In Vivo Protective Effects of Nootkatone against Particles-Induced Lung Injury Caused by Diesel Exhaust Is Mediated via the NF-κB Pathway
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals and Treatments
2.2. Diesel Exhaust Particles (DEP) and Animal Treatments
- Group 1: Normal saline administered by gavage 1 h prior to pulmonary exposure to vehicle;
- Group 2: Normal saline administered by gavage 1 h prior to pulmonary exposure to DEP (30 μg/mouse);
- Group 3: Nootkatone (90 mg/kg) administered by gavage 1 h prior to pulmonary exposure to vehicle;
- Group 4: Nootkatone (90 mg/kg) administered by gavage 1 h prior to pulmonary exposure to DEP (30 μg/mouse).
2.3. Airway Reactivity to Methacholine
2.4. Collection and Analysis of Bronchoalveolar (BAL) Fluid
2.5. Histology
2.6. Measurement of 8-Isoprostane, Reduced Glutathione (GSH), Total Nitric Oxide (NO) and Tumor Necrosis Factor (TNFα) in Lung Homogenates
2.7. Western Blot Analysis
2.8. DNA Damage Assessment by COMET Assay
2.9. Statistics
3. Results
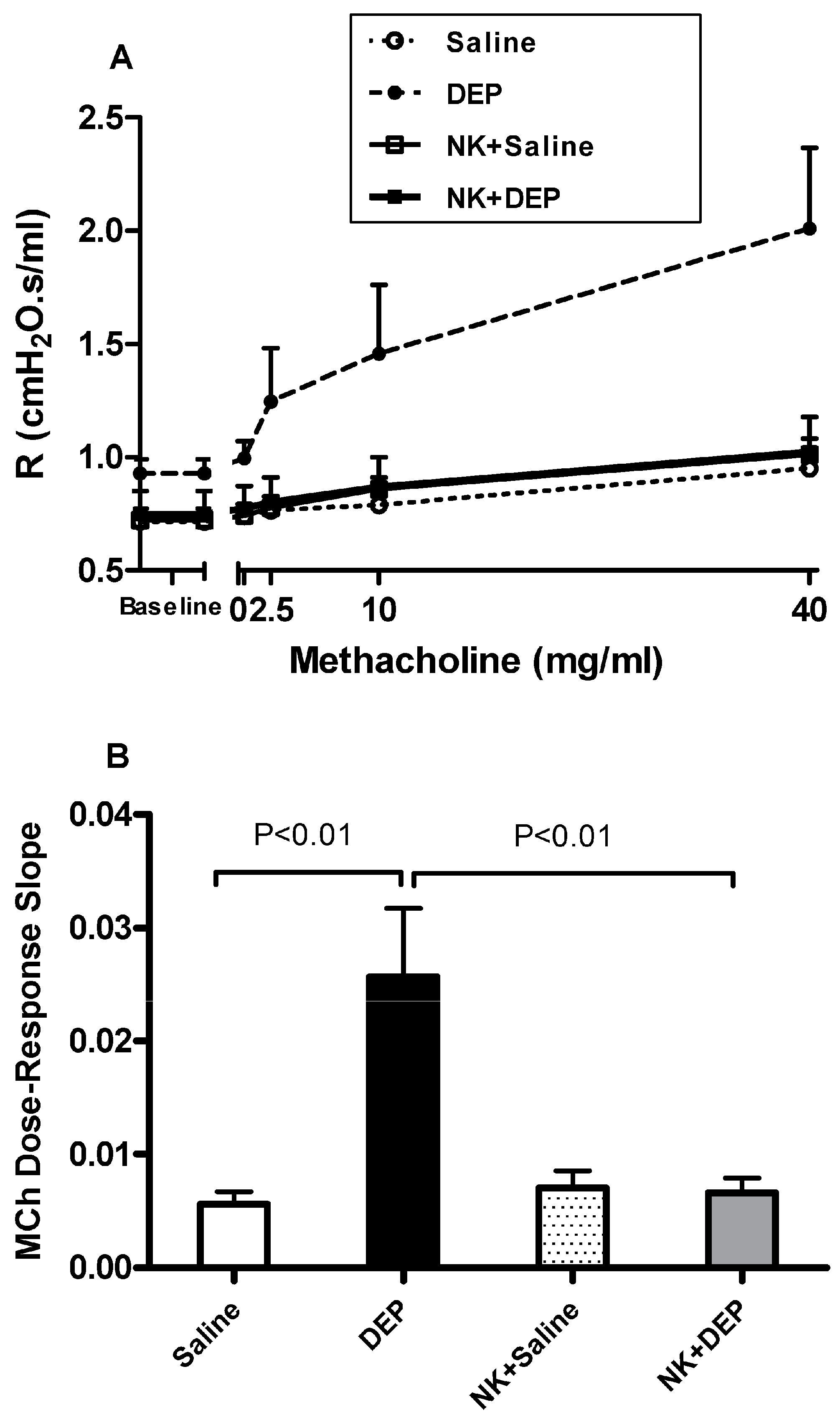
3.1. Airway Hyperreactivity to Methacholine
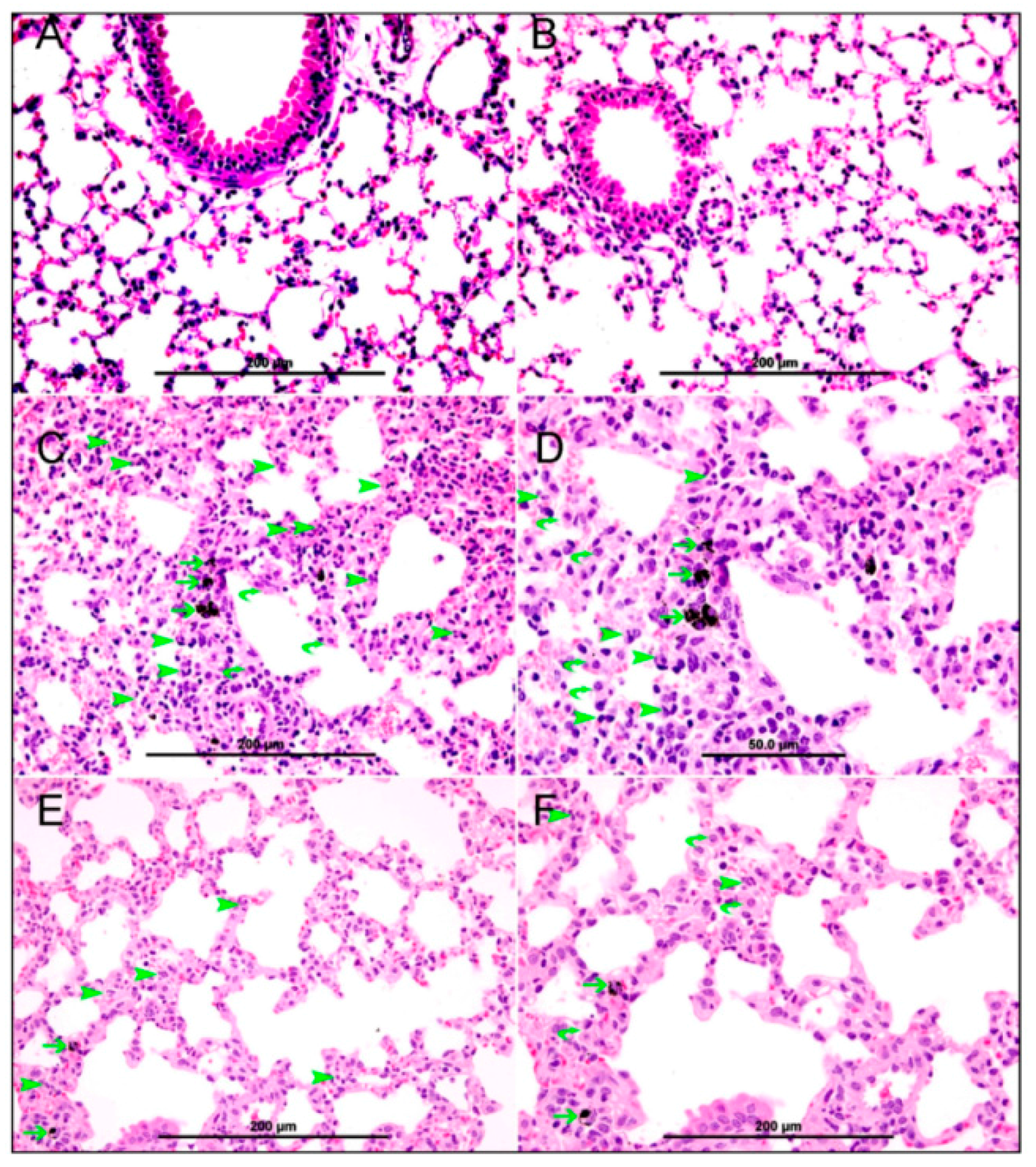
3.2. Lung Histology
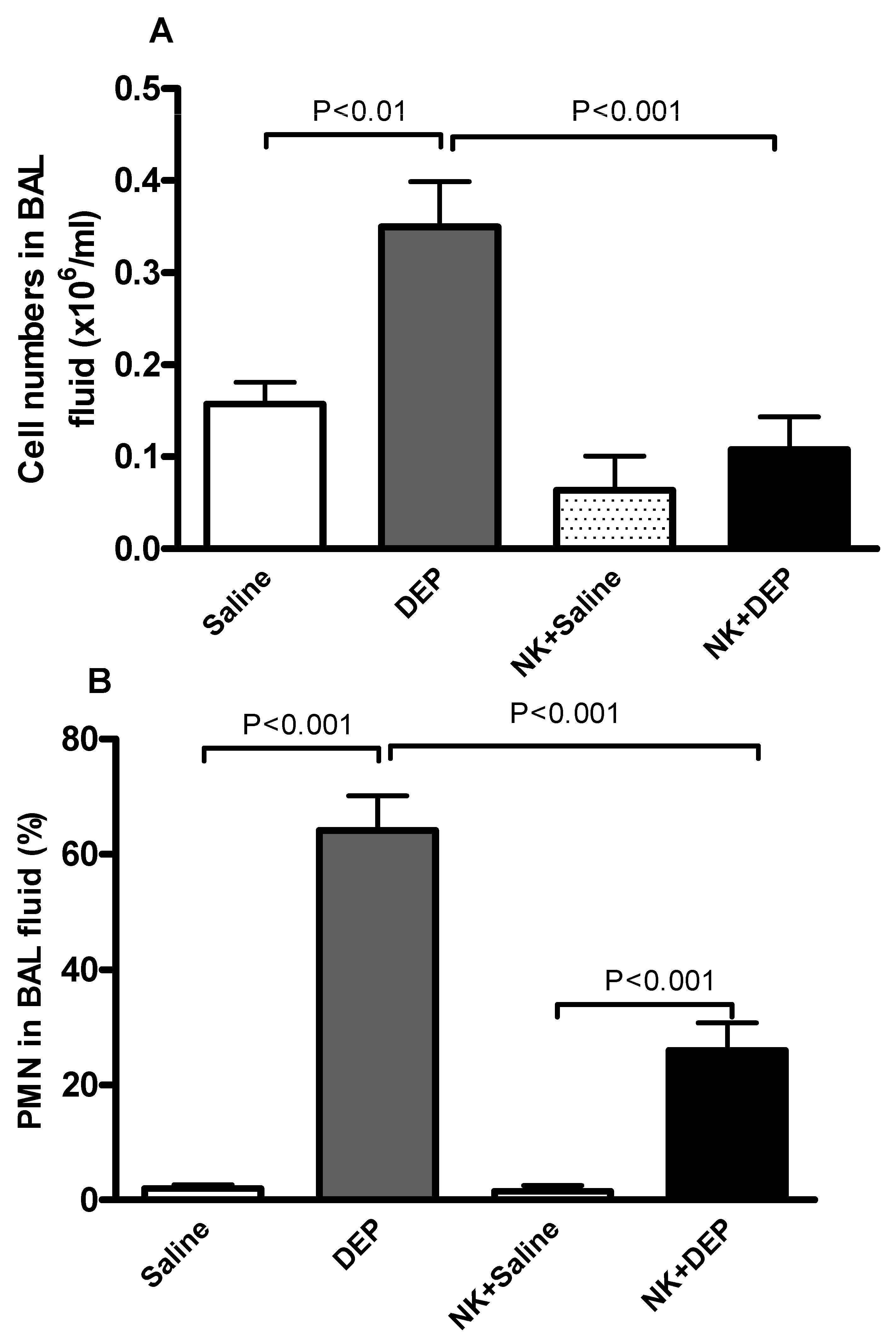
3.3. Cell Composition and Number in BAL Fluid
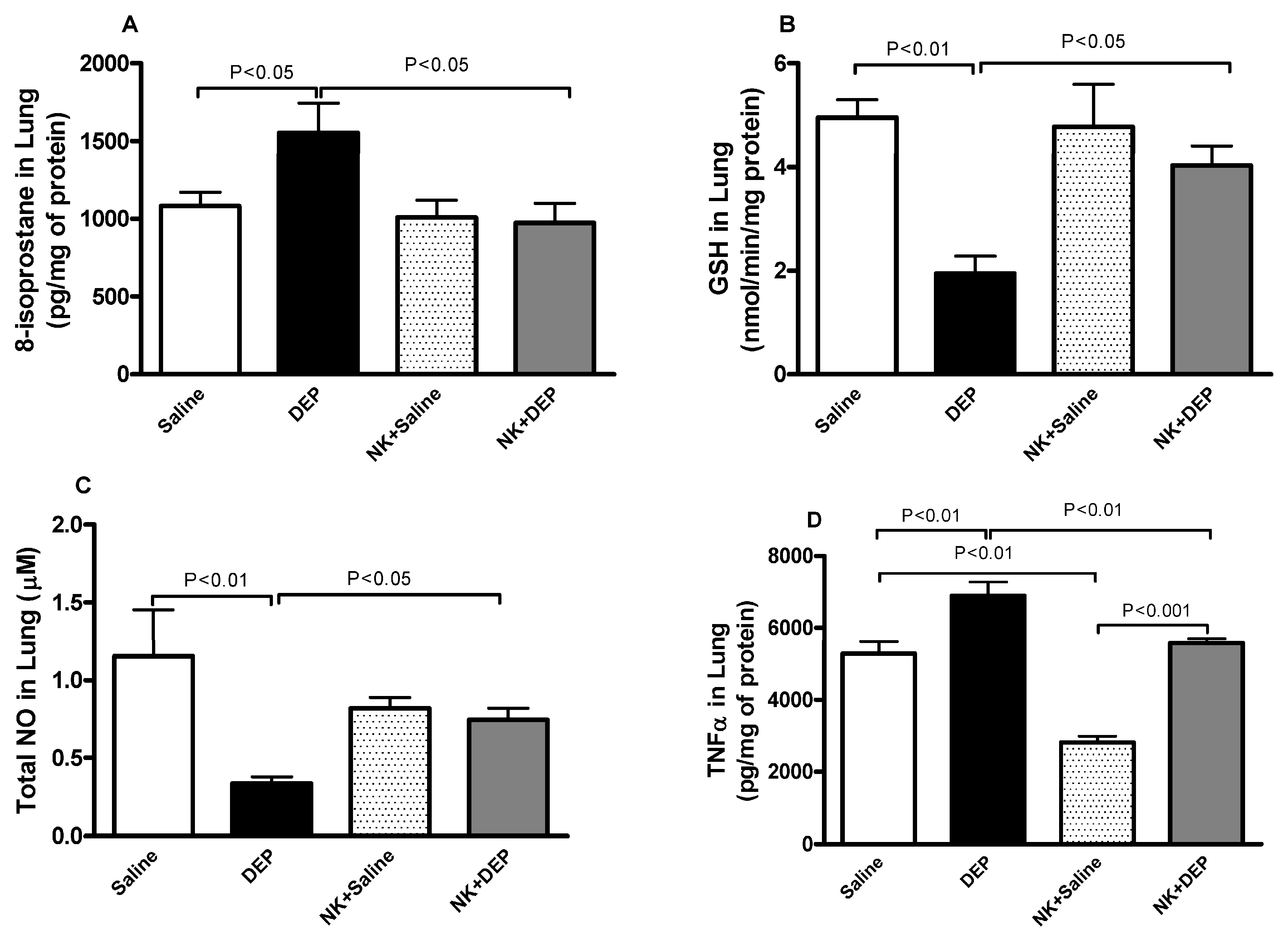
3.4. 8-Isoprostane, GSH, Total NO and TNFα in Lung Homogenates
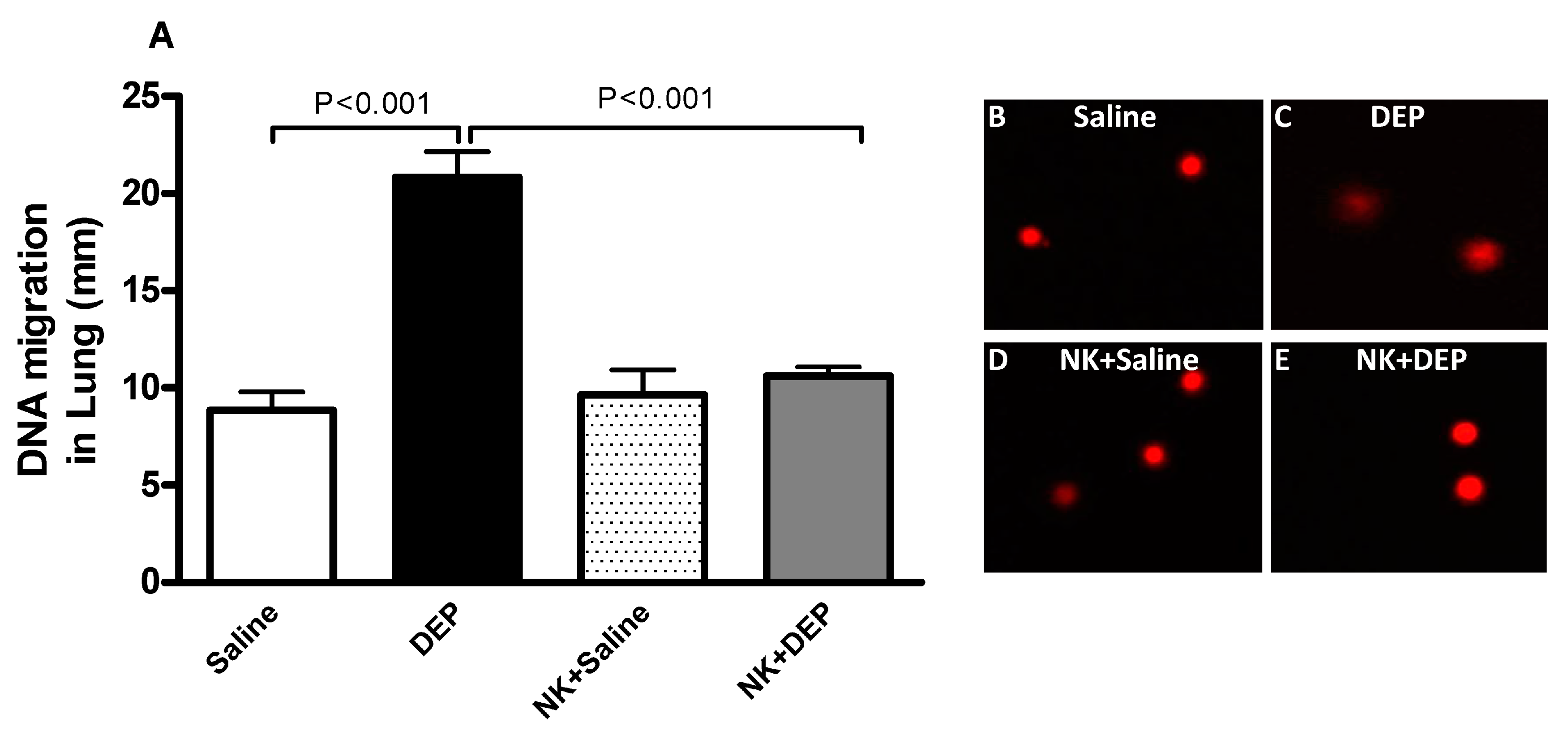
3.5. Lung DNA Damage
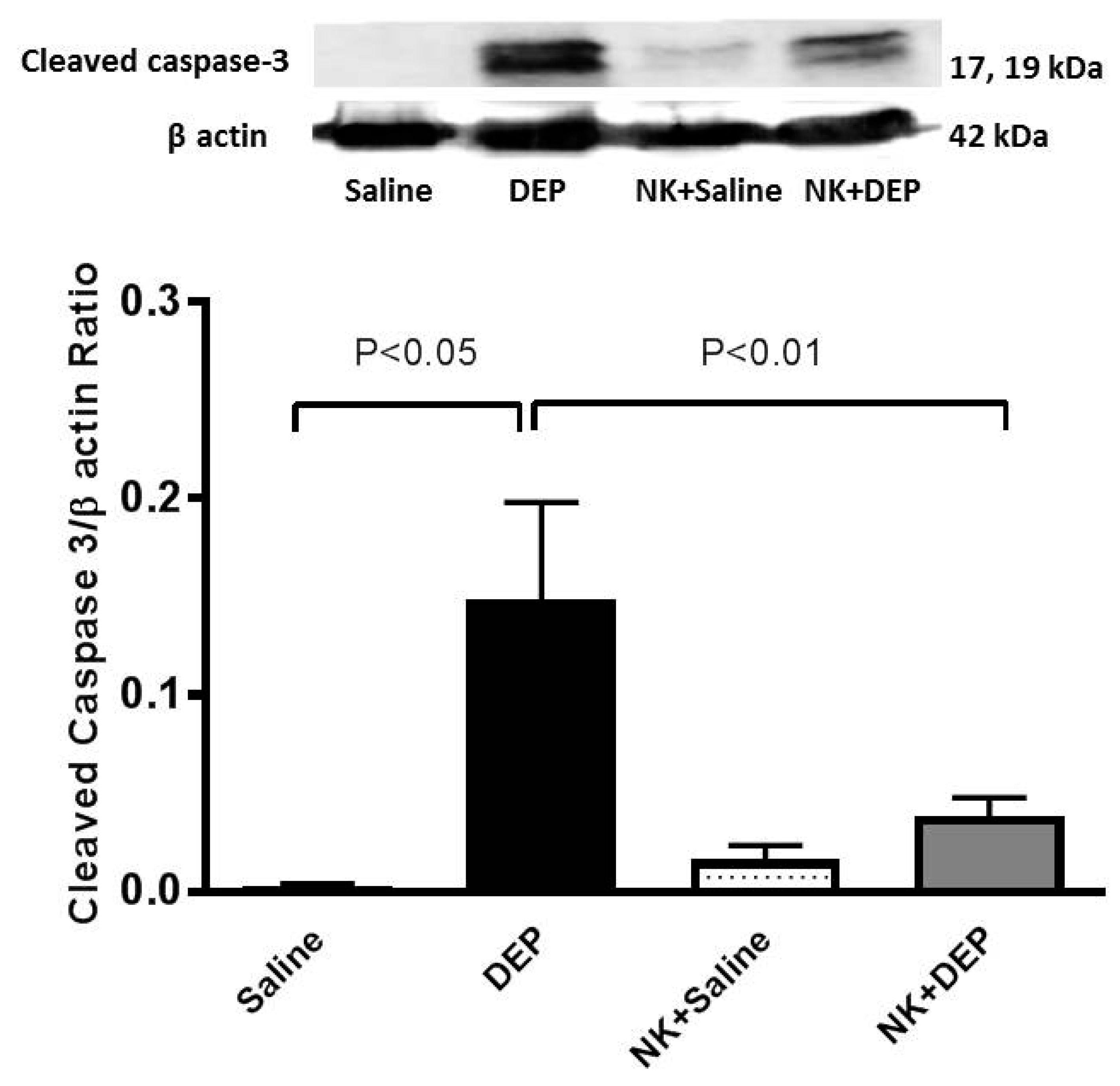
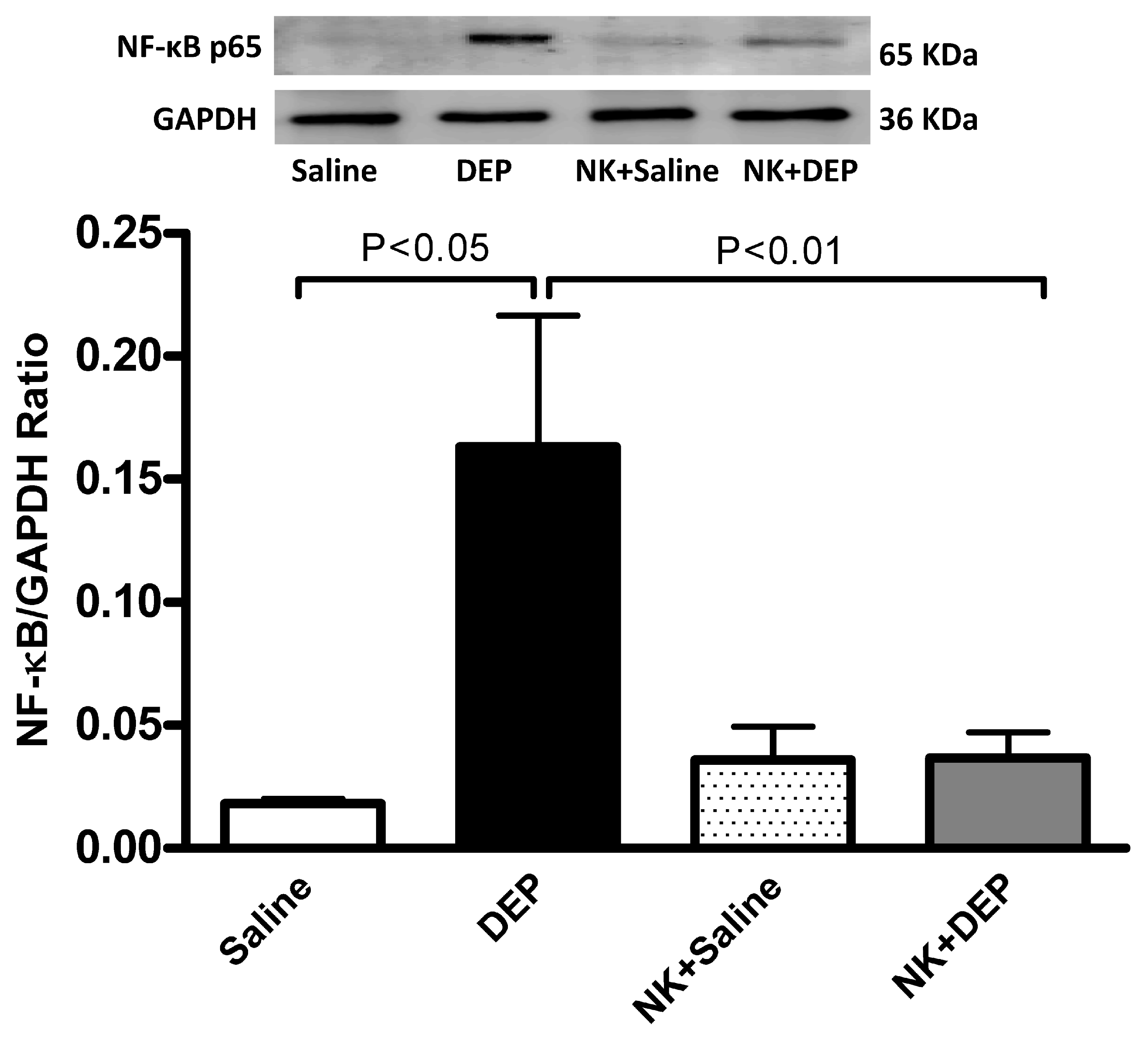
3.6. Western Blot Analysis for the Detection of Caspase-3 and NF-κB
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Atkinson, R.W.; Kang, S.; Anderson, H.R.; Mills, I.C.; Walton, H.A. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: A systematic review and meta-analysis. Thorax 2014, 69, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, B.; Ke, W.; Feng, B.; Lin, H.; Xiao, J.; Zeng, W.; Li, X.; Tao, J.; Yang, Z.; et al. Associations of Short-Term and Long-Term Exposure to Ambient Air Pollutants with Hypertension: A Systematic Review and Meta-Analysis. Hypertension 2016, 68, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.; Bisig, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Diesel exhaust: Current knowledge of adverse effects and underlying cellular mechanisms. Arch. Toxicol. 2016, 90, 1541–1553. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Holme, J.A.; Rosas, I.; Schwarze, P.E.; Alfaro-Moreno, E. Recent advances in particulate matter and nanoparticle toxicology: A review of the in vivo and in vitro studies. Biomed. Res. Int. 2013, 2013, 279371. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.; Blomberg, A.; Rudell, B.; Kelly, F.; Sandstrom, T.; Holgate, S.T.; Frew, A. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am. J. Respir. Crit. Care Med. 1999, 159, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Laumbach, R.J.; Kipen, H.M.; Ko, S.; Kelly-McNeil, K.; Cepeda, C.; Pettit, A.; Ohman-Strickland, P.; Zhang, L.; Zhang, J.; Gong, J.; et al. A controlled trial of acute effects of human exposure to traffic particles on pulmonary oxidative stress and heart rate variability. Part. Fibre Toxicol. 2014, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Barregard, L.; Nielsen, J.; Gudmundsson, A.; Wierzbicka, A.; Axmon, A.; Jonsson, B.A.; Karedal, M.; Albin, M. Effects of diesel exposure on lung function and inflammation biomarkers from airway and peripheral blood of healthy volunteers in a chamber study. Part. Fibre Toxicol. 2013, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Al-Salam, S.; Zia, S.; Marzouqi, F.; Al-Dhaheri, A.; Subramaniyan, D.; Dhanasekaran, S.; Yasin, J.; Ali, B.H.; Kazzam, E.E. Contrasting actions of diesel exhaust particles on the pulmonary and cardiovascular systems and the effects of thymoquinone. Br. J. Pharmacol. 2011, 164, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Al-Salam, S.; Yuvaraju, P.; Beegam, S.; Ali, B.H. Emodin mitigates diesel exhaust particles-induced increase in airway resistance, inflammation and oxidative stress in mice. Respir. Physiol. Neurobiol. 2015, 215, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.Y.; Park, M.K.; Leikauf, G.D.; Park, C.S.; Jang, A.S. Diesel exhaust particle-induced airway responses are augmented in obese rats. Int. J. Toxicol. 2014, 33, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, R.H.; Berger, R.G. Nootkatone. Adv. Biochem. Eng. Biotechnol. 2015, 148, 391–404. [Google Scholar] [PubMed]
- Murase, T.; Misawa, K.; Haramizu, S.; Minegishi, Y.; Hase, T. Nootkatone, a characteristic constituent of grapefruit, stimulates energy metabolism and prevents diet-induced obesity by activating AMPK. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E266–E275. [Google Scholar] [CrossRef] [PubMed]
- Tsoyi, K.; Jang, H.J.; Lee, Y.S.; Kim, Y.M.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Kwak, J.H.; Lee, D.U.; Chang, K.C. (+)-Nootkatone and (+)-valencene from rhizomes of Cyperus rotundus increase survival rates in septic mice due to heme oxygenase-1 induction. J. Ethnopharmacol. 2011, 137, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Al-Salam, S.; Beegam, S.; Yuvaraju, P.; Ali, B.H. Thrombosis, systemic and cardiac oxidative stress and DNA damage induced by pulmonary exposure to diesel exhaust particles, and the effect of nootkatone thereon. Am. J. Physiol. Heart Circ. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Al Salam, S.; Dhanasekaran, S.; Sudhadevi, M.; Ali, B.H. Pulmonary exposure to diesel exhaust particles promotes cerebral microvessel thrombosis: Protective effect of a cysteine prodrug l-2-oxothiazolidine-4-carboxylic acid. Toxicology 2009, 263, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Al Maskari, S.; Ali, B.H.; Al Amri, I.S. Cardiovascular and lung inflammatory effects induced by systemically administered diesel exhaust particles in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L664–L670. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Zia, S.; Subramaniyan, D.; Fahim, M.A.; Ali, B.H. Exacerbation of thrombotic events by diesel exhaust particle in mouse model of hypertension. Toxicology 2011, 285, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Subramaniyan, D.; Zia, S.; Yasin, J.; Ali, B.H. Airway resistance, inflammation and oxidative stress following exposure to diesel exhaust particle in angiotensin II-induced hypertension in mice. Toxicology 2012, 292, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Al-Salam, S.; Subramaniyan, D.; Yasin, J.; Yuvaraju, P.; Beegam, S.; Ali, B.H. Influence of experimental type 1 diabetes on the pulmonary effects of diesel exhaust particles in mice. Toxicol. Lett. 2013, 217, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Dhanasekaran, S.; Yasin, J.; Ba-Omar, H.; Fahim, M.A.; Kazzam, E.E.; Ali, B.H. Evaluation of the direct systemic and cardiopulmonary effects of diesel particles in spontaneously hypertensive rats. Toxicology 2009, 262, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.D.; Coalson, J.J.; Peters, J.; Chaparro, A.; Techasaensiri, C.; Cantwell, A.M.; Kannan, T.R.; Baseman, J.B.; Dube, P.H. Analysis of pulmonary inflammation and function in the mouse and baboon after exposure to Mycoplasma pneumoniae CARDS toxin. PLoS ONE 2009, 4, e7562. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Salam, S.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Ali, B.H. Pancreatic effects of diesel exhaust particles in mice with type 1 diabetes mellitus. Cell. Physiol. Biochem. 2014, 33, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic. Res. 2005, 39, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Wennmalm, A.; Benthin, G.; Edlund, A.; Jungersten, L.; Kieler-Jensen, N.; Lundin, S.; Westfelt, U.N.; Petersson, A.S.; Waagstein, F. Metabolism and excretion of nitric oxide in humans. An experimental and clinical study. Circ. Res. 1993, 73, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.F.; Goncales, T.A.; Steinmetz, A.; Moura, D.J.; Saffi, J.; Gomez, R.; Barros, H.M. Cocaine induces DNA damage in distinct brain areas of female rats under different hormonal conditions. Clin. Exp. Pharmacol. Physiol. 2014, 41, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Olive, P.L.; Banath, J.P.; Fjell, C.D. DNA strand breakage and DNA structure influence staining with propidium iodide using the alkaline comet assay. Cytometry 1994, 16, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Speit, G. The contribution of cytotoxicity to DNA-effects in the single cell gel test (comet assay). Toxicol. Lett. 1997, 90, 183–188. [Google Scholar] [CrossRef]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; Fahim, M.A.; Ali, B.H. Cerium Oxide Nanoparticles in Lung Acutely Induce Oxidative Stress, Inflammation, and DNA Damage in Various Organs of Mice. Oxid. Med. Cell. Longev. 2017, 2017, 9639035. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; Yasin, J.; Kazzam, E.E.; Ali, B.H. Oxidative stress, inflammation, and DNA damage in multiple organs of mice acutely exposed to amorphous silica nanoparticles. Int. J. Nanomed. 2016, 11, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Hazucha, M.J.; Bromberg, P.A.; Lay, J.C.; Bennett, W.; Zeman, K.; Alexis, N.E.; Kehrl, H.; Rappold, A.G.; Cascio, W.E.; Devlin, R.B. Pulmonary responses in current smokers and ex-smokers following a two hour exposure at rest to clean air and fine ambient air particles. Part. Fibre Toxicol. 2013, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.B.; Ljungman, P.L.; Wilker, E.H.; Gold, D.R.; Schwartz, J.D.; Koutrakis, P.; Washko, G.R.; O’Connor, G.T.; Mittleman, M.A. Short-term exposure to air pollution and lung function in the Framingham Heart Study. Am. J. Respir. Crit. Care Med. 2013, 188, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, G.M.; Green, D.; Bellmeyer, A.; Baker, C.M.; Burgess, Z.; Rajamannan, N.; Christman, J.W.; Foiles, N.; Kamp, D.W.; Ghio, A.J.; et al. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J. Clin. Investig. 2007, 117, 2952–2961. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Al, D.R.; Alamiri, J.; Al, H.S.; Al, S.H.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Ali, B.H. Diesel Exhaust Particles Induce Impairment of Vascular and Cardiac Homeostasis in Mice: Ameliorative Effect of Emodin. Cell. Physiol. Biochem. 2015, 36, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.L.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C.; et al. Air pollution and cardiovascular disease—A statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef] [PubMed]
- United Nations Environment Program; World Health Organization (WHO). Air Pollution in the world’s megacities. A Report from the U.N. Environment Programme and WHO. Environment 1994, 36, 25–37. [Google Scholar]
- Driscoll, K.E.; Costa, D.L.; Hatch, G.; Henderson, R.; Oberdorster, G.; Salem, H.; Schlesinger, R.B. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: Uses and limitations. Toxicol. Sci. 2000, 55, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa, E.; Bai, N.; Morimoto, K.; Gray, C.; Mui, T.; Yatera, K.; Zhang, X.; Xing, L.; Li, Y.; Laher, I.; et al. Particulate matter exposure induces persistent lung inflammation and endothelial dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L79–L85. [Google Scholar] [CrossRef] [PubMed]
- Laks, D.; de Oliveira, R.C.; de Andre, P.A.; Macchione, M.; Lemos, M.; Faffe, D.; Saldiva, P.H.; Zin, W.A. Composition of diesel particles influences acute pulmonary toxicity: An experimental study in mice. Inhal. Toxicol. 2008, 20, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.H.; Lee, D.U.; Kim, Y.S.; Kim, H.P. Anti-allergic activity of sesquiterpenes from the rhizomes of Cyperus rotundus. Arch. Pharm. Res. 2011, 34, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.J.; Lee, D.U.; Kwak, J.H.; Lee, S.M.; Kim, Y.S.; Jung, Y.S. Antiplatelet effects of Cyperus rotundus and its component (+)-nootkatone. J. Ethnopharmacol. 2011, 135, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Lee, D.U. Nootkatone from the rhizomes of Cyperus rotundus protects against ischemia-reperfusion mediated acute myocardial injury in the rat. Int. J. Pharm. 2016, 12, 845–850. [Google Scholar]
- Li, X.Y.; Hao, L.; Liu, Y.H.; Chen, C.Y.; Pai, V.J.; Kang, J.X. Protection against fine particle-induced pulmonary and systemic inflammation by omega-3 polyunsaturated fatty acids. Biochim. Biophys. Acta 2017, 1861, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Rahman, I. Current concepts on oxidative/carbonyl stress, inflammation and epigenetics in pathogenesis of chronic obstructive pulmonary disease. Toxicol. Appl. Pharmacol. 2011, 254, 72–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hummel, S.G.; Fischer, A.J.; Martin, S.M.; Schafer, F.Q.; Buettner, G.R. Nitric oxide as a cellular antioxidant: A little goes a long way. Free Radic. Biol. Med. 2006, 40, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U. Nitric oxide and oxidative stress in vascular disease. Pflug. Arch. 2010, 459, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Nurkiewicz, T.R.; Porter, D.W.; Hubbs, A.F.; Stone, S.; Chen, B.T.; Frazer, D.G.; Boegehold, M.A.; Castranova, V. Pulmonary nanoparticle exposure disrupts systemic microvascular nitric oxide signaling. Toxicol. Sci. 2009, 110, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhao, D.; Jing, L.; Cui, G.; Jin, M.; Li, Y.; Liu, X.; Liu, Y.; Du, H.; Guo, C.; et al. Cardiovascular Toxicity of Different Sizes Amorphous Silica Nanoparticles in Rats after Intratracheal Instillation. Cardiovasc. Toxicol. 2013, 13, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Lee, J.H.; Jung, Y.S. (+)-Nootkatone inhibits tumor necrosis factor alpha/interferon gamma-induced production of chemokines in HaCaT cells. Biochem. Biophys. Res. Commun. 2014, 447, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Moller, P.; Danielsen, P.H.; Karottki, D.G.; Jantzen, K.; Roursgaard, M.; Klingberg, H.; Jensen, D.M.; Christophersen, D.V.; Hemmingsen, J.G.; Cao, Y.; et al. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat. Res. Rev. Mutat. Res. 2014, 762, 133–166. [Google Scholar] [CrossRef] [PubMed]
- Nowsheen, S.; Yang, E.S. The intersection between DNA damage response and cell death pathways. Exp. Oncol. 2012, 34, 243–254. [Google Scholar] [PubMed]
- Savitskaya, M.A.; Onishchenko, G.E. Mechanisms of Apoptosis. Biochemistry 2015, 80, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Zin, W.A.; Silva, A.G.; Magalhaes, C.B.; Carvalho, G.M.; Riva, D.R.; Lima, C.C.; Leal-Cardoso, J.H.; Takiya, C.M.; Valenca, S.S.; Saldiva, P.H.; et al. Eugenol attenuates pulmonary damage induced by diesel exhaust particles. J. Appl. Physiol. 2012, 112, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Sakaguchi, I.; Mori, M.; Ikeda, N.; Kato, Y.; Minamino, M.; Watabe, K. Induction of apoptosis by Citrus paradisi essential oil in human leukemic (HL-60) cells. In Vivo 2003, 17, 553–559. [Google Scholar] [PubMed]
- Schuliga, M. NF-kappaB Signaling in Chronic Inflammatory Airway Disease. Biomolecules 2015, 5, 1266–1283. [Google Scholar] [CrossRef] [PubMed]
- Traboulsi, H.; Guerrina, N.; Iu, M.; Maysinger, D.; Ariya, P.; Baglole, C.J. Inhaled Pollutants: The Molecular Scene behind Respiratory and Systemic Diseases Associated with Ultrafine Particulate Matter. Int. J. Mol. Sci. 2017, 18, 243. [Google Scholar] [CrossRef] [PubMed]
- Pourazar, J.; Mudway, I.S.; Samet, J.M.; Helleday, R.; Blomberg, A.; Wilson, S.J.; Frew, A.J.; Kelly, F.J.; Sandstrom, T. Diesel exhaust activates redox-sensitive transcription factors and kinases in human airways. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L724–L730. [Google Scholar] [CrossRef] [PubMed]
- Totlandsdal, A.I.; Cassee, F.R.; Schwarze, P.; Refsnes, M.; Lag, M. Diesel exhaust particles induce CYP1A1 and pro-inflammatory responses via differential pathways in human bronchial epithelial cells. Part. Fibre Toxicol. 2010, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, C.B.; Riva, D.R.; DePaula, L.J.; Brando-Lima, A.; Koatz, V.L.; Leal-Cardoso, J.H.; Zin, W.A.; Faffe, D.S. In vivo anti-inflammatory action of eugenol on lipopolysaccharide-induced lung injury. J. Appl. Physiol. 2010, 108, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.F.; Ye, X.; Malik, A.B. In vivo inhibition of nuclear factor-kappa B activation prevents inducible nitric oxide synthase expression and systemic hypotension in a rat model of septic shock. J. Immunol. 1997, 159, 3976–3983. [Google Scholar] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemmar, A.; Al-Salam, S.; Beegam, S.; Yuvaraju, P.; Hamadi, N.; Ali, B.H. In Vivo Protective Effects of Nootkatone against Particles-Induced Lung Injury Caused by Diesel Exhaust Is Mediated via the NF-κB Pathway. Nutrients 2018, 10, 263. https://doi.org/10.3390/nu10030263
Nemmar A, Al-Salam S, Beegam S, Yuvaraju P, Hamadi N, Ali BH. In Vivo Protective Effects of Nootkatone against Particles-Induced Lung Injury Caused by Diesel Exhaust Is Mediated via the NF-κB Pathway. Nutrients. 2018; 10(3):263. https://doi.org/10.3390/nu10030263
Chicago/Turabian StyleNemmar, Abderrahim, Suhail Al-Salam, Sumaya Beegam, Priya Yuvaraju, Naserddine Hamadi, and Badreldin H. Ali. 2018. "In Vivo Protective Effects of Nootkatone against Particles-Induced Lung Injury Caused by Diesel Exhaust Is Mediated via the NF-κB Pathway" Nutrients 10, no. 3: 263. https://doi.org/10.3390/nu10030263




