1. Introduction
Human milk contains more than 200 nondigestible milk human oligosaccharides (HMO), whereas cow’s milk contains virtually no oligosaccharides, which explains the greater abundance of intestinal bifidobacteria observed in infants who were fed human milk compared with standard formula (SF) [
1,
2,
3]. Nutritional regimen greatly shapes intestinal microbiota composition, with impact on future human health and specific clinical outcomes [
4]. This may be particularly important in early life when initial microbiological colonization drives immune imprinting. Immune development affects the subsequent risk of allergy and of infections; at the same time atopy correlates with increased risk of wheezing and asthma, although data are controversial [
5].
Selected prebiotics, such as galactooligosaccharides (GOS) and inulin, have a bifidogenic effect when added to milk formula [
4]. Most effects have been observed with two chemical groups, namely inulin-type fructans and GOS. Those effects have clinical implications and preliminary evidence shows that addition of prebiotics to milk formula results in better stool pattern, reduces the risk of gastroenteritis and respiratory infections (RIs), reduces the incidence of atopic eczema and promotes mineral absorption [
4]. Further research is necessary before routine use of prebiotics can be recommended for the prevention of allergy in formula-fed infants. Whether the use of prebiotics should be restricted to infants at high risk of allergy or may have an effect in low-risk populations and whether it may have an effect on other allergic diseases including asthma are unclear [
6]. Furthermore, the cause-effect relationship with intestinal microbiota and the true clinical importance of such effects are not yet fully elucidated. The Committee on Nutrition of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition stated that “there is no sufficient evidence of clinical benefits to recommend the addition of prebiotics to infant foods” [
6].
The main limitations of studies on prebiotics include small sample size, difficulties of having a proper control population of breastfed infants, a lack of stringent inclusion and exclusion criteria (such as the specific risk of study populations), a lack of standardised endpoints, and the absence of parallel clinical and microbiological data [
7,
8,
9,
10,
11].
GOS selectively enhances the growth of Bifidobacteriaceae and Lactobacillaceae, and increases the colonic production of short-chain fatty acids that decrease luminal pH, which may reduce the growth of enteropathogens [
4]. Infant formula containing the prebiotic mixture GOS/fructooligosaccharide (FOS) compared with a standard infant formula, was associated with a significant reduction in the incidence of gastroenteritis, but not of upper respiratory tract infections although the number of children with multiple antibiotic courses per year was lower in children receiving prebiotics [
8]. One randomized controlled trial (RCT) found a similar number of episodes of diarrhoea and fewer episodes of physician-diagnosed respiratory tract infections, fever episodes and fewer antibiotic prescription in the group of infants fed extensively hydrolysed whey formula supplemented either with 0.8 g GOS/FOS or with maltodextrin as placebo [
9]. Two RCTs investigated the role of prebiotic supplementation of infant formula for the prevention of allergic disease and food hypersensitivity [
10,
11]. One of the included RCT investigated the effect of GOS/FOS on the cumulative incidence of atopic dermatitis during the first six months of life in infants at risk for allergy, and showed a reduction of atopic dermatitis (AD) in the first year of life [
10]. However there are conflicting data about the protective effect beyond the first year of life [
10,
12]. In a large multicentre, randomised, double-blind and placebo-controlled trial in 365 healthy term infants enrolled before eight weeks of age, the administration of GOS-containing infant formula without other HMOs, despite producing a definite prebiotic effect, did not change the incidence of infections or allergic manifestations in the first year of life [
13]. The relation between respiratory illnesses in early life and the development of asthma and atopy in childhood is incompletely understood. However no study evaluated whether prebiotics modify the history of common infections in a population with high risk of atopy.
Polydextrose (PDX) is a peculiar HMO whose effects in vivo and in vitro are less investigated than GOS. In contrast to other prebiotics with smaller molecular weights, PDX is fermented and remains available as a carbon source for microbiota with a sustained production of short-chain fatty acids (acetate, propionate, butyrate) [
14]. PDX-containing infant formula increases ileal lactobacilli and propionic and lactic acid concentrations, and decreases pH with associated changes in ileal cytokine expression [
14].GOS and PDX have generally-recognised-as-safe (GRAS) status and is often added to commercial infant formula [
15].
The aim of the Prebiotics in Prevention of Atopy (PIPA) study was to evaluate whether the addition of GOS/PDX to infant formula administered to young infants at risk of atopy prevents AD and reduces the risk of respiratory infections (RIs) and acute diarrhea in the first two years of life. A microbiological substudy was conducted in parallel in order to analyze the composition of intestinal microbiota in relation to nutritional intervention. We also included a control group of breastfed infants as breastfeeding (BF) is the gold standard of nutrition in infants until six months.
2. Materials and Methods
2.1. Clinical Trials.Gov Identifier
NCT02116452. The study protocol was approved by the ethics committee of the University of Padua. This double blind, randomized controlled trial investigated the effects of a GOS/PDX supplemented formula compared with a standard formula (SF) in preventing and modifying the history of AD in the first two years of life in infants at risk of atopy. Secondary endpoints included incidence of common infections (RIs and acute diarrhea in infants at risk of atopy).
2.2. Patient Selection Criteria
Infants who met all of the following criteria were eligible in the study: (1) gestational age > 37 and <42 weeks; (2) birth weight > 2500 g; (3) at risk of atopy (with at least one parent with physician-diagnosed asthma, allergic rhinoconjunctivitis, AD, allergic urticaria and food allergy) [
16]; and (4) informed consent. Infants were screened for risk of atopy at delivery using a validated questionnaire [
11]. Exclusion criteria were as follows: congenital immunodeficiency, severe congenital disorders or malformations, infants born to mothers with diabetes, long-term intake (>7 consecutive days) of probiotics or prebiotics, and intake of ≥50 mL of formula different from that included in the study for at least a week.
2.3. Randomisation
At birth, infants were randomised to either the SF (control group) or study formula (treatment arm) to be taken until 48 weeks of life. Only infants who had taken the formula were included in the analysis. Randomised infants who ended up being exclusively breastfed and did not receive artificial milk formula until at least six months of age and continued BF constituted the parallel comparison group.
Figure 1 summarises the study population according to the CONSORT (CONsolidated Standards of Reporting Trials) flow diagram.
2.4. Study Product
At birth, patients were randomized to receive 50/50 GOS/PDX formula (prebiotic formula [PF]) or SF until 48 weeks of life. The two formulas were identical except for the addition of a mixture of 4 g/L of GOS/PDX in a ratio of 50/50. The entire amount of formula needed for each child was provided by Mead Johnson Nutrition (Evansville, IN, USA) in a similar package to keep the randomized group blind both to the physicians and parents. Patients who were exclusively breastfed up to 24 weeks of life constituted the parallel comparison group of breastfed infants.
2.5. Patient Follow-up
At enrolment, infants at 24 (visit 1), 36 (visit 2), 48 (visit 3) and 96 (visit 4) weeks of age were evaluated by specifically trained physicians in the pneumology and allergology ambulatory of the University of Padua. All of the mothers were provided with a follow-up patient diary. Parental compliance with feeding recommendations was assessed based on diary entries in addition to structured interviews on health problems reported since the last medical visit.
2.6. Outcomes
Primary endpoints comprised the incidence and severity of AD at 36 weeks and 48 weeks of life. The AD was diagnosed according to the criteria described by Halken et al. [
17] and Muraro et al. [
18]. Only patients with a definitive physician diagnosis were included as cases in the statistical analysis.
Primary outcome parameters to define AD included the cumulative number of patients with at least one episode of AD at 36 weeks and 48 weeks of life, and the severity of AD at 36 and 48 weeks of life, as graded using the SCORAD (SCORing Atopic Dermatitis) score. All patients with atopic dermatitis was evaluated by food allergy prick tests to define food sensitization. Secondary endpoints to analyze AD included the cumulative number of patients with at least one episode of AD at 96 weeks of life; the severity of AD at 96 weeks of life graded using the SCORAD score; the mean number of episodes of AD at 36, 48 and 96 weeks of life; the total duration of AD (in days) at 48 weeks and 96 weeks of life; the use of drugs to treat AD at 48 weeks and 96 weeks of life (quantity and type). Secondary endpoints were also comprised of parameters to analyze the occurrence of (RIs). RIs were defined as any infection of three upper and lower respiratory tract diagnosed by a physician [
19]. Recurrent RIs (RRIs) will be defined as more than 3 episodes/year of RIs requiring medical attention [
5]. Parameters considered to estimate RIs included the cumulative number of infants with at least one episode of RI at 48 weeks and 96 weeks of life, the cumulative number of infants with RRIs at 96 weeks of life, the mean number of RI episodes at 48 and 96 weeks of life, the mean duration of RIs, the cumulative number of infants with wheezing lower RIs at 48 weeks and 96 weeks, number of patients with at least one antibiotic prescription, the number of parents who lost workdays within the first 96 weeks of life of infants, as well as the number of days lost at work by parents for children infections, and the number of infants with at least one episode of acute diarrhea at 48 weeks and 96 weeks of life. Acute gastroenteritis (AG) is defined as a reduction in the consistency of stools (loose or liquid) and/or an increase in the frequency of evacuations (typically three or more/24 h), with or without fever, vomiting, dehydration, or compromised general status during less than 7 days. Clinical outcomes were reported by parents in the weekly diary and confirmed by primary care physicians (family paediatricians or FPs). FPs belonged to the established Italian network Pedianet, covering a well defined number of patients in the Veneto Region of Italy.
2.7. Microbiological and Faecal Markers
A substudy was conducted to evaluate gut microbiota composition in 147 infants (45%). Stool samples were collected at baseline (before starting the nutritional intervention, with age ranging from one to six months) and at 9–12 months of age. Gut microbiota composition was investigated using fluorescence in situ hybridisation. The bacterial species analyzed were Bifidobacteria, Clostridium cluster I, Clostridium cluster IX, Firmicutes, Bacteroides and Faecalibacterium prausnitzii. The species were selected based on the possible relationships with clinical outcomes reported in previous papers [
20,
21].
Microbiological data were obtained at baseline (before nutritional shift) and at 9–12 months of age corresponding to a minimum period of three months of specific nutritional regimen. The loads of microbial species in the post-intervention period compared with baseline were measured to investigate their possible relationship with the type of feeding.
Fecal samples were fixed in Carnoy’s solution and embedded in paraffin, which was cut longitudinally into four sections, placed on Super- Frost slides (Thermo Scientific, Monza MB, Italy), and incubated with hybridization solution (20 mM Tris-HCl, 0.9 M NaCl, 0.1% SDS, and 1% formamide, pH 7.4) with EUB mix positive control oligonucleotide probes (100 ng of EUB I, EUBII, and EUBIII). For specific bacteria groups, the slides were incubated with hybridisation buffer with 25 ng of their respective fluorescent in situ hybridization (FISH) probes for 45 min at 50 °C and visualised using a Nikon 80i Eclipse epifluorescence microscope with a Nikon DS-U2 color camera and NIS-Elements imaging software (Nikon, Tokyo, Japan). Bacteria were quantified by count. The oligonucleotide probes used in this study were synthesized by MWG Eurofins (MWG Operon Ebersberg, Ebersberg, Germany). All of the samples were analysed with the Eub338 mix probe conjugated with fluorescein isothiocyanate (FITC, green signal) at the end (positive control that reacts with all bacteria), and a species-specific probe was conjugated with a single fluorescent carbocyanine molecule (Cy3, red signal).
2.8. Statistical Methods
Sample size calculation was calculated a priori. It was based on the difference in AD incidence between GOS/PDX and SF at 48 weeks. Assuming that the proportions of infants expected to develop AD in the PF and SF groups being are equal to 30% and 50%, respectively, considering type I and II errors of 0.05 and 0.20 and a dropout rate of 10%, the estimated sample size was 120 infants per randomized arm (240 in total for the formula groups). As a result the power of the study was calculated to emphasize a difference of 20%.
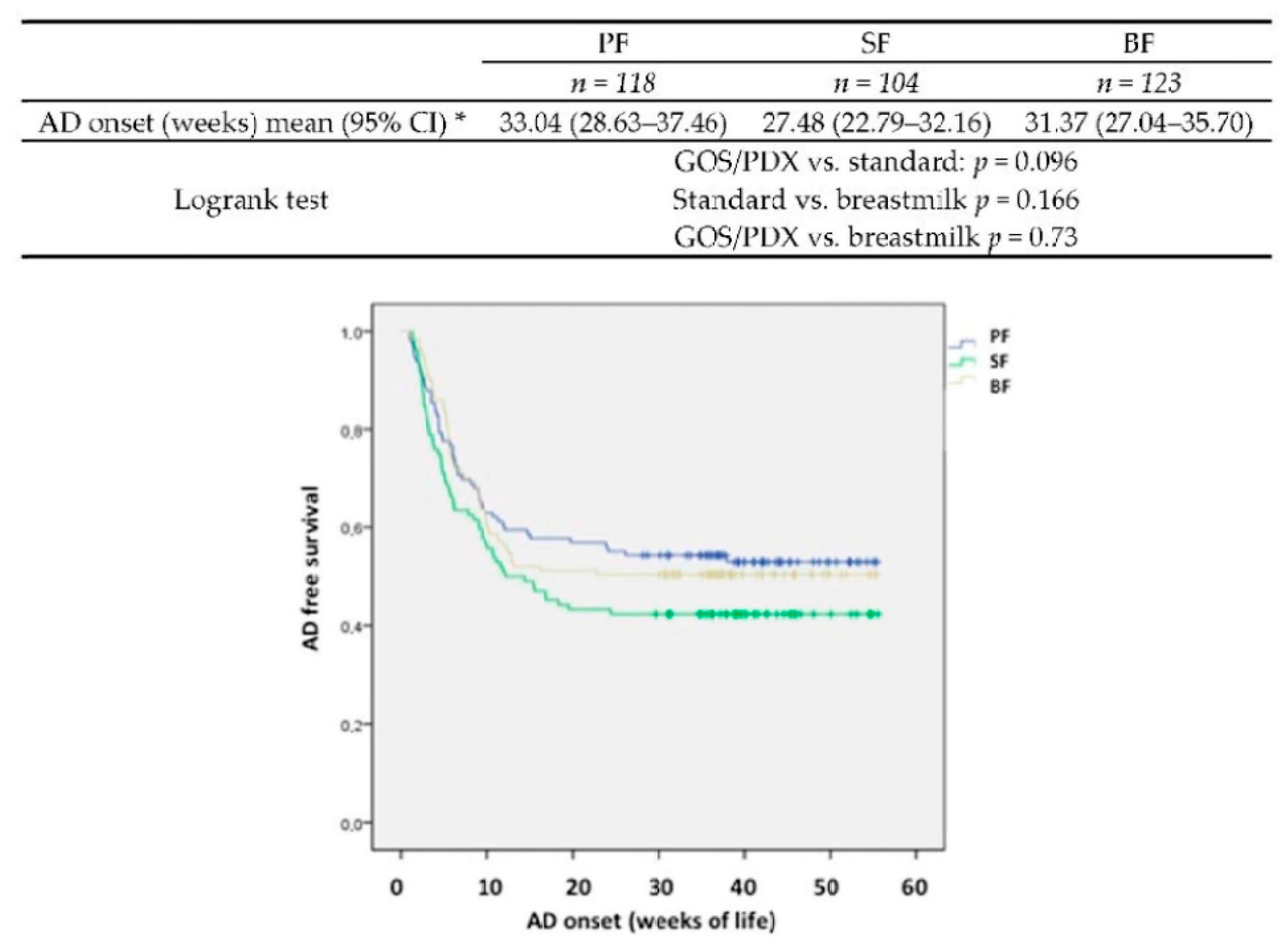
The analysis and results reported in this paper reflect the final study design and are based on the per protocol (PP) population including all children completing the study. Intention-to-treat analysis (ITT) confirmed the data trend. Descriptive statistics were reported for all of the recorded variables; frequencies and percentages for qualitative variables, medians and interquartile ranges for ordinal variables and means and standard deviations for quantitative variables were applied. The chi-square test and one-way analysis of variance were conducted to determine the differences among the three groups (PF, SF and BF) in the qualitative and quantitative variables, respectively. In case of significant or nearly significant overall tests (p < 0.10), post-hoc tests were performed to identify pairwise differences. Bonferroni correction was applied for multiplicity.
4. Discussion
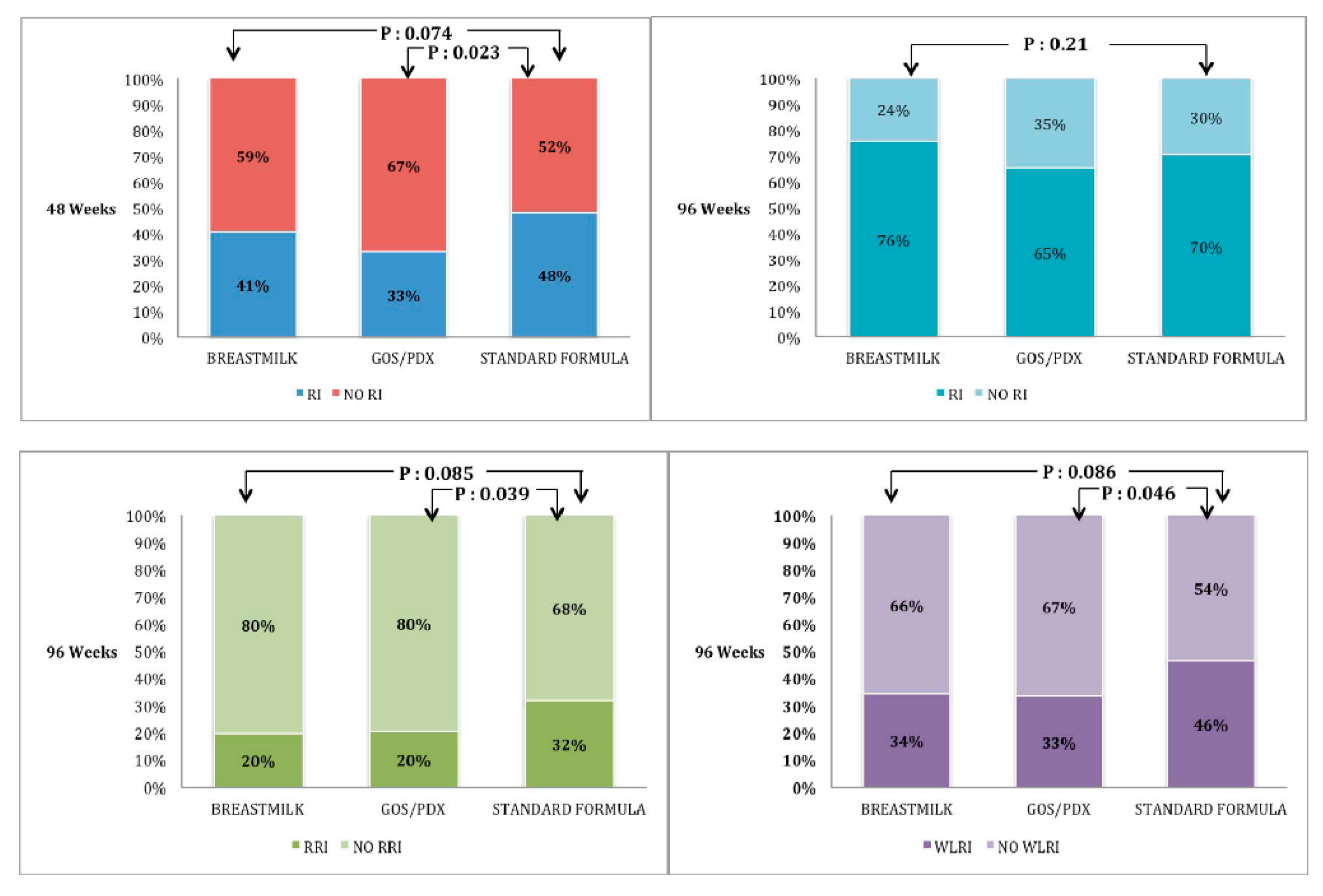
This is a double blind randomised clinical trial including also a parallel comparison group of BF infants. As for atopic dermatitis, which represents the primary objective, our study does not show a significant reduction of incidence of its occurrence in the PF group (configuring itself as a negative study). However it is important to underline that the risk of AD in PF compared with SF is reduced by 35%. A major clinical effect observed in PF-fed infants was the reduction of RIs. This effect may be important in at-risk population of infants born to atopic parents considering the vicious circle between atopy and infections. Children at risk of atopy have a greater risk of RIs [
22], and in turn recurrent RIs trigger asthma [
23]. In particular, WLRIs, such as bronchiolitis, are significantly associated with asthma later in life. In our cohort of infants, approximately 25% were free from RIs until 96 months of life, which agrees with that reported in the general population [
5]. However, we observed a high rate of WLRIs with about 40% of infants presenting at least one such episode, with higher incidence than that expected in the general population [
5]. WLRIs, representing the bridge between RIs and asthma [
24], were highly prevalent in our cohort probably due to the atopic risk of infant and/or close physician surveillance. The effect of GOS/PDX on RIs was dependent on the assumed time since it was not observed when the enriched formula was discontinued. However, even this transient effect had a prolonged impact since the prevalence of RRIs and WLRI at 96 weeks was reduced in the PF group compared with the SF group. As for acute gastroenteritis, we obtained further proof of the well-known protective effect of BF. The rate of AG was not different in the PF and SF groups.
The mechanisms of the protective effect of prebiotic against RIs are not clearly elucidated but are likely to involve modifications of intestinal microbiota. In this study, we explored the modifications in intestinal microbiota in parallel with the clinical effects and confirmed that prebiotics increase the load of Bifidobacteria. The role of intestinal microbiota and RIs has already been established in chronic conditions, such as cystic fibrosis and asthma. In a previous study involving children with cystic fibrosis (CF), we found a disrupted intestinal microbiota with reduced diversity in intestinal microbiota composition and Bifidobacterial load was strongly reduced compared with non-CF children [
8]. Our study directly showed for the first time that Bifidobacteria were increased in children without RIs regardless of nutrition, supporting the protective role of Bifidobacteria against RIs. More specifically, our data support the relationship between intestinal microbiota and direct protection from RIs involving Bifidobacteria. Therefore, the well-known “bifidogenic effect” of prebiotics has a clinical implication resulting in protection against RIs. The reduction of repeated antibiotic courses may well contribute to maintaining eubiosys in children receiving prebiotic-enriched formula.
Previous studies showed a positive association between Clostridia and atopic sensitisation [
25,
26], but the opposite [
20,
27] or no association was also reported [
28,
29]. In our study, Clostridium cluster I (also known as
Clostridium butyricum) load was increased in AD-free infants after the intervention period but not at baseline. This pattern is similar to that reported by Nakayama et al. [
27], but in contrast to that by Penders et al. [
20]. In our study, Clostridium cluster I load was specifically increased in prebiotics but not by other nutritional groups (including BF). Interestingly, non-toxigenic Clostridium I strains are currently used as probiotics in Asian countries [
29] in combination with immunotherapy in asthmatic patients [
30] in order to alleviate intestinal inflammation in ulcerative colitis in food allergy [
31] and to treat intestinal dysbacteriosis in neonates [
32].
The present study is singular in this field from a methodological point of view, in particular for the following aspects: the accuracy in defining the standardised endpoints; the use of expert, fixed and blinded clinicians for the follow-up of infants; the use of stringent inclusion and exclusion criteria (such as the high risk of atopy of the studied population); the high statistical power of the study set to emphasize differences of 20%, which is more than considered in other studies, with nutritional intervention; and the presence of parallel clinical and microbiological data. Limitations of this study are mainly: the small size of the final population; the non-randomised BF control group; and the high percentage of mixed-fed (BF plus FF) infants, according to common infants feeding habits.