Methyl-Donor and Cofactor Nutrient Intakes in the First 2–3 Years and Global DNA Methylation at Age 4: A Prospective Cohort Study
Abstract
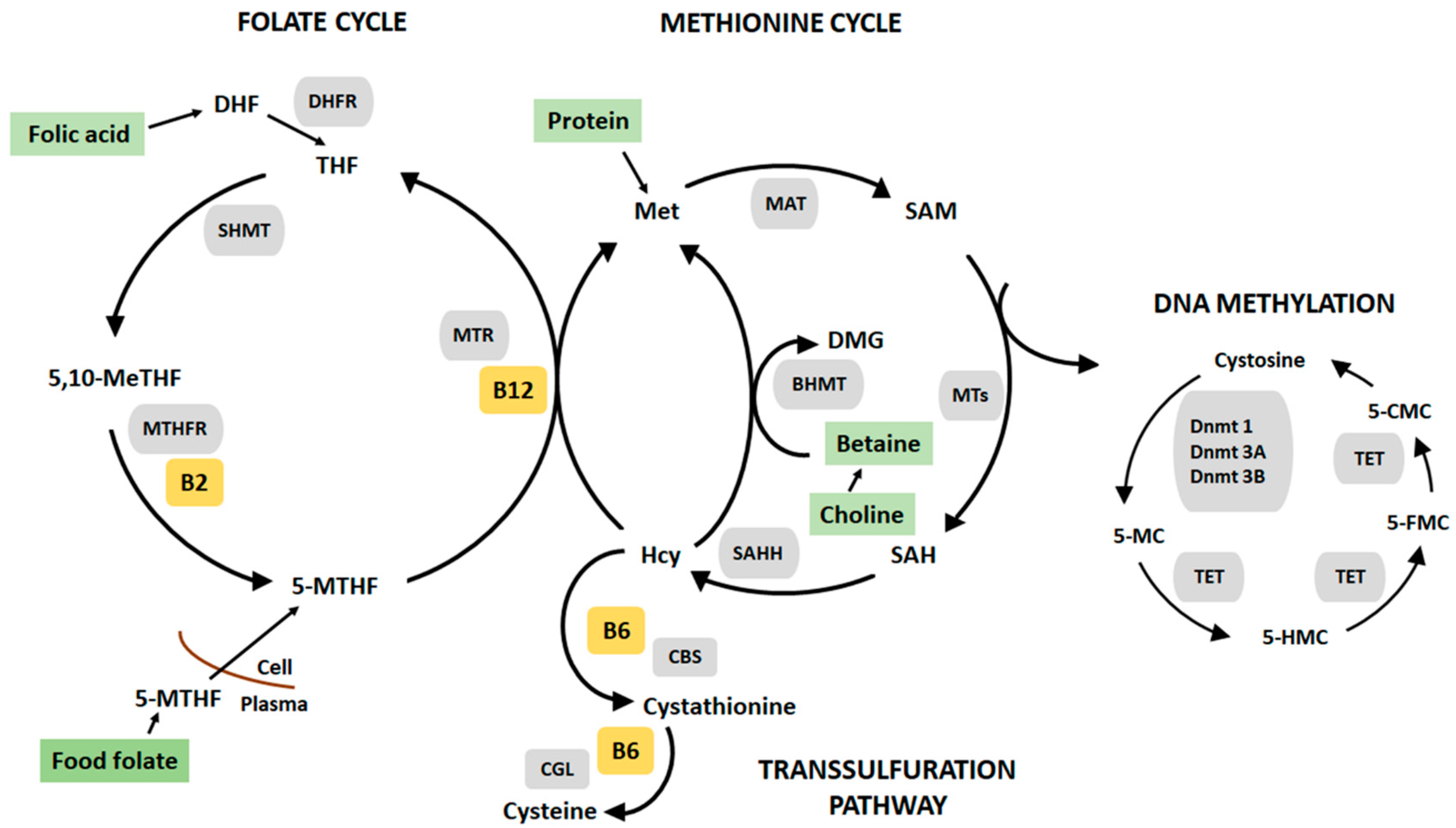
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Assessment
2.2.1. Infant Feeding Questionnaires
2.2.2. 24-h Dietary Recalls
2.2.3. Four-day Weighed Food Records
2.2.4. Food Frequency Questionnaire
2.3. Dietary Analysis
2.3.1. Quantifying Energy and Nutrient Intake from Breastmilk
2.3.2. Dietary analysis of Protein, Folate, Vitamin B2, B6 and B12 Intake
2.3.3. Dietary Analysis of Methionine and Choline
2.3.4. Methionine
2.3.5. Choline
2.3.6. Quantifying Nutrient Intake from FFQs
2.3.7. Maternal Supplementation
2.4. DNA Collection
2.5. DNA Extraction
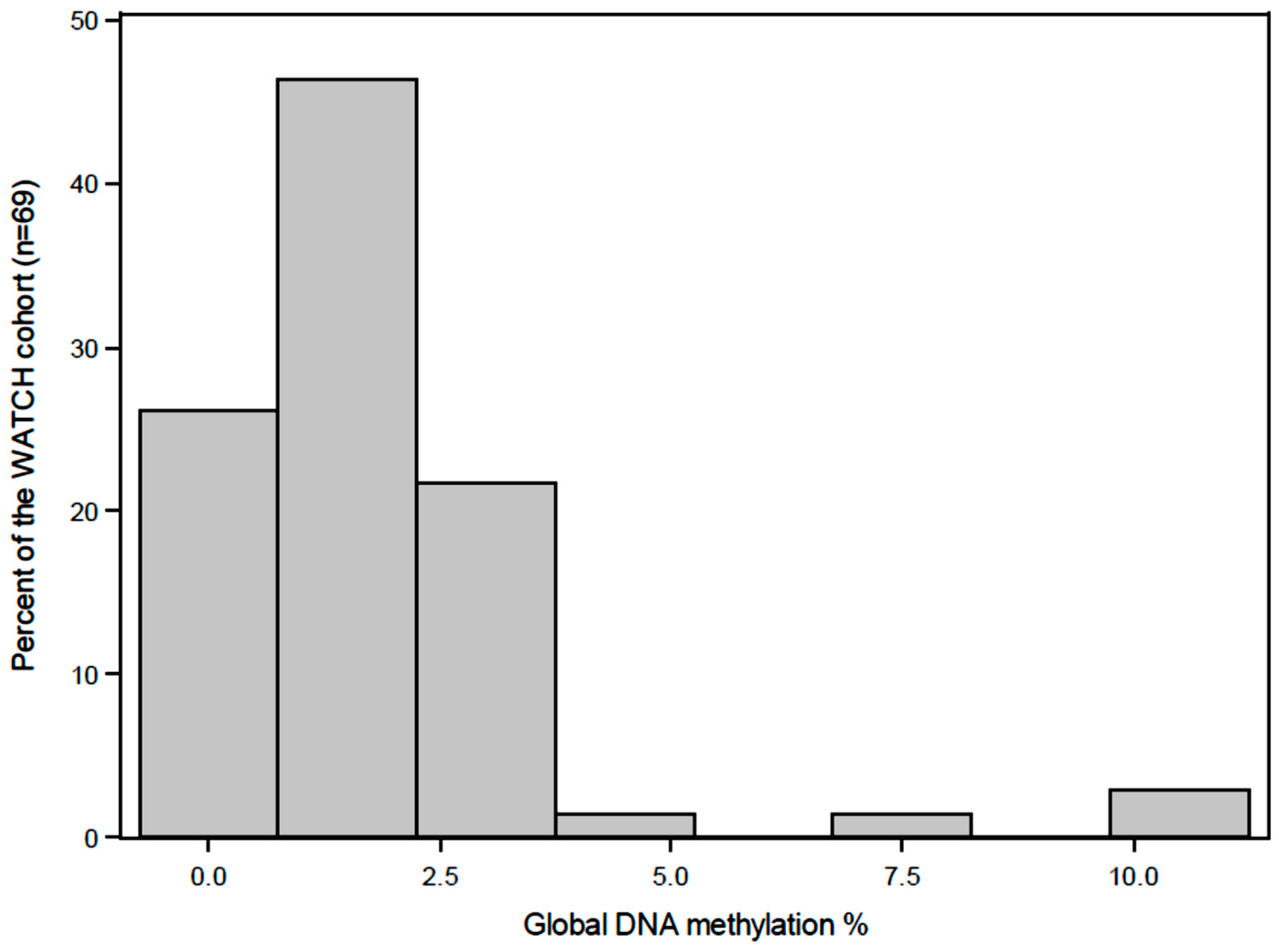
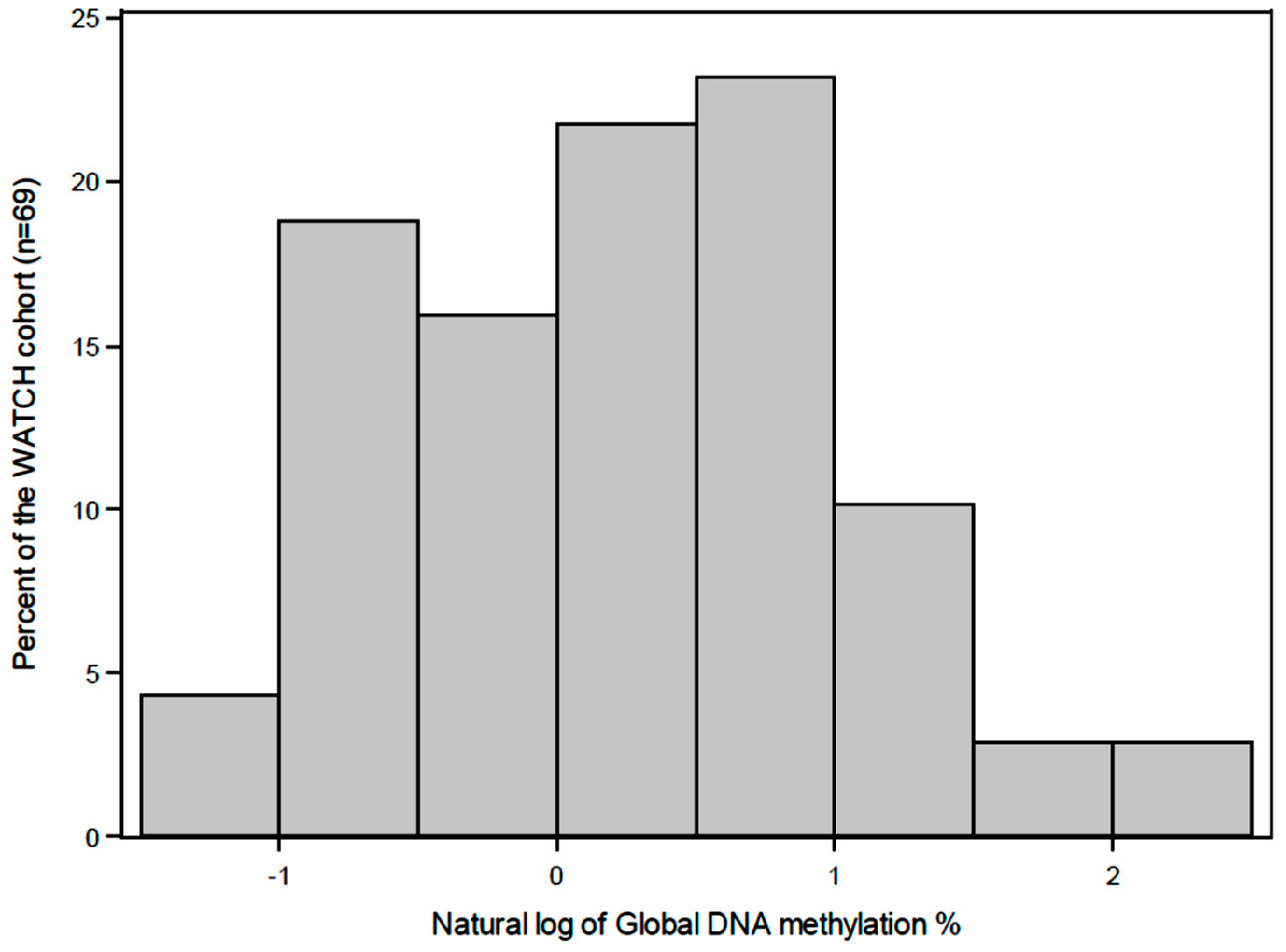
2.6. Quantification of Global DNA Methylation
2.7. Participant Characteristics
2.8. Statistical Analysis
3. Results
4. Discussion
4.1. Impact of Postnatal Nutrition on DNA Methylation
4.2. Gender Differences DNA Methylation
4.3. Global and Locus Specific DNA Methylation
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABS | Australian Bureau of Statistics |
| ACAES | Australian Child and Adolescent Eating Survey |
| APD | accredited practicing dietitian |
| AUSNUT | Australian Nutrition Tables |
| Avy | agouti viable yellow |
| BMI | body mass index |
| CCCEH | Columbia Centre for Children’s Environmental Health |
| CNF | Canadian Nutrient File |
| CpG | cytosine phosphate guanine |
| 24hr-DR | 24 hour dietary recalls |
| DNMT | de novo methylase |
| EER | estimated energy intake |
| EI | energy intake |
| ELISA | enzyme-linked immunosorbent assay |
| FFQ | food frequency questionnaire |
| FSANZ | Food standards Australia and New Zealand |
| IGF2 | insulin-like growth factor 2 |
| JHH | John Hunter Hospital |
| LINE-1 | long interspersed nuclear elements-1 |
| 5-mC | 5-methylcytosine |
| MTHFR | methylenetetrahydrofolate reductase |
| NSW | New South Wales |
| NUTTAB | Nutrition tables |
| OD | optical density |
| SAM | S-adenosyl-methionine |
| SAS | Statistical Analysis System |
| SEIFA | socio-economic Indexes for Areas |
| tDMRs | tissue-specific differentially methylated regions |
| USDA | United States Department of Agriculture |
| WATCH | Women and their Children’s Health |
| 4d-WFR | 4 day weighed food records |
| WHO | World Health Organization |
References
- Robertson, K.D. DNA methylation and human disease. Nat. Rev. Genet. 2005, 6, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Dean, W.; Santos, F.; Stojkovic, M.; Zakhartchenko, V.; Walter, J.; Wolf, E.; Reik, W. Conservation of methylation reprogramming in mammalian development: Aberrant reprogramming in cloned embryos. Proc. Natl. Acad. Sci. USA 2001, 98, 13734–13738. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.; Hendrich, B.; Reik, W.; Dean, W. Dynamic Reprogramming of DNA methylation in the early mouse embryo. Dev. Biol. 2002, 241, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Gicquel, C.; El-Osta, A.; le Bouc, Y. Epigenetic regulation and fetal programming. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Alisch, R.S.; Barwick, B.G.; Chopra, P.; Myrick, L.K.; Satten, G.A.; Conneely, K.N.; Warren, S.T. Age-associated DNA methylation in pediatric populations. Genome Res. 2012, 22, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, K.D.; Connor, C.M.; Campan, M.; Long, T.I.; Weisenberger, D.J.; Biniszkiewicz, D.; Jaenisch, R.; Laird, P.W.; Akbarian, S. DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PLoS ONE 2007, 2, e895. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.; Pliushch, G.; el Hajj, N.E.; Galetzka, D.; Puhl, A.; Schorsch, M.; Frauenknecht, K.; Riepert, T.; Tresch, A.; Muller, A.M.; et al. Spatial, temporal and interindividual epigenetic variation of functionally important DNA methylation patterns. Nucleic Acids Res. 2010, 38, 3880–3890. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammaliand. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Li, E.; Bestor, T.H.; Jaenisch, R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 1992, 69, 915–926. [Google Scholar] [CrossRef]
- Wang, I.J.; Chen, S.L.; Lu, T.P.; Chuang, E.Y.; Chen, P.C. Prenatal smoke exposure, DNA methylation and childhood atopic dermatitis. Clin. Exp. Allergy 2013, 43, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Kresovich, J.K.; Zheng, Y.; Cardenas, A.; Joyce, B.T.; Rifas-Shiman, S.L.; Oken, E.; Gillman, M.W.; Hivert, M.F.; Baccarelli, A.A.; Hou, L. Cord blood DNA methylation and adiposity measures in early and mid-childhood. Clin. Epigenet. 2017, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Barton, S.J.; Ngo, S.; Costello, P.; Garratt, E.; El-Heis, S.; Antoun, E.; Clarke-Harris, R.; Murray, R.; Bhatt, T.; Burdge, G.; et al. DNA methylation of Th2 lineage determination genes at birth is associated with allergic outcomes in childhood. Clin. Exp. Allergy 2017, 47, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Winter, P.D.; Osmond, C.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 2, 577–580. [Google Scholar] [CrossRef]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Friso, S.; Choi, S.W.; Girelli, D.; Mason, J.B.; Dolnikowski, G.G.; Bagley, P.J.; Olivieri, O.; Jacques, P.F.; Rosenberg, I.H.; Corrocher, R.; et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc. Natl. Acad. Sci. USA 2002, 99, 5606–5611. [Google Scholar] [CrossRef] [PubMed]
- Catoni, G.L. S-Adenosylmethionine: A new intermediate formed enzymatically from l-methionine and adenosinetriphosphate. J. Biol. Chem. 1953, 204, 403–416. [Google Scholar] [PubMed]
- De La Haba, G.; Cantoni, G.L. The enzymatic synthesis of S-adenosyl-l-homocysteine from adenosine and homocysteine. J. Biol. Chem. 1959, 234, 603–608. [Google Scholar] [PubMed]
- Jones, P.L.; Veenstra, G.J.; Wade, P.A.; Vermaak, D.; Kass, S.U.; Landsberger, N.; Strouboulis, J.; Wolffe, A.P. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 1998, 19, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.; Ng, H.H.; Johnson, C.A.; Laherty, C.D.; Turner, B.M.; Eisenman, R.N.; Bird, A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 1998, 393, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Rzehak, P.; Covic, M.; Saffery, R.; Reischl, E.; Wahl, S.; Grote, V.; Weber, M.; Xhonneux, A.; Langhendries, J.P.; Ferre, N.; et al. DNA-methylation and body composition in preschool children: Epigenome-Wide-Analysis in the European Childhood Obesity Project (CHOP)-Study. Sci. Rep. 2017, 7, 14349. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, S.J.; Peters, T.J.; Buckley, M.; Zhou, J.; Jones, P.A.; Gibson, R.A.; Makrides, M.; Muhlhausler, B.S.; Molloy, P.L. DNA methylation in blood from neonatal screening cards and the association with BMI and insulin sensitivity in early childhood. Int. J. Obes. (Lond.) 2017, 42, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Volberg, V.; Yousefi, P.; Huen, K.; Harley, K.; Eskenazi, B.; Holland, N. CpG Methylation across the adipogenic PPARgamma gene and its relationship with birthweight and child BMI at 9 years. BMC Med. Genet. 2017, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Wolff, G.L.; Kodell, R.L.; Moore, S.R.; Cooney, C.A. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998, 12, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Salas, P.; Moore, S.E.; Baker, M.S.; Bergen, A.W.; Cox, S.E.; Dyer, R.A.; Fulford, A.J.; Guan, Y.; Laritsky, E.; Silver, M.J.; et al. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat. Commun. 2014, 5, 3746. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Salas, P.; Moore, S.E.; Cole, D.; da Costa, K.A.; Cox, S.E.; Dyer, R.A.; Fulford, A.J.; Inni, S.M.; Waterland, R.A.; Zeisel, S.H.; et al. DNA methylation potential: Dietary intake and blood concentrations of one-carbon metabolites and cofactors in rural African women. Am. J. Clin. Nutr. 2013, 97, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Kovacheva, V.P.; Mellott, T.J.; Davison, J.M.; Wagner, N.; Lopez-Coviella, I.; Schnitzler, A.C.; Blusztajn, J.K. Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by up-regulation of Dnmt1 expression. J. Biol. Chem. 2007, 282, 31777–31788. [Google Scholar] [CrossRef] [PubMed]
- Hure, A.J.; Collins, C.E.; Smith, R. A longitudinal study of maternal folate and vitamin B12 status in pregnancy and postpartum, with the same infant markers at 6 months of age. Matern. Child Health J. 2012, 16, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Martino, D.; Loke, Y.J.; Gordon, L.; Ollikainen, M.; Cruickshank, M.N.; Saffery, R.; Craig, J.M. Longitudinal, genome-scale analysis of DNA methylation in twins from birth to 18 months of age reveals rapid epigenetic change in early life and pair-specific effects of discordance. Genome Biol. 2013, 14, R42. [Google Scholar] [CrossRef] [PubMed]
- Vidovic, A.; Vidovic Juras, D.; Vucicevic Boras, V.; Lukac, J.; Grubisic-Ilic, M.; Rak, D.; Sabioncello, A. Determination of leucocyte subsets in human saliva by flow cytometry. Arch. Oral Biol. 2012, 57, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, S.; Ghosh, M.; Duca, R.C.; Bekaert, B.; Freson, K.; Huybrechts, I.; Langie, S.A.S.; Koppen, G.; Devlieger, R.; Godderis, L. Maternal intake of methyl-group donors affects DNA methylation of metabolic genes in infants. Clin. Epigenet. 2017, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Hure, A.J.; Collins, C.E.; Giles, W.B.; Wright, I.M.R.; Smith, R. Protocol for the Women And Their Children's Health (WATCH) Study: A cohort of pregnancy and beyond. J. Epidemiol. 2012, 22, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Margo, E.-A. Monitoring child health in NSW: The New South Wales Child Health Survey 2001 and beyond. N. S. W. Public Health Bull. 2002, 13, 239–240. [Google Scholar]
- Australian Bureau of Statistics. National Nutrition Survey: Confidentialised Unit Record File; Revised September 1998; Australian Bureau of Statistics: Canberra, Australia, 1995.
- Hector, D.; Webb, K.; Lymer, S. Report on Breastfeeding in NSW 2004; CPHN/NSW, Health Department NSW: Sydney, Australia, 2004; Available online: http://sydney.edu.au/science/molecular_bioscience/cphn/pdfs/breastfeeding_report_revised.pdf (accessed on 26 February 2018).
- Webb, K.; Marks, G.; Lund-Adams, M.; Rutishauser, I.; Abraham, B. Towards a National System for Monitoring Breastfeeding in Australia: Recommendations for Population Indicators, Definitions and Next Steps; Australian Food and Nutrition Monitoring Unit, Commonwealth Department of Health and Aged Care: Canberra, Australia, 2001. [Google Scholar]
- World Health Organization. Indicators for Assessing Infant and Young Child Feeding Practices; World Health Organization (WHO): Geneva, Switzerland, 2008. [Google Scholar]
- Watson, J.F.; Collins, C.E.; Sibbritt, D.W.; Dibley, M.J.; Garg, M.L. Reproducibility and comparative validity of a food frequency questionnaire for Australian children and adolescents. Int. J. Behav. Nutr. Phys. Act. 2009, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M.; Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids; United States National Academy Press: Washington, DC, USA, 2002. [Google Scholar]
- Schoen, S.; Sichert-Hellert, W.; Kersting, M. Validation of energy requirement equations for estimation of breast milk consumption in infants. Public Health Nutr. 2009, 12, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Food Standards Australia and New Zealand (FSANZ). AUSNUT 2013—Australian Food, Supplement and Nutrient Database for Estimation of Population Nutrient Intakes; FSANZ: Canberra, Australia, 2014.
- Food Standards Australia and New Zealand (FSANZ). AUSNUT 2007—Australian Food, Supplement and Nutrient Database for Estimation of Population Nutrient Intakes; FSANZ: Canberra, Australia, 2008.
- Food Standards Australia and New Zealand (FSANZ). NUTTAB 2010—Australian Food Composition Tables; FSANZ: Canberra, Australia, 2010.
- Commonwealth Scientific Industrial Research Organisation (Australia) PHNRF; The University of South Australia. Australian National Children’s Nutrition and Physical Activity Surveys-Main Findings 2007; Australia Department of Health and Ageing: Canberra, Australia, 2008. [Google Scholar]
- Food Standards Australia and New Zealand (FSANZ). Iodine Fortification 2016 9/10/17. Available online: http://www.foodstandards.gov.au/consumer/nutrition/iodinefort/Pages/default.aspx (accessed on 26 February 2018).
- Food Standards Australia and New Zealand (FSANZ). Monitoring of Folic Acid Fortification 2016. Available online: http://www.foodstandards.gov.au/science/monitoringnutrients/monitoringfort/Pages/default.aspx (accessed on 26 February 2018).
- US Department of Agriculture ARS, Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, Release 282016 2017 12 April. Available online: https://ndb.nal.usda.gov/ndb/nutrients/index (accessed on 26 February 2018).
- Health Canada. Canadian Nutrient File2015 2017 12 April. Available online: http://www.hc-sc.gc.ca/fn-an/alt_formats/pdf/nutrition/fiche-nutri-data/user_guide_utilisation-eng.pdf (accessed on 26 February 2018).
- Agostoni, C.; Carratu, B.; Boniglia, C.; Riva, E.; Sanzini, E. Free amino acid content in standard infant formulas: Comparison with human milk. J. Am. Coll. Nutr. 2000, 19, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.K.; Lin, S.P.; Lee, H.C.; Wang, T.J.; Shih, Y.S.; Huang, F.Y.; Yeung, C.Y. Free amino acids in full-term and pre-term human milk and infant formula. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.Y.; Bhagwat, S.A.; Williams, J.R.; Howe, J.C. USDA Database for the Choline Content of Common Foods, Release 2 2008 2017 12 April. Available online: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Choline/Choln02.pdf (accessed on 26 February 2018).
- Joubert, B.R.; den Dekker, H.T.; Felix, J.F.; Bohlin, J.; Ligthart, S.; Beckett, E.; Tiemeier, H.; van Meurs, J.B.; Uitterlinden, A.G.; Hofman, A.; et al. Maternal plasma folate impacts differential DNA methylation in an epigenome-wide meta-analysis of newborns. Nat. Commun. 2016, 7, 10577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, R.; Gemma, C.; Beyan, H.; Hawa, M.I.; Bazeos, A.; Leslie, R.D. Buccals are likely to be a more informative surrogate tissue than blood for epigenome-wide association studies. Epigenetics 2013, 8, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, N.; Dieriks, B.V.; Lill, C.; Faull, R.L.; Curtis, M.A.; Dragunow, M. Global changes in DNA methylation and hydroxymethylation in Alzheimer’s disease human brain. Neurobiol. Aging 2014, 35, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, J.; Garg, G.; Kumar, A.; Patowary, A.; Karthikeyan, G.; Ramakrishnan, L.; Brahmachari, V.; Sengupta, S. Detection of altered global DNA methylation in coronary artery disease patients. DNA Cell Biol. 2008, 27, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; James, S.R.; Link, P.A.; McCann, S.E.; Hong, C.C.; Davis, W.; Nesline, M.K.; Ambrosone, C.B.; Karpf, A.R. Association between global DNA hypomethylation in leukocytes and risk of breast cancer. Carcinogenesis 2009, 30, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M.; Gama-Sosa, M.A.; Huang, L.H.; Midgett, R.M.; Kuo, K.C.; McCune, R.A.; Gehrke, C. Amount and distribution of 5-methylcytosine in human DNA from different types of tissues of cells. Nucleic Acids Res. 1982, 10, 2709–2721. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics. Socio-Economic Indexes for Areas (SEIFA); Australian Bureau of Statistics: Canberra, Australia, 2011.
- LeMay, R. NSW Mothers to Get State-Wide Database. 2005. Available online: http://www.zdnet.com/article/nsw-mothers-to-get-state-wide-database/ (accessed on 26 February 2018).
- Sinha, P.; Singh, K.; Sachan, M. High resolution methylation analysis of the HoxA5 regulatory region in different somatic tissues of laboratory mouse during development. Gene Expr. Patterns 2017, 23–24, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Slieker, R.C.; Roost, M.S.; van Iperen, L.; Suchiman, H.E.; Tobi, E.W.; Carlotti, F.; de Koning, E.J.; Slagboom, P.E.; Heijmans, B.T.; Chuva de Sousa Lopes, S.M. DNA Methylation Landscapes of Human Fetal Development. PLoS Genet. 2015, 11, e1005583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timms, J.A.; Relton, C.L.; Rankin, J.; Strathdee, G.; McKay, J.A. DNA methylation as a potential mediator of environmental risks in the development of childhood acute lymphoblastic leukemia. Epigenomics 2016, 8, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, S.; Duca, R.C.; Devlieger, R.; Freson, K.; Straetmans, D.; van Herck, E.; Huybrechts, I.; Koppen, G.; Godderis, L. Maternal Methyl-Group Donor Intake and Global DNA (Hydroxy)Methylation before and during Pregnancy. Nutrients 2016, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-M.; Johnson, A.; Tarapore, P.; Janakiram, V.; Zhangm, X.; Leungm, Y.-K. Environmental epigenetics and its implication on disease risk and health outcomes. ILAR J. 2012, 53, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef] [PubMed]
- Tobi, E.W.; Lumey, L.H.; Talens, R.P.; Kremer, D.; Putter, H.; Stein, A.D. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum. Mol. Genet. 2009, 18, 4046–4053. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, Z.; Feil, R.; Constancia, M.; Fraga, M.; Junien, C.; Carel, J.C.; Boileau, P.; le Bouc, Y.; Deal, C.L.; Lillycrop, K.; et al. Child health, developmental plasticity and epigenetic programming. Endocr. Rev. 2011, 32, 159–224. [Google Scholar] [CrossRef] [PubMed]
- Urdinguio, R.G.; Torró, M.I.; Bayón, G.F.; Álvarez-Pitti, J.; Fernández, A.F.; Redon, P.; Fraga, M.F.; Lurbe, E. Longitudinal study of DNA methylation during the first 5 years of life. J. Transl. Med. 2016, 14, 160. [Google Scholar] [CrossRef] [PubMed]
- Herbstman, J.B.; Wang, S.; Perera, F.P.; Lederman, S.A.; Vishnevetsky, J.; Rundle, A.G.; Hoepner, L.A.; Qu, L.; Tang, D. Predictors and Consequences of global DNA methylation in cord blood and at three years. PLoS ONE 2013, 8, e72824. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Jones, M.J.; Chen, E.; Neumann, S.M.; Fraser, H.B.; Miller, G.E.; Kobor, M.S. Discordance of DNA methylation variance between two accessible human tissues. Sci. Rep. 2015, 5, 8257. [Google Scholar] [CrossRef] [PubMed]
- Montrose, L.; Ward, T.J.; Semmens, E.O.; Cho, Y.H.; Brown, B.; Noonan, C.W. Dietary intake is associated with respiratory health outcomes and DNA methylation in children with asthma. Allergy Asthma Clin. Immunol. 2017, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Rozek, L.S.; Mora-Plazas, M.; Duchin, O.; Marin, C.; Forero, Y.; Baylin, A.; Villamor, E. Micronutrient status and global DNA methylation in school-age children. Epigenetics 2012, 7, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Axume, J.; Smith, S.S.; Pogribny, I.P.; Moriarty, D.J.; Caudill, M.A. The MTHFR 677TT genotype and folate intake interact to lower global leukocyte DNA methylation in young Mexican American women. Nutr. Res. 2007, 27, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Huen, K.; Yousefi, P.; Bradman, A.; Yan, L.; Harley, K.G.; Kogut, K.; Eskenazi, B.; Holland, N. Effects of age, sex and persistent organic pollutants on DNA methylation in children. Environ. Mol. Mutagen. 2014, 55, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Boeke, C.E.; Baccarelli, A.; Kleinman, K.P.; Burris, H.H.; Litonjua, A.A.; Rifas-Shiman, S.L.; Tarantini, L.; Gillman, M.W. Gestational intake of methyl donors and global LINE-1 DNA methylation in maternal and cord blood: Prospective results from a folate-replete population. Epigenetics 2012, 7, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.S.; Hughes, I.A.; Reyes, F.I.; Faiman, C. Pituitary-gonadal relations in infancy: 2. Patterns of serum gonadal steroid concentrations in man from birth to two years of age. J. Clin. Endocrinol. Metab. 1976, 42, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Weisz, J.; Ward, I.L. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses and neonatal offspring. Endocrinology 1980, 106, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Ghahramani, N.M.; Ngun, T.C.; Chen, P.Y.; Tian, Y.; Krishnan, S.; Muir, S.; Rubbi, L.; Arnold, A.P.; de Vries, G.J.; Forger, N.G.; et al. The effects of perinatal testosterone exposure on the DNA methylome of the mouse brain are late-emerging. Biol. Sex Differ. 2014, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, S.J.; Zhou, J.; Peters, T.J.; Buckley, M.; Sutcliffe, B.; Oytam, Y.; Gibson, R.A.; McPhee, A.; Yelland, L.N.; Makrides, M.; et al. Effect of prenatal DHA supplementation on the infant epigenome: Results from a randomized controlled trial. Clin. Epigenet. 2016, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, B.C.; Maheshwari, H.G.; He, L.; Reed, M.; Lozykowski, M.; Okada, S.; Cataldo, L.; Coschigamo, K.; Wagner, T.E.; et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc. Natl. Acad. Sci. USA 1997, 94, 13215–13220. [Google Scholar] [CrossRef] [PubMed]
- Lister, R.; Pelizzola, M.; Dowen, R.H.; Hawkins, R.D.; Hon, G.; Tonti-Filippini, J.; Nery, J.R.; Lee, L.; Ye, Z.; Ngo, Q.-M.; et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 2009, 462, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.S.; Estécio, M.R.H.; Doshi, K.; Kondo, Y.; Tajara, E.H.; Issa, J.-P.J. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004, 32, e38. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.S.; Doshi, K.D.; Choi, S.W.; Mason, J.B.; Mannari, R.K.; Gharybian, V.; Luna, R.; Rashid, A.; Shen, L.; Estecio, M.R.; et al. DNA methylation changes after 5-aza-2′-deoxycytidine therapy in patients with leukemia. Cancer Res. 2006, 66, 5495–5503. [Google Scholar] [CrossRef] [PubMed]
- Estécio, M.R.H.; Gharibyan, V.; Shen, L.; Ibrahim, A.E.K.; Doshi, K.; He, R.; Jelinek, J.; Yang, A.S.; Yan, P.S.; Huang, T.H.M.; et al. LINE-1 Hypomethylation in Cancer Is Highly Variable and Inversely Correlated with Microsatellite Instability. PLoS ONE 2007, 2, e399. [Google Scholar] [CrossRef] [PubMed]

| Nutrient | Food Sources |
|---|---|
| Choline | Cauliflower, eggs, flax seeds, lentils, liver, peanuts, soybeans and wheat germ. |
| Folate and folic acid | Asparagus, cheese, eggs, fortified breads and cereals, legumes, liver, peanuts, oranges and spinach. |
| Methionine | Dairy products, eggs, fish, meat, poultry and rice. |
| Vitamin B2 (Riboflavin) | Cheese, eggs, meat and milk. |
| Vitamin B6 (Pyridoxine) | Bananas, fish, grains, legumes, liver, meat, potatoes and poultry. |
| Vitamin B12 (Cobalamin) | Eggs, fish, meat, poultry, dairy products |
| Characteristics | Median (IQR) | Range |
|---|---|---|
| Mother | ||
| Maternal age (y) | 30(8) | 18–41 |
| Education | n | % |
| No formal qualification | 1 | 1.4 |
| Year 10 or equivalent | 13 | 18 |
| Year 12 or equivalent | 13 | 18 |
| Trade/apprenticeship | 3 | 4.2 |
| Certificate/diploma | 12 | 17 |
| University degree | 23 | 32 |
| Higher university degree | 6 | 8.5 |
| Missing | 2 | |
| Income | n | % |
| No income | 7 | 9.7 |
| $1–299 | 26 | 36 |
| 300–699 | 25 | 35 |
| 700–999 | 11 | 15 |
| Unsure | 3 | 4.2 |
| Missing | 1 | |
| Marital status | n | % |
| Never married | 23 | 32 |
| Married | 46 | 64 |
| Divorced | 2 | 2.8 |
| Widowed | 1 | 1.4 |
| Missing | 1 | |
| Maternal smoking | n | % |
| Yes | 7 | 10 |
| No | 62 | 90 |
| Missing | 4 | |
| Live births (37 weeks’ gestation) | n | % |
| None | 1 | 1.6 |
| 1–2 | 46 | 75 |
| 3–4 | 14 | 23.1 |
| 5 | 1 | 1.6 |
| Missing | 11 | |
| Live preterm births (≤36 weeks’ gestation) | n | % |
| None | 39 | 53.4 |
| 1 | 6 | 8.2 |
| 2 | 1 | 1.4 |
| Missing | 27 | |
| Child | ||
| Gender | n | % |
| Male | 32 | 44 |
| Female | 41 | 56 |
| Median (IQR) | Range | |
| Birth weight (g) | 3560 (690) | 1960–5080 |
| Birth length (cm) | 51 (4) | 48–57.5 |
| Head circumference (cm) | 35 (2) | 31–39.5 |
| Characteristic | Global DNA Methylation Quintiles (Minimum and Maximum) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| (0.313–0.597) | (0.598–1.036) | (1.035–1.552) | (1.554–2.611) | (2.614–10.752) | |
| Mother | |||||
| Maternal age (years, median) | 29 | 31.5 | 31 | 27 | 31 |
| Maternal smoking (%) | 14.3 | 0 | 15.4 | 0 | 14.3 |
| Children | |||||
| Males (%) | 36 | 21 | 54 | 43 | 57 |
| Females (%) | 64 | 77 | 46 | 57 | 42 |
| Birthweight (grams, median) | 3442 | 3560 | 3310 | 3680 | 3260 |
| Model 1 | N | Nutrient 2 | Outcome 3 | Association (95% CI) | P-Value | R-Square |
|---|---|---|---|---|---|---|
| 1 | 57 | Methionine | DNA methylation % | −0.001 (−0.05 to 0.05) | 0.95 | 0.08 |
| 2 | 57 | Vitamin B2 (Riboflavin) | DNA methylation % | 0.002 (−0.04 to 0.05) | 0.94 | 0.08 |
| 3 | 57 | Vitamin B6 (Pyridoxine) | DNA methylation % | −0.007 (−0.05 to 0.04) | 0.74 | 0.08 |
| 4 | 57 | Vitamin B12 (Cobalamin) | DNA methylation % | 0.024 (−0.02 to 0.07) | 0.28 | 0.10 |
| 5 | 57 | Choline | DNA methylation % | −0.000 (−0.05 to 0.05) | 0.99 | 0.07 |
| 6 | 57 | Folate | DNA methylation % | −0.016 (−0.06 to 0.03) | 0.44 | 0.09 |
| Model 1 | N | Nutrient | Outcome 2 | Association (95% CI) | P-Value | R-Square |
|---|---|---|---|---|---|---|
| 1 | 57 | Methionine | DNA methylation % | −0.017 (−0.18 to 0.14) | 0.83 | 0.08 |
| 2 | 57 | Vitamin B2 (Riboflavin) | DNA methylation % | −0.037 (−0.2 to 0.13) | 0.66 | 0.08 |
| 3 | 57 | Vitamin B6 (Pyridoxine) | DNA methylation % | −0.086 (−0.24 to 0.07) | 0.26 | 0.10 |
| 4 | 57 | Vitamin B12 (Cobalamin) | DNA methylation % | 0.100 (−0.06 to 0.26) | 0.23 | 0.10 |
| 5 | 57 | Choline | DNA methylation % | 0.037 (−0.12 to 0.19) | 0.64 | 0.08 |
| 6 | 57 | Folate | DNA methylation % | −0.062 (−0.21 to 0.09) | 0.41 | 0.09 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, R.M.; Smith, R.; Collins, C.E.; Mossman, D.; Wong-Brown, M.W.; Chan, E.-C.; Evans, T.-J.; Attia, J.R.; Smith, T.; Butler, T.; et al. Methyl-Donor and Cofactor Nutrient Intakes in the First 2–3 Years and Global DNA Methylation at Age 4: A Prospective Cohort Study. Nutrients 2018, 10, 273. https://doi.org/10.3390/nu10030273
Taylor RM, Smith R, Collins CE, Mossman D, Wong-Brown MW, Chan E-C, Evans T-J, Attia JR, Smith T, Butler T, et al. Methyl-Donor and Cofactor Nutrient Intakes in the First 2–3 Years and Global DNA Methylation at Age 4: A Prospective Cohort Study. Nutrients. 2018; 10(3):273. https://doi.org/10.3390/nu10030273
Chicago/Turabian StyleTaylor, Rachael M., Roger Smith, Clare E. Collins, David Mossman, Michelle W. Wong-Brown, Eng-Cheng Chan, Tiffany-Jane Evans, John R. Attia, Tenele Smith, Trent Butler, and et al. 2018. "Methyl-Donor and Cofactor Nutrient Intakes in the First 2–3 Years and Global DNA Methylation at Age 4: A Prospective Cohort Study" Nutrients 10, no. 3: 273. https://doi.org/10.3390/nu10030273





