Selenium and Mercury Interactions in Apex Predators from the Gulf of Trieste (Northern Adriatic Sea)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Analyses
3. Results and Discussion
3.1. Seawater
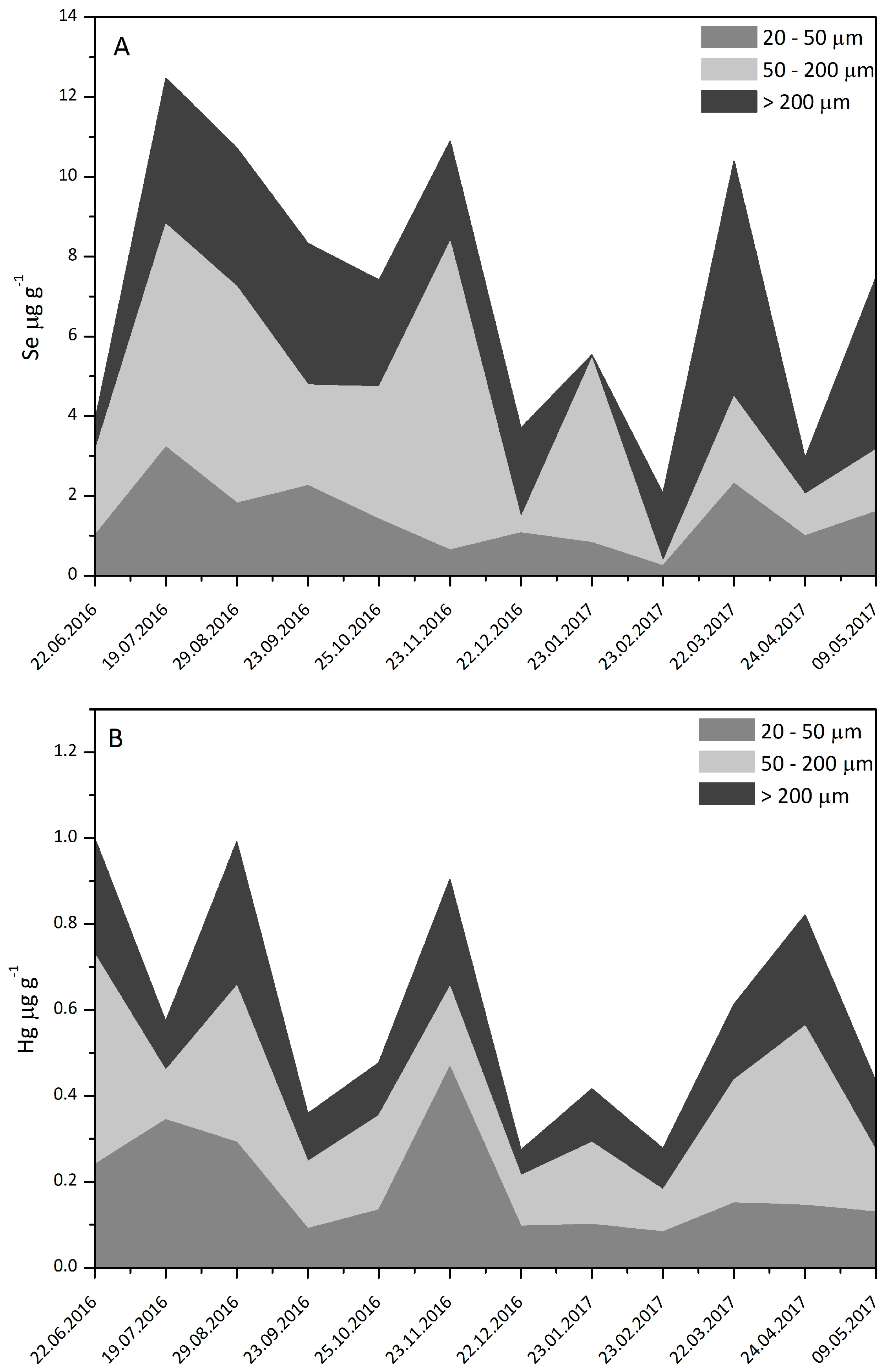
3.2. Plankton
3.3. Sediments
3.4. Fish
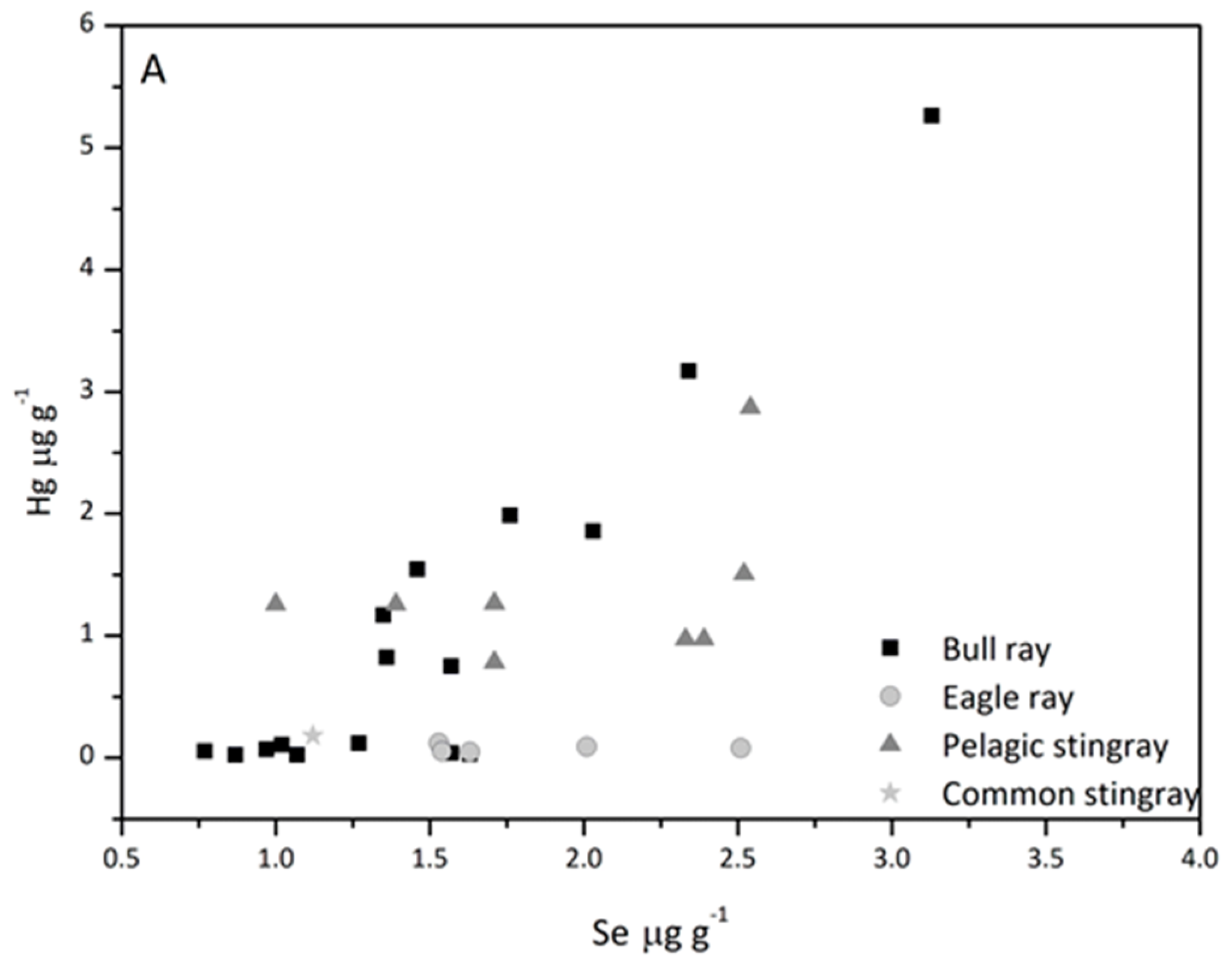
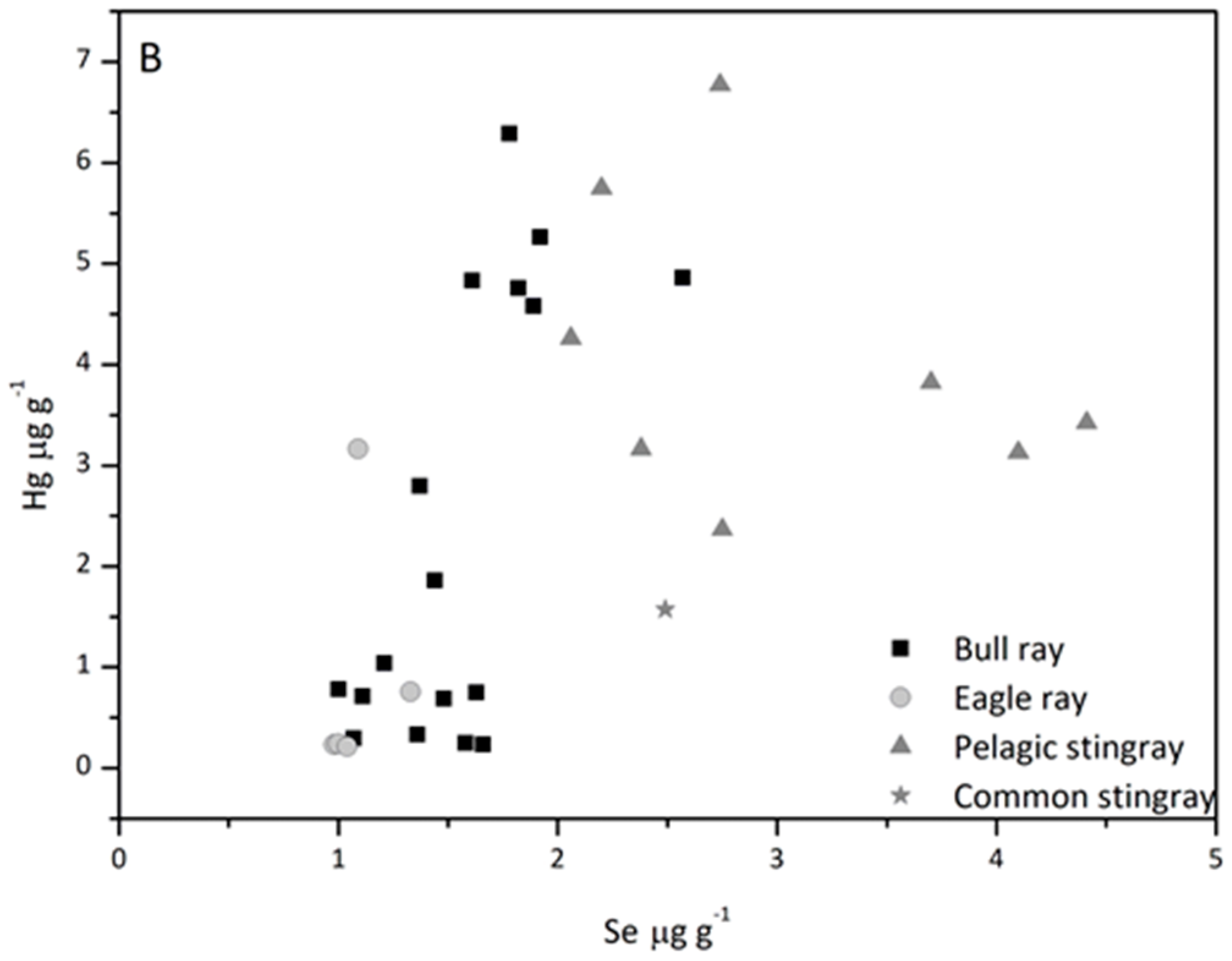
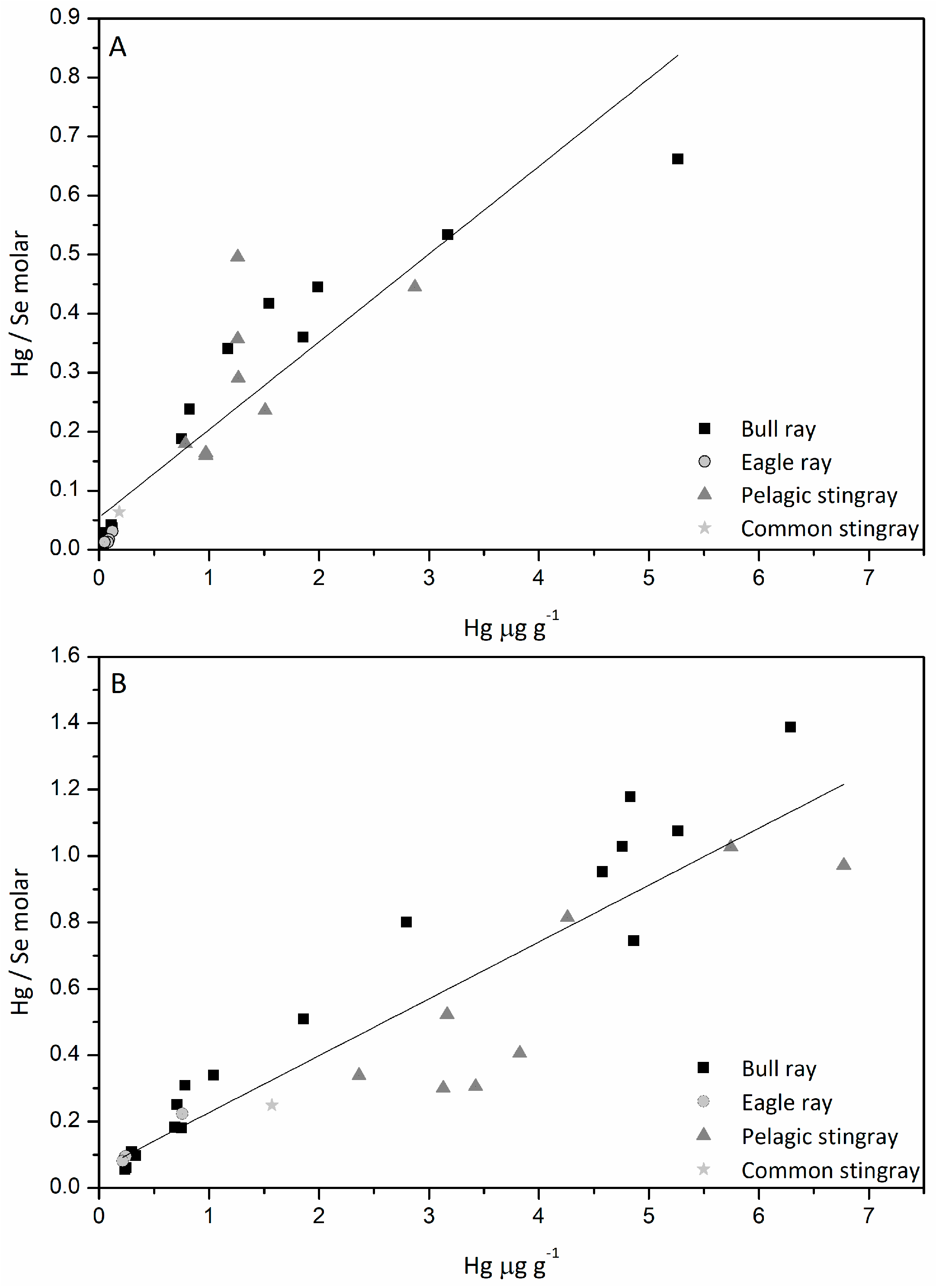
3.5. Hg/Se Ratio
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koerman, J.H.; Peeters, W.H.; Koudstaal-Hol, C.H.; Tjioe, P.S.; de Goeij, J.J. Mercury-selenium correlations in marine mammals. Nature 1973, 245, 385–386. [Google Scholar] [CrossRef]
- Ganther, H.E.; Sunde, M.I. Factors in fish modifying methylmercury toxicity and metabolism. Biol. Trace Elem. Res. 2007, 119, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.C.V. Selenium health benefit values as seafood safety criteria. EcoHealth 2008, 5, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.C.V.; Blackwell, J.L., III; Raymond, L.J. Importance of molar ratios in selenium-dependent protection against methylmercury toxicity. Biol. Trace Elem. Res. 2007, 11, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.A.; Ralston, N.V.C.; Whanger, P.D.; Oldfield, J.E.; Mosher, W.D. Selenium and mercury in interactions with emphasis of fish tissue. Environ. Bioindic. 2009, 4, 318–334. [Google Scholar] [CrossRef]
- Peterson, S.A.; Ralston, N.V.C.; Peck, D.V.; Sickle, J.V.; Robertson, J.D.; Spate, V.I.; Morris, J.S. How might selenium moderate the toxic effects of mercury in stream fish of the western US? Environ. Sci. Technol. 2009, 43, 3919–3925. [Google Scholar] [CrossRef] [PubMed]
- Cuvin-Aralar, M.L.A.; Furness, R.W. Mercury and selenium interactions: A review. Ecotoxicol. Environ. Saf. 1991, 21, 507–519. [Google Scholar] [CrossRef]
- Yang, D.-Y.; Chen, Y.-W.; Gunn, J.M.; Belzile, N. Selenium and mercury in organisms: Interactions and mechanisms. Environ. Rev. 2008, 16, 71–92. [Google Scholar] [CrossRef]
- Carvalho, C.M.L.; Chew, E.-H.; Hashemy, S.I.; Lu, J.; Holmgren, A. Inhibition of the human thioredoxin system: A molecular mechanism of mercury toxicity. J. Biol. Chem. 2008, 18, 11913–11923. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.C. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology 2010, 278, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.C.; Azenkeng, A.; Ralston, C.R.; Raymond, L.J. Selenium-health benefit values as seafood safety criteria. In Seafood Science Advances in Chemistry, Technology and Applications; Kim, S.-K., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 433–457. [Google Scholar]
- Penglase, S.; Hamre, K.; Ellingsen, S. Selenium and mercury have a synergistic negative effect on fish reproduction. Aquat. Toxicol. 2014, 149, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Wang, F.Y. Mercury-selenium compounds and their toxicological significance: Toward a molecular understanding of the mercury-selenium antagonism. Environ. Toxicol. Chem. 2009, 28, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Yamashita, Y.; Suzuki, T.; Kani, Y.; Mizusawa, N.; Imamura, S.; Takemoto, K.; Hara, T.; Hossain, M.A.; Yabu, T.; et al. Selenoneine, a novel selenium-containing compound, mediates detoxification mechanism against methylmercury accumulation and toxicity in zebrafish embryo. Mar. Biotechnol. 2013, 15, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-Y.; Ye, X.; Chen, Y.W.; Belzile, N. Inverse relationship between selenium and mercury in tissues of young walleye (Stizosedion vitreum) from Canadian boreal lakes. Sci. Total Environ. 2010, 408, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.-X. Selenium induces the demethylation of mercury in marine fish. Environ. Pollut. 2017. [Google Scholar] [CrossRef] [PubMed]
- Horvat, M.; Degenek, N.; Lipej, L.; Tratnik Snoj, J.; Faganeli, J. Trophic transfer and accumulation of mercury in ray species in coastal waters affected by historic mercury mining (Gulf of Trieste, Northern Adriatic Sea). Environ. Sci. Pollut. Res. 2014, 21, 4163–4176. [Google Scholar] [CrossRef] [PubMed]
- Šlejkovec, Z.; Stanjko, A.; Falnoga, I.; Lipej, L.; Mazej, D.; Horvat, M.; Faganeli, J. Bioaccumulation of arsenic species in rays from the northern Adriatic Sea. Int. J. Mol. Sci. 2014, 15, 22073–22091. [Google Scholar] [CrossRef] [PubMed]
- Pethybridge, H.; Cossa, D.; Butler, E.C.V. Mercury in 16 demersal sharks from southeast Australia; biotic nada biotic sources of variation and consumer health implications. Mar. Environ. Res. 2010, 69, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Horvat, M.; Covelli, S.; Faganeli, J.; Logar, M.; Mandic, V.; Rajar, R.; Sirca, A.; Zagar, D. Mercury in contaminated coastal environments: A case study: The Gulf of Trieste. Sci. Total Environ. 1999, 237–238, 43–56. [Google Scholar] [CrossRef]
- Kosta, L.; Ravnik, V.; Byrne, A.R.; Štirn, J.; Dermelj, M.; Stegnar, P. Some trace elements in the waters, marine organisms and sediments of the Adriatic by neutron activation analysis. J. Radioanal. Chem. 1978, 44, 317–332. [Google Scholar] [CrossRef]
- Stegnar, P.; Kosta, L.; Planinc, R.; Štirn, J. Baseline Studies and Monitoring of Metals, Particularly Mercury and Cadmium, in Marine Organisms; FAO/UNEP Report: Roma, Italy, 1980. [Google Scholar]
- Mavrič, B.; Jenko, R.; Makovec, T.; Lipej, L. On the occurence of the pelagic stingray, Dasyatis violacea (Bonaparte, 1832), in the Gulf of Trieste (Northern Adriatic). Ann. Ser. Hist. Nat. 2004, 14, 181–186. [Google Scholar]
- Dulčič, J.; Lipej, L.; Orlando Bonaca, M.; Jenko, R.; Grbec, B.; Guélorget, O.; Capape, C. The bull ray, Pteromylaeus bovinus (Myliobatidae), in the Northern Adriatic Sea. Cybium 2008, 32, 119–123. [Google Scholar]
- Lipej, L.; Mavrič, B.; Paliska, D.; Capapé, C. Feeding habits of the pelagic stingray Pteroplatytrygon violacea (Chondrichthyes: Dasyatidae) in the Adriatic Sea. J. Mar. Biol. Assoc. UK 2013, 93, 285–290. [Google Scholar] [CrossRef]
- Capape, C.; Guelorget, O.; Vergne, Y.; Marques, A.; Quingnard, J.P. Skates and rays (Chondrychthyes) from waters off Languedocian coast (Southern France, Northern Mediterranean): A historical survey and present status. Anna. Ser. Hist. Nat. 2006, 16, 165–178. [Google Scholar]
- Bloom, N.S.; Crecelius, E.A. Determination of mercury in seawater at subnanogram per liter levels. Mar. Chem. 1983, 14, 49–59. [Google Scholar] [CrossRef]
- Koron, N.; Faganeli, J.; Falnoga, I.; Mazej, D.; Klun, K.; Kovac, N. Association of macroaggregates and metals in coastal waters (Gulf of Trieste, Northern Adriatic Sea). Mar. Chem. 2013, 157, 185–193. [Google Scholar] [CrossRef]
- Tinta, T.; Vojvoda, J.; Talaber, I.; Vodopivec, M.; Malfatti, F.; Turk, V. Bacterial community shift is induced by dynamic environmental parameters in a changing coastal ecosystem (Northern Adriatic, NE Mediterranean Sea)—A 2 years time scale study. Environ. Microbiol. 2015, 17, 3581–3596. [Google Scholar] [CrossRef] [PubMed]
- Mozetič, P.; Fonda Umani, S.; Cataletto, B.; Malej, A. Seasonal and inter-annual plankton variability in the Gulf of Trieste (Northern Adriatic). ICES J. Mar. Sci. 1998, 55, 711–722. [Google Scholar] [CrossRef]
- Millero, F.J. Chemical Oceanography, 3rd ed.; CRC Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Faganeli, J.; Horvat, M.; Covelli, S.; Fajon, V.; Logar, M.; Lipej, L.; Cermelj, B. Mercury and methylmercury in the Gulf of Trieste (Northern Adriatic Sea). Sci. Total Environ. 2003, 304, 315–326. [Google Scholar] [CrossRef]
- Čebulec, O. Chemical Characterization of Zooplankton. Bachelor’s Thesis, University of Ljubljana, Ljubljana, Slovenia, 2014. [Google Scholar]
- Lipej, L.; Mozetič, P.; Turk, V.; Malej, A. The trophic role of the marine cladoceran Penilia avirostris in the Gulf of Trieste. Hydrobiologia 1997, 360, 197–203. [Google Scholar] [CrossRef]
- Ogorelec, B.; Mišič, M.; Faganeli, J. Marine geology of the Gulf of Trieste (Northern Adriatic): Sedimentological aspects. Mar. Geol. 1991, 99, 79–92. [Google Scholar] [CrossRef]
- Covelli, S.; Faganeli, J.; Horvat, M.; Brambati, A. Mercury contamination of coastal sediments as the result of long-term cinnabar mining activity (Gulf of Trieste, northern Adriatic sea). Appl. Geochem. 2001, 16, 541–558. [Google Scholar] [CrossRef]
- Commission of the European Communities. EU Commission Decision 466/2001/EC of March 8, 2001 Setting Maximum Levels for Certain Contaminants in Foodstuffs; Commission of the European Communities: Brussels, Belgium, 2001. [Google Scholar]
- Storelli, M.M.; Marcotrigiano, G.O. Mercury speciation and relationship between mercury and selenium in liver of Galeus melastomus from the Mediterranean Sea. Bull. Environ. Contamin. Toxicol. 2002, 69, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Horvat, M.; Zvonarić, T.; Stegnar, P.; Prosenc, A.; Konda, D.; Sabadin, A. Relation between total mercury, methylmercury and selenium in fish muscle from the Adriatic Sea. In Heavy Metals in the Environment; Vernet, J.-P., Ed.; CRC Press: Geneva, Switzerland, 1989; Volume 1, pp. 370–373. [Google Scholar]
 sediments were collected, and the oceanographic buoy Vida (sampling site F0) where the seawater and plankton samples were collected.
sediments were collected, and the oceanographic buoy Vida (sampling site F0) where the seawater and plankton samples were collected.
 sediments were collected, and the oceanographic buoy Vida (sampling site F0) where the seawater and plankton samples were collected.
sediments were collected, and the oceanographic buoy Vida (sampling site F0) where the seawater and plankton samples were collected.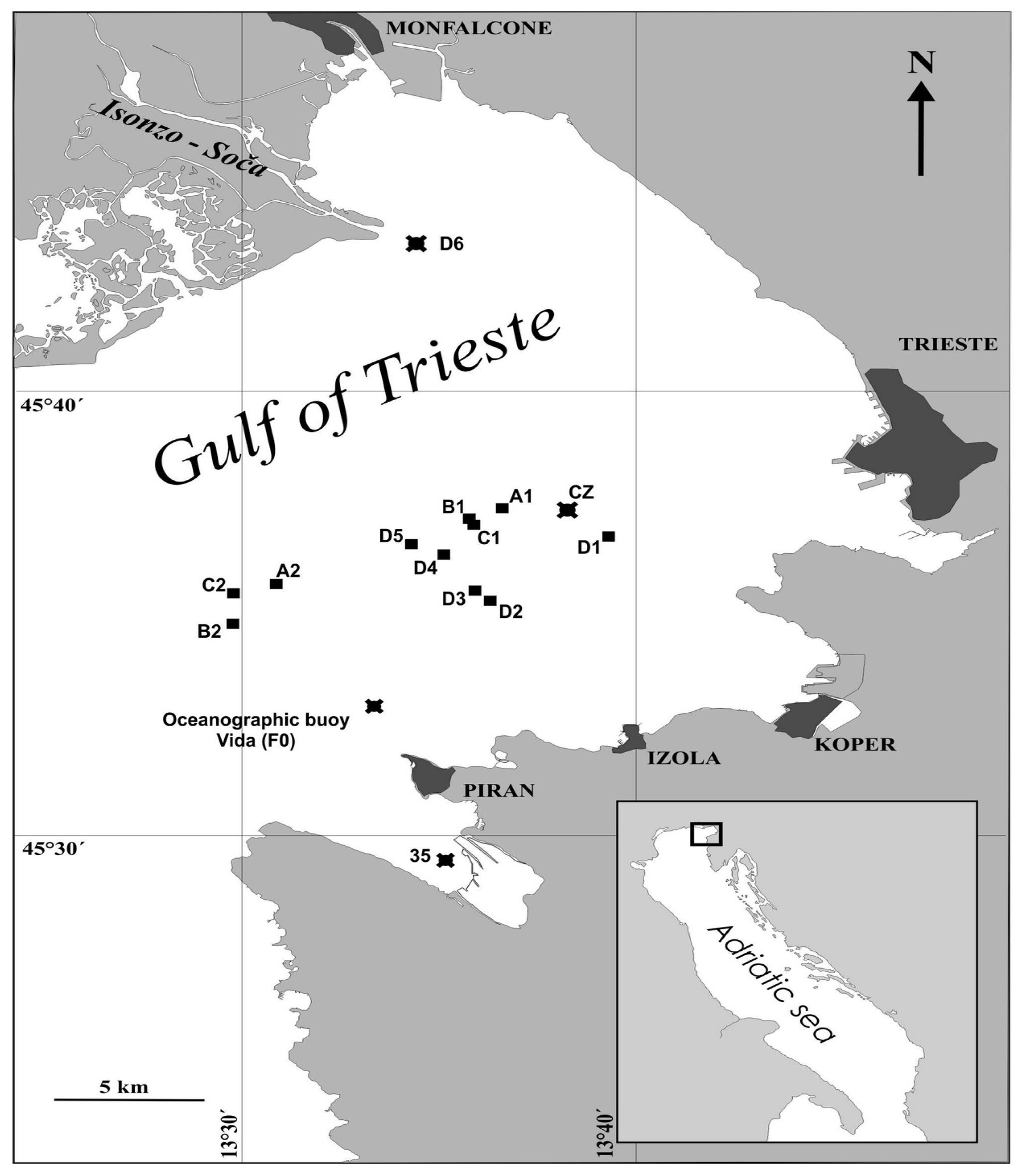
| Species | n (m, f, juv) | Disc Width (mm) | Disc Length (mm) | Weight (kg) |
|---|---|---|---|---|
| Eagle ray (Myliobatis aquila) | 5 (1, 1, 3) | 273–380, 310 ± 50 | 142–225, 176 ± 38,9 | 0.26–0.98, 0.50 ± 1.31 |
| Bull ray (Pteromylaeus bovinus) | 17 (6, 9, 2) | 450–2220, 727 ± 422 | 760–2940, 1714 ± 606 | 1.50–116.0, 47.0 ± 43.0 |
| Pelagic stingray (Dasyatis violacea) | 8 (5, 3, 0) | 437–600, 531 ± 61 | 1010–1392, 1240 ± 146 | 2.40–7.56, 4.83 ± 1.78 |
| Common stingray (Dasyatis pastinaca) | 1 (1, 0, 0) | 455 | 367 | 4.00 |
| Se | Hg | Hg/Se | |
|---|---|---|---|
| Seawater dissolved | 1.58–2.37 | BDL—5.03 10‒3 a | <0.01 |
| Seawater colloidal | |||
| (>5 kDa) | 0.032–0.04 | BDL—1.98 b | 0.43 |
| Seawater particulate | |||
| (0.45–20 µm) | 158 | BDL—4.35 | <1.3 |
| Microplankton | |||
| 20–50 µm | 639.9–3223.2 | 8.0–540.7 | 0.01–0.22 |
| 50–200 µm | 371.3–632.0 | 120.6–422.1 | 0.01–0.36 |
| Mesoplankton | |||
| (>200 µm) | BDL-5901.3 | 10.05–341.7 | <0.12 |
| Sediment surface | |||
| (0–1 cm) | 275.9–2599.1 | 110.5–2291.4 | 0.05–0.35 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faganeli, J.; Falnoga, I.; Horvat, M.; Klun, K.; Lipej, L.; Mazej, D. Selenium and Mercury Interactions in Apex Predators from the Gulf of Trieste (Northern Adriatic Sea). Nutrients 2018, 10, 278. https://doi.org/10.3390/nu10030278
Faganeli J, Falnoga I, Horvat M, Klun K, Lipej L, Mazej D. Selenium and Mercury Interactions in Apex Predators from the Gulf of Trieste (Northern Adriatic Sea). Nutrients. 2018; 10(3):278. https://doi.org/10.3390/nu10030278
Chicago/Turabian StyleFaganeli, Jadran, Ingrid Falnoga, Milena Horvat, Katja Klun, Lovrenc Lipej, and Darja Mazej. 2018. "Selenium and Mercury Interactions in Apex Predators from the Gulf of Trieste (Northern Adriatic Sea)" Nutrients 10, no. 3: 278. https://doi.org/10.3390/nu10030278






