, G.A.; Blair, S.N. Validity of U.S. Nutritional surveillance: National health and nutrition examination survey caloric energy intake data, 1971–2010. PLoS ONE 2013, 8, e76632. [Google Scholar] [CrossRef] [PubMed]
- Freedman, L.S.; Commins, J.M.; Moler, J.E.; Arab, L.; Baer, D.J.; Kipnis, V.; Midthune, D.; Moshfegh, A.J.; Neuhouser, M.L.; Prentice, R.L.; et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am. J. Epidemiol. 2014, 180, 172–188. [Google Scholar]
- Schoeller, D.; Archer, E.; Dawson, J.A.; Heymsfield, S. Implausible results from the use of invalid methods. J. Nutr. 2015, 145, 150. [Google Scholar] [CrossRef] [PubMed]
- Schoeller, D.A.; Thomas, D.; Archer, E.; Heymsfield, S.B.; Blair, S.N.; Goran, M.I.; Hill, J.O.; Atkinson, R.L.; Corkey, B.E.; Foreyt, J.; et al. Self-report-based estimates of energy intake offer an inadequate basis for scientific conclusions. Am. J. Clin. Nutr. 2013, 97, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Freedman, L.S.; Tooze, J.A.; Kirkpatrick, S.I.; Boushey, C.; Neuhouser, M.L.; Thompson, F.E.; Potischman, N.; Guenther, P.M.; Tarasuk, V.; et al. Addressing current criticism regarding the value of self-report dietary data. J. Nutr. 2015, 145, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Freedman, L.S.; Schatzkin, A.; Midthune, D.; Kipnis, V. Dealing with dietary measurement error in nutritional cohort studies. J. Natl. Cancer Inst. 2011, 103, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Stampfer, M.J.; Rimm, E.; Ascherio, A.; Rosner, B.A.; Spiegelman, D.; Willett, W.C. Dietary fat and coronary heart disease: A comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am. J. Epidemiol. 1999, 149, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Cade, J.E. Measuring diet in the 21st century: Use of new technologies. Proc. Nutr. Soc. 2017, 76, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Cade, J.E.; Warthon-Medina, M.; Albar, S.; Alwan, N.A.; Ness, A.; Roe, M.; Wark, P.A.; Greathead, K.; Burley, V.J.; Finglas, P.; et al. Diet@net: Best practice guidelines for dietary assessment in health research. BMC Med. 2017, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Black, A.E. Physical activity levels from a meta-analysis of doubly labeled water studies for validating energy intake as measured by dietary assessment. Nutr. Rev. 1996, 54, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.J.; Davies, P.S. The validity of self-reported energy intake as determined using the doubly labelled water technique. Br. J. Nutr. 2001, 85, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Prentice, R.L.; Huang, Y.; Tinker, L.F.; Beresford, S.A.; Lampe, J.W.; Neuhouser, M.L. Statistical aspects of the use of biomarkers in nutritional epidemiology research. Stat. Biosci. 2009, 1, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Willet, W.; Rimm, E.B.; Hu, F.B. Response to E Archer. Am. J. Clin. Nutr. 2017, 106, 950–951. [Google Scholar]
- Hebert, J.R.; Hurley, T.G.; Steck, S.E.; Miller, D.R.; Tabung, F.K.; Peterson, K.E.; Kushi, L.H.; Frongillo, E.A. Considering the value of dietary assessment data in informing nutrition-related health policy. Adv. Nutr. 2014, 5, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. Programme User Guide for UK Core Sample Data; Public Health England: London, UK, 2014.
- NatCen Social Research MHNRaUCL. National Diet and Nutrition Survey 1–4, 2008/09–2011/12, 7th ed.; UK Data Service: Colchester, UK, 2015. [Google Scholar]
- Whitton, C.; Nicholson, S.K.; Roberts, C.; Prynne, C.J.; Pot, G.K.; Olson, A.; Fitt, E.; Cole, D.; Teucher, B.; Bates, B.; et al. National diet and nutrition survey: UK food consumption and nutrient intakes from the first year of the rolling programme and comparisons with previous surveys. Br. J. Nutr. 2011, 106, 1899–1914. [Google Scholar] [CrossRef] [PubMed]
- Newby, P.K.; Tucker, K.L. Empirically derived eating patterns using factor or cluster analysis: A review. Nutr. Rev. 2004, 62, 177–203. [Google Scholar] [CrossRef] [PubMed]
- Field, A. Discovering Statistics Using Spss; Sage Publications: London, UK, 2009. [Google Scholar]
- Northstone, K.; Emmett, P.; Rogers, I. Dietary patterns in pregnancy and associations with socio-demographic and lifestyle factors. Eur. J. Clin. Nutr. 2008, 62, 471–479. [Google Scholar] [CrossRef] [PubMed]
- North, K.; Emmett, P. Multivariate analysis of diet among three-year-old children and associations with socio-demographic characteristics. The Avon Longitudinal Study of Pregnancy and Childhood (ALSPAC) Study Team. Eur. J. Clin. Nutr. 2000, 54, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Pot, G.K.; Stephen, A.M.; Dahm, C.C.; Key, T.J.; Cairns, B.J.; Burley, V.J.; Cade, J.E.; Greenwood, D.C.; Keogh, R.H.; Bhaniani, A.; et al. Dietary patterns derived with multiple methods from food diaries and breast cancer risk in the UK dietary cohort consortium. Eur. J. Clin. Nutr. 2014, 68, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Bennette, C.; Vickers, A. Against quantiles: Categorization of continuous variables in epidemiologic research, and its discontents. BMC Med. Res. Methodol. 2012, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.H. Multivariable Analysis: A Practical Guide for Clinicians and Public Health Researchers; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Committee on Medical Aspects of Food Policy. Dietary reference values for food energy and nutrients for the United Kingdom. Report of the panel on dietary reference values of the committee on medical aspects of food policy. Rep. Health Soc. Subj. 1991, 41, 1–210. [Google Scholar]
- Scientific Advisory Committee on Nutrition. Dietary Reference Values for Food Energy and Nutrients for the United Kingdom; TSO: London, UK, 2011. [Google Scholar]
- England, P.H. Government Dietary Recommendations; Public Health England: London, UK, 2016.
- Public Health England. National Diet and Nutrition Survey Results from Years 1, 2, 3 and 4 (Combined) of the Rolling Programme (2008/09–2011/12); Public Health England: London, UK, 2014.
- Public Health England. 3.8 Million People in England Now Have Diabetes. Available online: https://www.gov.uk/government/news/38-million-people-in-england-now-have-diabetes (accessed on 15 March 2017).
- Public Health England. Sugar Reduction: The Evidence for Action; Public Health England: London, UK, 2015.
- Rayner, M.; Scarborough, P. The burden of food related ill health in the UK. J. Epidemiol. Community Health 2005, 59, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.E. An Investigation of Dietary Patterns in UK Adults as a Method for developing a Brief Diet Quality Assessment Tool. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2017. Available online: http://etheses.whiterose.ac.uk/ (accessed on 15 October 2017).
- Office of National Statistics. The National Statistics Socio-Economic Classification User Manual; Palgrave MacMillan: Basingstoke, UK, 2005.
- Markussen, M.S.; Veierod, M.B.; Sakhi, A.K.; Ellingjord-Dale, M.; Blomhoff, R.; Ursin, G.; Andersen, L.F. Evaluation of dietary patterns among Norwegian postmenopausal women using plasma carotenoids as biomarkers. Br. J. Nutr. 2015, 113, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Marmot, M.; Bell, R. Fair society, healthy lives. Public Health 2012, 126 (Suppl. 1), S4–S10. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Mishra, G.D. Dietary patterns and cardiovascular risk markers in the UK low income diet and nutrition survey. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Northstone, K.; Emmett, P.M.; Rogers, I. Dietary patterns in pregnancy and associations with nutrient intakes. Br. J. Nutr. 2008, 99, 406–415. [Google Scholar] [CrossRef] [PubMed]
- McCourt, H.J.; Draffin, C.R.; Woodside, J.V.; Cardwell, C.R.; Young, I.S.; Hunter, S.J.; Murray, L.J.; Boreham, C.A.; Gallagher, A.M.; Neville, C.E.; et al. Dietary patterns and cardiovascular risk factors in adolescents and young adults: The northern Ireland young hearts project. Br. J. Nutr. 2014, 112, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- McCann, S.E.; Marshall, J.R.; Brasure, J.R.; Graham, S.; Freudenheim, J.L. Analysis of patterns of food intake in nutritional epidemiology: Food classification in principal components analysis and the subsequent impact on estimates for endometrial cancer. Public Health Nutr. 2001, 4, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.; Ashwell, M. Dietary patterns among British adults: Compatibility with dietary guidelines for salt/sodium, fat, saturated fat and sugars. Public Health Nutr. 2011, 14, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
| Food Group Variable | |
|---|---|
| 1. 1% milk | 31. High fibre breakfast cereals |
| 2. Beef, veal, and dishes | 32. Lamb and dishes |
| 3. Butter | 33. Liver products and dishes |
| 4. Other margarine, fats, and oils | 34. Meat pies and pastries |
| 5. Other milk and cream | 35. Nuts and seeds |
| 6. PUFA margarine oils | 36. Oily fish |
| 7. Semi skimmed milk | 37. Other bread |
| 8. Whole milk | 38. Other meat and meat products |
| 9. Wholemeal bread | 39. Pasta rice and other cereals |
| 10. Ice cream | 40. Pork and dishes |
| 11. White fish coated or fried | 41. Salad and other raw vegetables |
| 12. Beer, lager, cider, and perry | 42. Sausages |
| 13. Crisps and savoury snacks | 43. White bread |
| 14. Fruit juice including smoothies | 44. Yogurt, fromage frais, and dairy desserts |
| 15. Soft drinks (low calorie) | 45. Other breakfast cereals |
| 16. Soft drinks (not low calorie) | 46. Other potatoes, potato salads and dishes |
| 17. Spirits and liqueurs | 47. Other white fish, shellfish, fish dishes |
| 18. Sugar confectionery | 48. Vegetables not raw |
| 19. Tea coffee and water | 49. Chicken and turkey dishes |
| 20. Wine | 50. Puddings |
| 21. Bacon and ham | 51. Brown granary and wheat germ bread |
| 22. Biscuits | 52. Skimmed milk |
| 23. Buns, cakes, pastries, and fruit pies | 53. Sugars, preserves, and sweet spreads |
| 24. Burgers and kebabs | 54. Dry weight beverages * |
| 25. Cheese | 55. Low fat spread not polyunsaturated ** |
| 26. Chips (fried), roast potatoes, potato products | 56. Low fat spread polyunsaturated ** |
| 27. Chocolate confectionery | 57. Reduced fat spread not polyunsaturated *** |
| 28. Coated chicken and turkey | 58. Reduced fat spread polyunsaturated *** |
| 29. Eggs and egg dishes | 59. Savoury sauces, pickles, gravies, condiments * |
| 30. Fruit | 60. Soup homemade and retail * |
| Characteristics | n | % | Mean (Standard Deviation) |
|---|---|---|---|
| Males | 901 | 43.3 | |
| Females | 1182 | 56.7 | |
| Age (years) | 49 (17) | ||
| White | 1877 | 91.5 | |
| Non-white | 168 | 8.5 | |
| NSSEC Group | |||
| Higher managerial and professional | 309 | 14.9 | |
| Lower managerial and professional | 553 | 26.6 | |
| Intermediate occupations | 191 | 9.2 | |
| Small employers | 222 | 10.7 | |
| Low supervisory and technical occupations | 203 | 9.8 | |
| Semi-routine occupations | 289 | 13.9 | |
| Routine occupations | 235 | 11.3 | |
| Never worked | 43 | 2.1 | |
| Smoker | 488 | 24 | |
| Non-smoker | 1557 | 76 | |
| Equivalised household income (£) | 1777 | 32,035 (23,570) | |
| BMI (kg/m2) | 1902 | 27.7 (5.4) | |
| Total energy intake diet only (kcal) | 2083 | 1803 (575) |
| Food Variables (Average g/Day) | Principal Component (Dietary Pattern) | |||
|---|---|---|---|---|
| 1 (SFFFD) | 2 (FVOF) | 3 (MPB) | 4 (SFD) | |
| Soft drinks not low calorie | 0.51 | 0.03 | 0.10 | 0.03 |
| Crisps and savoury snacks | 0.48 | −0.01 | 0.00 | 0.24 |
| Soft drinks low calorie | 0.42 | 0.11 | −0.07 | 0.07 |
| Coated chicken and turkey | 0.40 | −0.02 | −0.07 | 0.06 |
| Tea, coffee, and water | −0.38 | 0.20 | 0.05 | 0.25 |
| Burgers and kebabs | 0.37 | −0.08 | 0.004 | −0.01 |
| High fibre breakfast cereals | −0.35 | 0.09 | −0.05 | 0.14 |
| Chips, fried and roast potatoes, and potato products | 0.32 | −0.28 | 0.25 | 0.07 |
| Wholemeal bread | −0.28 | 0.16 | −0.08 | 0.16 |
| Sugar confectionery | 0.27 | 0.18 | −0.05 | 0.24 |
| Pasta, rice, and other cereals | 0.26 | 0.19 | −0.20 | −0.05 |
| Soup homemade and retail | −0.20 | 0.06 | −0.04 | 0.02 |
| Chicken and turkey dishes | 0.17 | 0.14 | −0.12 | −0.09 |
| Low fat spread polyunsaturated | −0.06 | 0.03 | 0.03 | 0.03 |
| Fruit | −0.30 | 0.56 | −0.12 | 0.19 |
| Salad and other raw vegetables | −0.14 | 0.51 | −0.05 | 0.02 |
| Yogurt, fromage frais, and dairy desserts | −0.13 | 0.39 | −0.13 | 0.22 |
| Oily fish | −0.19 | 0.38 | −0.01 | −0.06 |
| Vegetables not raw | −0.23 | 0.34 | 0.22 | 0.00 |
| Fruit juice including smoothies | 0.07 | 0.27 | 0.11 | 0.00 |
| Nuts and seeds | 0.02 | 0.27 | −0.06 | 0.13 |
| Skimmed milk | −0.03 | 0.25 | −0.06 | 0.02 |
| White fish coated or fried | −0.07 | −0.25 | 0.04 | 0.06 |
| Other margarine, fats, and oils | −0.04 | 0.21 | 0.09 | 0.03 |
| Other bread | 0.02 | 0.21 | 0.05 | −0.05 |
| Other white fish, shellfish, and fish dishes | −0.06 | 0.20 | −0.12 | −0.09 |
| Other milk and cream | 0.03 | 0.19 | 0.04 | −0.04 |
| Brown, granary, and wheat germ bread | 0.01 | 0.15 | −0.03 | 0.00 |
| PUFA margarine and oils | −0.05 | 0.09 | 0.04 | 0.02 |
| Other potatoes, potato salads and dishes | −0.23 | 0.14 | 0.47 | 0.03 |
| Sauces, pickles, and gravies | 0.06 | 0.18 | 0.47 | −0.05 |
| White bread | 0.30 | −0.27 | 0.44 | 0.13 |
| Butter | −0.09 | −0.02 | 0.35 | 0.04 |
| Sugar, preserves, and sweet spreads | −0.10 | −0.26 | 0.34 | 0.29 |
| Bacon and ham | 0.18 | 0.05 | 0.34 | 0.09 |
| Beer, lager, cider, and perry | 0.22 | −0.07 | 0.29 | −0.16 |
| Meat pies and pastries | 0.09 | −0.14 | 0.28 | 0.04 |
| Sausages | 0.06 | −0.11 | 0.27 | 0.03 |
| Whole milk | −0.07 | −0.18 | 0.25 | −0.03 |
| Pork and dishes | −0.01 | 0.03 | 0.21 | −0.05 |
| Eggs and egg dishes | −0.07 | 0.16 | 0.20 | −0.15 |
| Other meat and meat products | 0.02 | 0.05 | 0.18 | −0.02 |
| Beef, veal, and dishes | 0.06 | −0.10 | −0.15 | −0.004 |
| Liver and dishes | −0.08 | −0.002 | 0.10 | −0.04 |
| Low fat spread not polyunsaturated | −0.01 | 0.03 | −0.03 | −0.03 |
| Biscuits | 0.04 | 0.04 | −0.13 | 0.47 |
| Semi skimmed milk | −0.17 | −0.12 | −0.03 | 0.43 |
| Chocolate confectionery | 0.35 | 0.13 | 0.02 | 0.40 |
| Buns, cakes, pastries, and fruit pies | −0.12 | 0.11 | 0.19 | 0.38 |
| Wine | 0.03 | 0.28 | 0.13 | −0.31 |
| Puddings | −0.13 | 0.01 | 0.12 | 0.28 |
| Cheese | 0.01 | 0.15 | 0.12 | 0.28 |
| Ice cream | 0.03 | 0.07 | −0.002 | 0.24 |
| Reduced fat spread not polyunsaturated | 0.12 | −0.14 | 0.19 | 0.21 |
| Spirits and liqueurs | 0.17 | 0.08 | 0.15 | −0.20 |
| Reduced fat spread polyunsaturated | −0.08 | −0.13 | 0.01 | 0.16 |
| Other breakfast cereals | 0.001 | −0.04 | 0.001 | 0.15 |
| Dry weight beverages | −0.07 | −0.04 | −0.06 | 0.09 |
| Lamb and dishes | −0.02 | −0.02 | 0.03 | −0.08 |
| Dietary Pattern Label | Foods with Moderate/Strong Positive Factor Loadings (≥0.25) | Foods with Moderate/Strong Negative Factor Loadings (≤−0.25) |
|---|---|---|
| ‘Snacks, fast food, fizzy drinks’ (SFFFD) | Soft drinks (not low calorie) Crisps and savoury snacks Soft drinks (low calorie) Coated chicken and turkey Burgers and kebabs Chocolate confectionery Chips (fried), roast potatoes, and potato products White bread Sugar confectionery Pasta, rice, and other cereals | Tea, coffee, and water High fibre breakfast cereals Fruit Wholemeal bread |
| ‘Fruit, vegetables, oily fish’ (FVOF) | Fruit Salad and other raw vegetables Yoghurt, fromage frais, and dairy deserts Oily fish Vegetables not raw Fruit juice including smoothies Wine Nuts and seeds Skimmed milk | Chips (fried), roast potatoes, and potato products White bread Sugar, preserves, and sweet spreads White fish, coated or fried |
| ‘Meat, potatoes, beer’ (MPB) | Other potatoes, potato salads and dishes Savoury sauces, pickles, gravies, and condiments White bread Butter Sugar, preserves, and sweet spreads Bacon and ham Beer, lager, cider, and perry Meat pies and pastries Sausages Whole milk | |
| ‘Sugary food, dairy’ (SFD) | Biscuits Semi skimmed milk Chocolate confectionery Buns, cakes, pastries, and fruit pies Sugars, preserves, and sweet spreads Puddings Cheese Tea, coffee, and water | Wine |
| Sample Characteristics (n) | Snacks, Fast Food, Fizzy Drinks (SFFFD) | Fruit, Veg, Oily Fish (FVOF) | Meat, Potatoes, Beer (MPB) | Sugary Foods, Dairy (SFD) | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p | Coefficient (95% CI) | p | Coefficient (95% CI) | p | Coefficient (95% CI) | p | |
| Sex | ||||||||
| Male (711) | 0.24 (0.16, 0.33) | <0.001 | −0.08 (−0.08, −0.08) | <0.001 | 0.63 (0.54, 0.72) | <0.001 | 0.19 (0.10, 0.29) | <0.001 |
| Female (914) | Reference * | |||||||
| Age year (2083) | −0.03 (−0.03, −0.03) | <0.001 | 0.002 (0.002, 0.002) | <0.001 | 0.01 (0.004, 0.01) | <0.001 | 0.003 (<0.001, 0.01) | <0.001 |
| Ethnicity | ||||||||
| Non-white (1495) | −0.14 (0.06, −0.27) | 0.72 | 0.22 (0.05, −0.39) | 0.01 | −0.56 (−0.73, −0.39) | <0.001 | −0.46 (−0.65, −0.30) | <0.001 |
| White (130) | Reference * | |||||||
| National Statistics Socio-economic Classification Group (NSSEC) | ||||||||
| Higher managerial and professional (260) | 0.12 (−0.20, 0.30) | 0.46 | 0.25 (0.24, 0.27) | <0.001 | 0.03 (−0.33, 0.37) | 0.89 | 0.45 (0.09, 0.81) | 0.02 |
| Lower managerial and professional (453) | 0.21 (−0.10, 0.51) | 0.18 | 0.14 (0.13, 0.15) | <0.001 | −0.04 (−0.37, 0.30) | 0.83 | 0.43 (0.08, 0.78) | 0.02 |
| Intermediate occupations 1 (149) | 0.21 (−0.11, 0.53) | 0.20 | −0.02 (−0.03, −0.01) | 0.002 | 0.04 (−0.31, 0.40) | 0.81 | 0.38 (0.01, 0.75) | 0.04 |
| Small employers (165) | 0.05 (−0.27, 0.34) | 0.76 | −0.03 (−0.04, −0.02) | <0.001 | 0.25 (−0.10, 0.61) | 0.16 | 0.37 (0.007, 0.74) | 0.05 |
| Low supervisory and technical occupations (160) | 0.16 (−0.16, 0.47) | 0.33 | −0.12 (−0.14, −0.11) | <0.001 | 0.09 (−0.27, 0.44) | 0.63 | 0.53 (0.16, 0.89) | 0.005 |
| Semi-routine occupations 2 (228) | 0.27 (−0.4, 0.56) | 0.09 | −0.11 (−0.12, −0.10) | <0.001 | 0.21 (−0.13, 0.56) | 0.23 | 0.52 (0.16, 0.87) | 0.004 |
| Routine occupations 3 (177) | 0.20 (−0.11, 0.52) | 0.21 | −0.36 (−0.37, −0.35) | <0.001 | 0.20 (−0.16, 0.55) | 0.27 | 0.25 (−0.11, 0.62) | 0.17 |
| Never worked (33) | Reference * | |||||||
| Smoking status | ||||||||
| Smoker (377) | 0.16 (0.06, 0.26) | 0.002 | −0.37 (−0.38, −0.37) | <0.001 | 0.21 (0.10, 0.33) | <0.001 | −0.21 (−0.33, −0.10) | <0.001 |
| Non-smoker (1248) | Reference * | |||||||
| Household income (1777) | 7.627 × 10−7 (−1.207 × 10, 2.733 × 10−6) | 0.45 | 7.112 × 10−6 (7.044 × 10−6, 7.181 × 10−6) | <0.001 | −9.357 × 10−7 (−3.146 × 10−6, 1.274 × 10−6) | 0.41 | −2.192 × 10−6 (−4.471 × 10−6, 8.576 × 10−8) | 0.06 |
| Body Mass Index (BMI) (1902) | 0.13 (0.005, 0.02) | 0.01 | −0.002 (−0.002, −0.001) | <0.001 | −0.003 (−0.12, 0.005) | 0.42 | −0.02 (−0.03, −0.01) | <0.001 |
| Snacks, Fast Foods, Fizzy Drinks | Fruit, Vegetables, Oily Fish | Meat, Potatoes, Beer | Sugary Foods, Dairy | |
|---|---|---|---|---|
| Coefficients (95% Confidence Intervals), * p < 0.05, ** p < 0.01, *** p < 0.001 | ||||
| Nutrient intake | ||||
| Vitamin C per 1000 kcal | −0.01 (−0.006, −0.004) *** | 0.01 (0.011, 0.014) *** | −0.003 (−0.004, −0.002) *** | −0.003 (−0.004, −0.002) *** |
| Vitamin D per 1000 kcal | −0.20 (−0.23, −0.16) *** | 0.17 (0.133, 0.20) *** | 0.01 (−0.03, 0.41) | −0.10 (−0.13, −0.6) *** |
| Vitamin E per 1000 kcal | −0.04 (−0.05, −0.02) *** | 0.08 (0.07, 0.10) *** | −0.06 (−0.7, −0.4) *** | −0.02 (−0.4, −0.01) ** |
| Vitamin B6 per 1000 kcal | −0.19 (−0.27, −0.11) ** | 0.09 (0.01, 0.18) * | −0.03 (−0.16, 0.05) | −0.40 (−0.48, −0.32) *** |
| Vitamin B12 per 1000 kcal | −0.10 (−0.12, −0.08) *** | 0.04 (0.02, 0.05) *** | −0.01 (−0.03, 0.01) | −0.05 (−0.07, −0.03) *** |
| Iron per 1000 kcal | −0.12 (−0.14, −0.10) *** | 0.11 (0.09, 0.14) *** | −0.08 (−0.10, −0.06) *** | −0.14 (−0.16, −0.12) *** |
| Folate per 1000 kcal | −0.01 (−0.006, −0.005) *** | 0.003 (0.003, 0.004) *** | −0.001 (−0.001, 0.001) | −0.003 (−0.004, −0.002) *** |
| Magnesium per 1000 kcal | −0.01 (−0.01, −0.008) *** | 0.01 (0.009, 0.011) *** | −0.01 (−0.006, −0.004) *** | −0.01 (−0.006, −0.004) *** |
| % Food Energy NMES | 0.05 (0.04, 0.05) *** | −0.01 (0.21, 0.007) *** | 0.02 (0.02, 0.03) *** | 0.03 (0.02, 0.03) *** |
| % Food Energy Sat Fat | −0.12 (−0.024, 0.001) | −0.04 (−0.05, −0.02) *** | 0.08 (0.07, 0.09) *** | 0.06 (0.05, 0.07) *** |
| % Food Energy Total Fat | 0.01 (0.01, 0.02) *** | −0.01 (−0.02, −0.002) ** | 0.04 (0.03, 0.05) *** | 0.01 (0.01, −0.02) *** |
| % Food Energy n-3 PUFA | −0.21 (−0.30, −0.12) *** | 0.45 (0.36, 0.54) *** | 0.08 (−0.01, 0.17) | −0.31 (−0.40, −0.22) *** |
| % Food Energy n-6 PUFA | 0.06 (0.03, 0.08) *** | 0.03 (0.002, 0.06) * | −0.004 (−0.03, 0.02) | −0.04 (−0.07, −0.01) ** |
| % Food Energy Starch | 0.01 (0.001, 0.02) * | −0.04 (−0.05, −0.03) *** | −0.02 (−0.03, -0.01) *** | −0.02 (−0.03, −0.01) *** |
| Non-starch polysaccharides (fibre) | −0.03 (−0.04, −0.02) *** | 0.11 (0.10, 0.12) *** | 0.03 (0.02, 0.04) *** | 0.07 (0.06, 0.08) *** |
| Nutrient level from biomarkers | ||||
| Vitamin C | −0.004 (−0.007, −0.001) * | 0.02 (0.01, 0.02) *** | −0.004 (−0.01, −0.001) * | 0.001 (−0.002, 0.004) |
| 25-Hydroxy Vitamin D | −0.002 (−0.004, 0.001) | 0.01 (0.004, 0.009) *** | −0.001 (−0.003, 0.002) | 0.0004 (−0.002, 0.003) |
| Retinol (Vitamin A) | −0.10 (−0.20, 0.004) | 0.13 (0.02, 0.23) * | 0.12 (−0.001, 0.21) | −0.18 (−0.29, −0.08) *** |
| Ferritin (iron) | 4.908 × 10−5 (−0.001, −0.001) | 0.001 (5.153 × 10−5, 0.001) * | 0.001 (0.001, 0.002) *** | −0.001 (−0.001, −3.55) * |
| Triglycerides | 0.01 (−0.05, 0.08) | −0.06 (−0.13, 0.002) | 0.13 (0.06, 0.19) *** | −0.02 (−0.09, 0.04) |
| Total cholesterol | −0.07 (−0.13, −0.02) ** | 0.04 (−0.01, 0.10) | −0.03 (−0.08, 0.03) | −0.07 (−0.13, −0.02) |
| Urinary sodium | 0.01 (0.005, 0.008) *** | −0.01 (−0.006, −0.003) *** | 0.002 (0.0004, 0.003) * | −0.002 (−0.003, 0.0001) * |
| Total carotenoids | −0.08 (−0.15, −0.02) * | 0.32 (0.26, 0.38) *** | −0.16 (−0.23, −0.09) *** | −0.03 (−0.09, 0.04) |
| Dietary Patterns | Coefficient (Unadjusted) | CI (95%) Lower | Upper | Coefficient (Adjusted) | CI (95%) Lower | Upper |
|---|---|---|---|---|---|---|
| Snacks, Fast Food, Fizzy Drinks (SFFFD) | −3.727 | −4.205 | −3.249 | −3.31 | −3.97 | −2.64 |
| Fruit, Vegetables, Oily Fish (FVOF) | 4.514 | 4.037 | 4.992 | 3.79 | 3.19 | 4.40 |
| Meat, Potatoes, Beer (MPB) | −1.183 | −1.660 | −0.705 | −1.76 | −2.43 | −1.09 |
| Sugary Foods, Dairy (SFD) | 2.425 | 1.948 | 2.903 | 1.31 | 0.69 | 1.93 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberts, K.; Cade, J.; Dawson, J.; Holdsworth, M. Empirically Derived Dietary Patterns in UK Adults Are Associated with Sociodemographic Characteristics, Lifestyle, and Diet Quality. Nutrients 2018, 10, 177. https://doi.org/10.3390/nu10020177
Roberts K, Cade J, Dawson J, Holdsworth M. Empirically Derived Dietary Patterns in UK Adults Are Associated with Sociodemographic Characteristics, Lifestyle, and Diet Quality. Nutrients. 2018; 10(2):177. https://doi.org/10.3390/nu10020177
Chicago/Turabian StyleRoberts, Katharine, Janet Cade, Jeremy Dawson, and Michelle Holdsworth. 2018. "Empirically Derived Dietary Patterns in UK Adults Are Associated with Sociodemographic Characteristics, Lifestyle, and Diet Quality" Nutrients 10, no. 2: 177. https://doi.org/10.3390/nu10020177





