Systematic Review of Vitamin D and Hypertensive Disorders of Pregnancy
Abstract
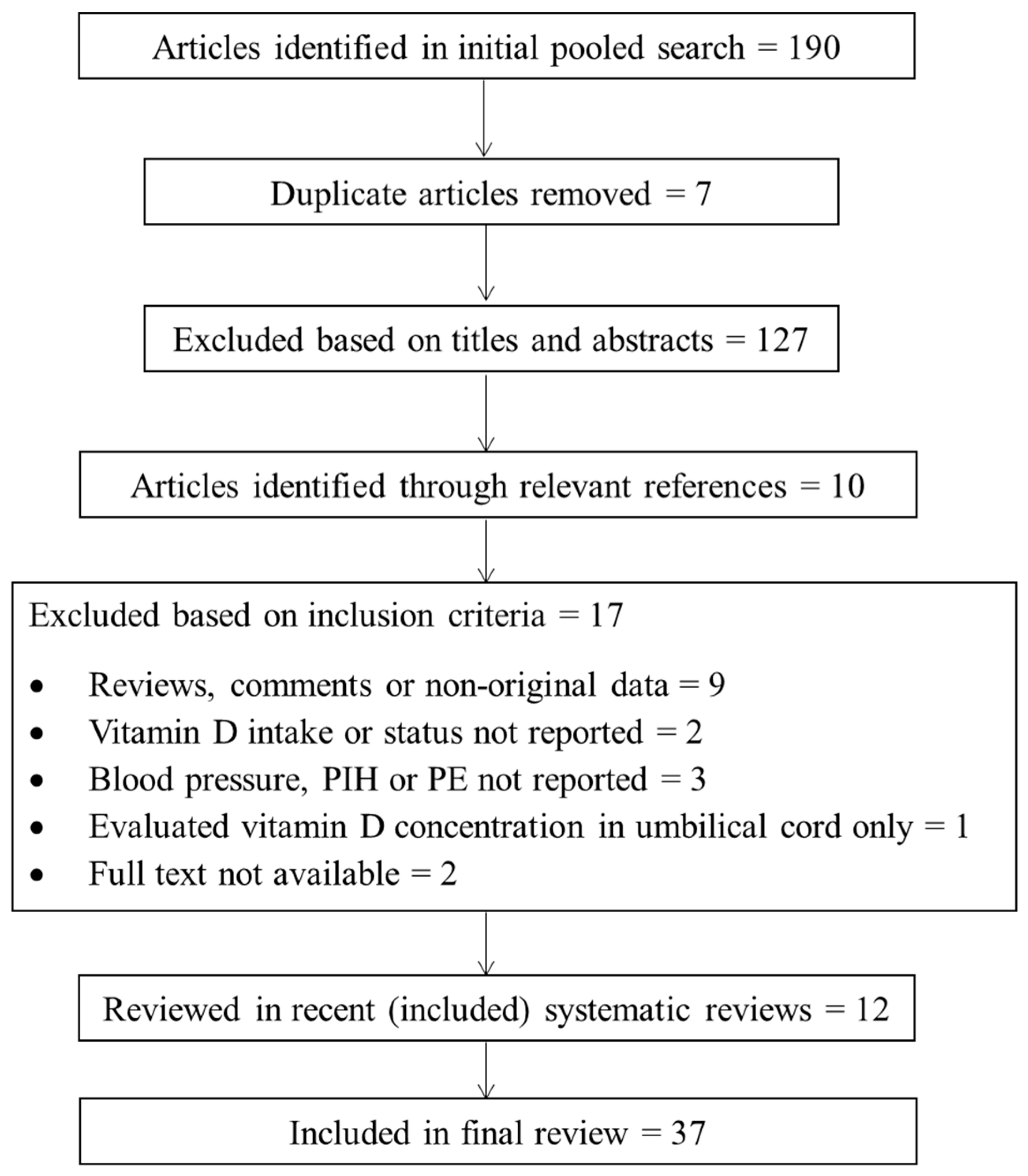
:1. Introduction
2. Objectives
2.1. PICO
2.2. Search Methods
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Data Collection
3. Results
3.1. Intervention Studies
3.1.1. Preeclampsia
3.1.2. Gestational Hypertension
3.2. Observational Studies
3.2.1. Vitamin D Status and Preeclampsia
3.2.2. Vitamin D Intake and Preeclampsia
3.2.3. Gestational Hypertension
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organisation. Who guidelines approved by the guidelines review committee. In Who Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Tranquilli, A.L.; Dekker, G.; Magee, L.; Roberts, J.; Sibai, B.M.; Steyn, W.; Zeeman, G.G.; Brown, M.A. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the isshp. Pregnancy Hypertens. 2014, 4, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Hutcheon, J.A.; Lisonkova, S.; Joseph, K.S. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Zakiyah, N.; Postma, M.J.; Baker, P.N.; van Asselt, A.D. On Behalf of the IMPROvED Consortium. Pre-eclampsia diagnosis and treatment options: A review of published economic assessments. Pharmacoeconomics 2015, 33, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Duley, L. Pre-eclampsia and the hypertensive disorders of pregnancy. Br. Med. Bull. 2003, 67, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Meads, C.A.; Cnossen, J.S.; Meher, S.; Juarez-Garcia, A.; Ter Riet, G.; Duley, L.; Roberts, T.E.; Mol, B.W.; van der Post, J.A.; Leeflang, M.M.; et al. Methods of prediction and prevention of pre-eclampsia: Systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol. Assess. 2008, 12, 1–270. [Google Scholar] [CrossRef]
- Snydal, S. Major changes in diagnosis and management of preeclampsia. J. Midwifery Womens Health 2014, 59, 596–605. [Google Scholar] [CrossRef] [PubMed]
- James, J.L.; Whitley, G.S.; Cartwright, J.E. Pre-eclampsia: Fitting together the placental, immune and cardiovascular pieces. J. Pathol. 2010, 221, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, D.; Evans, K.N.; Kilby, M.D.; Bulmer, J.N.; Innes, B.A.; Stewart, P.M.; Hewison, M. The ontogeny of 25-hydroxyvitamin D(3) 1alpha-hydroxylase expression in human placenta and decidua. Am. J. Pathol. 2002, 161, 105–114. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Zhang, J.Y.; Lucey, A.J.; Horgan, R.; Kenny, L.C.; Kiely, M. Impact of pregnancy on vitamin D status: A longitudinal study. Br. J. Nutr. 2014, 112, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Papapetrou, P.D. The interrelationship of serum 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D in pregnancy at term: A meta-analysis. Hormones 2010, 9, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Saffery, R.; Ellis, J.; Morley, R. A convergent model for placental dysfunction encompassing combined sub-optimal one-carbon donor and vitamin D bioavailability. Med. Hypotheses 2009, 73, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Tamblyn, J.A.; Susarla, R.; Jenkinson, C.; Jeffery, L.E.; Ohizua, O.; Chun, R.F.; Chan, S.Y.; Kilby, M.D.; Hewison, M. Dysregulation of maternal and placental vitamin D metabolism in preeclampsia. Placenta 2017, 50, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Tamblyn, J.A.; Hewison, M.; Wagner, C.L.; Bulmer, J.N.; Kilby, M.D. Immunological role of vitamin D at the maternal-fetal interface. J. Endocrinol. 2015, 224, R107–R121. [Google Scholar] [CrossRef] [PubMed]
- Saraf, R.; Morton, S.M.; Camargo, C.A., Jr.; Grant, C.C. Global summary of maternal and newborn vitamin D status—A systematic review. Matern. Child Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.; Hemmingway, A.; O’Callaghan, K.M. Vitamin D in pregnancy: Current perspectives and future directions. Ther. Adv. Musculoskelet. Dis. 2017, 9, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Choi, M.Y.; Longtine, M.S.; Nelson, D.M. Vitamin D effects on pregnancy and the placenta. Placenta 2010, 31, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Díaz, L.; Arranz, C.; Avila, E.; Halhali, A.; Vilchis, F.; Larrea, F. Expression and activity of 25-hydroxyvitamin D-1 alpha-hydroxylase are restricted in cultures of human syncytiotrophoblast cells from preeclamptic pregnancies. J. Clin. Endocrinol. Metab. 2002, 87, 3876–3882. [Google Scholar] [PubMed]
- Fischer, D.; Schroer, A.; Lüdders, D.; Cordes, T.; Bücker, B.; Reichrath, J.; Friedrich, M. Metabolism of vitamin D3 in the placental tissue of normal and preeclampsia complicated pregnancies and premature births. Clin. Exp. Obstet. Gynecol. 2007, 34, 80–84. [Google Scholar] [PubMed]
- Ma, R.; Gu, Y.; Zhao, S.; Sun, J.; Groome, L.J.; Wang, Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E928–E935. [Google Scholar] [CrossRef] [PubMed]
- Díaz, L.; Noyola-Martínez, N.; Barrera, D.; Hernández, G.; Avila, E.; Halhali, A.; Larrea, F. Calcitriol inhibits TNF-alpha-induced inflammatory cytokines in human trophoblasts. J. Reprod. Immunol. 2009, 81, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.; Lucas, R.M.; Halliday, J.; Ponsonby, A.L. Vitamin D deficiency and pregnancy: From preconception to birth. Mol. Nutr. Food Res. 2010, 54, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Lapillonne, A. Vitamin D deficiency during pregnancy may impair maternal and fetal outcomes. Med. Hypotheses 2010, 74, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.E.; Tough, S.C.; O’Beirne, M.; Rabi, D.M. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ 2013, 346, f1169. [Google Scholar] [CrossRef] [PubMed]
- Tabesh, M.; Salehi-Abargouei, A.; Tabesh, M.; Esmaillzadeh, A. Maternal vitamin D status and risk of pre-eclampsia: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2013, 98, 3165–3173. [Google Scholar] [CrossRef] [PubMed]
- De-Regil, L.M.; Palacios, C.; Lombardo, L.K.; Peña-Rosas, J.P. Vitamin D Supplementation for Women during Pregnancy. Cochrane Database Syst. Rev. 2016, 14, CD008873. [Google Scholar] [CrossRef]
- Wei, S.Q.; Qi, H.P.; Luo, Z.C.; Fraser, W.D. Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2013, 26, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Hyppönen, E.; Cavadino, A.; Williams, D.; Fraser, A.; Vereczkey, A.; Fraser, W.D.; Bánhidy, F.; Lawlor, D.; Czeizel, A.E. Vitamin D and pre-eclampsia: Original data, systematic review and meta-analysis. Ann. Nutr. Metab. 2013, 63, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Swiglo, B.A.; Murad, M.H.; Schünemann, H.J.; Kunz, R.; Vigersky, R.A.; Guyatt, G.H.; Montori, V.M. A case for clarity, consistency, and helpfulness: State-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J. Clin. Endocrinol. Metab. 2008, 93, 666–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Shakeri, H.; Esmaillzadeh, A. Vitamin D supplementation affects serum high-sensitivity C-reactive protein, insulin resistance, and biomarkers of oxidative stress in pregnant women. J. Nutr. 2013, 143, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Hossain, N.; Kanani, F.H.; Ramzan, S.; Kausar, R.; Ayaz, S.; Khanani, R.; Pal, L. Obstetric and neonatal outcomes of maternal vitamin D supplementation: Results of an open-label, randomized controlled trial of antenatal vitamin D supplementation in Pakistani women. J. Clin. Endocrinol. Metab. 2014, 99, 2448–2455. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Samimi, M.; Siavashani, M.A.; Mazloomi, M.; Tabassi, Z.; Karamali, M.; Jamilian, M.; Esmaillzadeh, A. Calcium-vitamin D co-supplementation affects metabolic profiles, but not pregnancy outcomes, in healthy pregnant women. Int. J. Prev. Med. 2016, 7, 49. [Google Scholar] [PubMed]
- Bärebring, L.; Bullarbo, M.; Glantz, A.; Leu Agelii, M.; Jagner, A.; Ellis, J.; Hulthén, L.; Schoenmakers, I.; Augustin, H. Preeclampsia and blood pressure trajectory during pregnancy in relation to vitamin D status. PLoS ONE 2016, 11, e0152198. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Q.; Audibert, F.; Hidiroglou, N.; Sarafin, K.; Julien, P.; Wu, Y.; Luo, Z.C.; Fraser, W.D. Longitudinal vitamin D status in pregnancy and the risk of pre-eclampsia. BJOG 2012, 119, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Shand, A.W.; Nassar, N.; Von Dadelszen, P.; Innis, S.M.; Green, T.J. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. BJOG 2010, 117, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Hossain, N.; Khanani, R.; Hussain-Kanani, F.; Shah, T.; Arif, S.; Pal, L. High prevalence of vitamin D deficiency in Pakistani mothers and their newborns. Int. J. Gynaecol. Obstet. 2011, 112, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.E.; Zhang, J.Y.; Kinsella, M.; Khashan, A.S.; Kenny, L.C. Vitamin D status is associated with uteroplacental dysfunction indicated by pre-eclampsia and small-for-gestational-age birth in a large prospective pregnancy cohort in ireland with low vitamin D status. Am. J. Clin. Nutr. 2016, 104, 354. [Google Scholar] [CrossRef] [PubMed]
- Achkar, M.; Dodds, L.; Giguère, Y.; Forest, J.C.; Armson, B.A.; Woolcott, C.; Agellon, S.; Spencer, A.; Weiler, H.A. Vitamin D status in early pregnancy and risk of preeclampsia. Am. J. Obstet. Gynecol. 2015, 212. [Google Scholar] [CrossRef] [PubMed]
- Scholl, T.O.; Chen, X.; Stein, T.P. Vitamin D, secondary hyperparathyroidism, and preeclampsia. Am. J. Clin. Nutr. 2013, 98, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Q.; Audibert, F.; Luo, Z.C.; Nuyt, A.M.; Masse, B.; Julien, P.; Fraser, W.D. Maternal plasma 25-hydroxyvitamin D levels, angiogenic factors, and preeclampsia. Am. J. Obstet. Gynecol. 2013, 208. [Google Scholar] [CrossRef] [PubMed]
- Bomba-Opon, D.A.; Brawura-Biskupski-Samaha, R.; Kozlowski, S.; Kosinski, P.; Bartoszewicz, Z.; Bednarczuk, T.; Wielgos, M. First trimester maternal serum vitamin D and markers of preeclampsia. J. Mater. Fetal Neonatal Med. 2014, 27, 1078–1079. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Fernandez, I.; Prieto, B.; Rodríguez, V.; Ruano, Y.; Escudero, A.I.; Álvarez, F.V. Role of vitamin D and sFlt-1/PlGF ratio in the development of early- and late-onset preeclampsia. Clin. Chem. Lab. Med. 2015, 53, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Woodham, P.C.; Brittain, J.E.; Baker, A.M.; Long, D.L.; Haeri, S.; Camargo, C.A., Jr.; Boggess, K.A.; Stuebe, A.M. Midgestation maternal serum 25-hydroxyvitamin D level and soluble fms-like tyrosine kinase 1/placental growth factor ratio as predictors of severe preeclampsia. Hypertension 2011, 58, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Zabul, P.; Wozniak, M.; Slominski, A.T.; Preis, K.; Gorska, M.; Korozan, M.; Wieruszewski, J.; Zmijewski, M.A.; Zabul, E.; Tuckey, R.; et al. A proposed molecular mechanism of high-dose vitamin D3 supplementation in prevention and treatment of preeclampsia. Int. J. Mol. Sci. 2015, 16, 13043–13064. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lee, M.; Jeyabalan, A.; Roberts, J.M. The relationship of hypovitaminosis D and IL-6 in preeclampsia. Am. J. Obstet. Gynecol. 2014, 210, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Wetta, L.A.; Biggio, J.R.; Cliver, S.; Abramovici, A.; Barnes, S.; Tita, A.T. Is midtrimester vitamin D status associated with spontaneous preterm birth and preeclampsia? Am. J. Perinatol. 2014, 31, 541–546. [Google Scholar] [PubMed]
- Gidlöf, S.; Silva, A.T.; Gustafsson, S.; Lindqvist, P.G. Vitamin D and the risk of preeclampsia—A nested case-control study. Acta Obstet. Gynecol. Scand. 2015, 94, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Schneuer, F.J.; Roberts, C.L.; Guilbert, C.; Simpson, J.M.; Algert, C.S.; Khambalia, A.Z.; Tasevski, V.; Ashton, A.W.; Morris, J.M.; Nassar, N. Effects of maternal serum 25-hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population. Am. J. Clin. Nutr. 2014, 99, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Lechtermann, C.; Hauffa, B.P.; Herrmann, R.; Schündeln, M.M.; Gellhaus, A.; Schmidt, M.; Grasemann, C. Maternal vitamin D status in preeclampsia: Seasonal changes are not influenced by placental gene expression of vitamin D metabolizing enzymes. PLoS ONE 2014, 9, e105558. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.; Ralph, J.L.; Johnson, L.; Scheett, A.; Wright, M.L.; Taylor, J.Y.; Ohm, J.E.; Uthus, E. First trimester vitamin D status and placental epigenomics in preeclampsia among Northern Plains primiparas. Life Sci. 2015, 129, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.J.; Wagner, C.L.; Hollis, B.W.; Baatz, J.E.; Johnson, D.D. Association of maternal vitamin D and placenta growth factor with the diagnosis of early onset severe preeclampsia. Am. J. Perinatol. 2013, 30, 167–172. [Google Scholar] [PubMed]
- Pena, H.R.; de Lima, M.C.; Brandt, K.G.; de Antunes, M.M.; da Silva, G.A. Influence of preeclampsia and gestational obesity in maternal and newborn levels of vitamin D. BMC Pregnancy Childbirth 2015, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Abedi, P.; Mohaghegh, Z.; Afshary, P.; Latifi, M. The relationship of serum vitamin D with pre-eclampsia in the Iranian women. Matern. Child Nutr. 2014, 10, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Mohaghegh, Z.; Abedi, P.; Dilgouni, T.; Namvar, F.; Ruzafza, S. The relation of preeclampsia and serum level of 25-hydroxyvitamin D in mothers and their neonates: A case control study in Iran. Horm. Metab. Res. 2015, 47, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.I.; Koch, C.A.; Tamanna, S.; Rouf, S.; Shamsuddin, L. Vitamin D deficiency and the risk of preeclampsia and eclampsia in Bangladesh. Horm. Metab. Res. 2013, 45, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Simhan, H.N.; Catov, J.M.; Roberts, J.M.; Platt, R.W.; Diesel, J.C.; Klebanoff, M.A. Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology 2014, 25, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.; Gurung, P.; Aggarwal, N.; Dutta, U.; Kochhar, R. Relationship between preeclampsia and vitamin D deficiency: A case control study. Arch. Gynecol. Obstet. 2015, 291, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Haugen, M.; Brantsaeter, A.L.; Trogstad, L.; Alexander, J.; Roth, C.; Magnus, P.; Meltzer, H.M. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology 2009, 20, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.H.; Rifas-Shiman, S.L.; Huh, S.Y.; Kleinman, K.; Litonjua, A.A.; Oken, E.; Rich-Edwards, J.W.; Camargo, C.A., Jr.; Gillman, M.W. Vitamin D status and hypertensive disorders in pregnancy. Ann. Epidemiol. 2014, 24, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Ning, Y.; Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Olsen, S.F.; Gillman, M.W. Diet during pregnancy and risk of preeclampsia or gestational hypertension. Ann. Epidemiol. 2007, 17, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaikh, G.K.; Ibrahim, G.H.; Fayed, A.A.; Al-Mandeel, H. Impact of vitamin D deficiency on maternal and birth outcomes in the Saudi population: A cross-sectional study. BMC Pregnancy Childbirth 2016, 16, 119. [Google Scholar] [CrossRef] [PubMed]
- Ringrose, J.S.; PausJenssen, A.M.; Wilson, M.; Blanco, L.; Ward, H.; Wilson, T.W. Vitamin D and hypertension in pregnancy. Clin. Investig. Med. 2011, 34, E147–E154. [Google Scholar] [CrossRef]
- Kazemian, E.; Dorosty-Motlagh, A.R.; Sotoudeh, G.; Eshraghian, M.R.; Ansary, S.; Omidian, M. Nutritional status of women with gestational hypertension compared with normal pregnant women. Hypertens. Pregnancy 2013, 32, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Arain, N.; Mirza, W.A.; Aslam, M. Review-vitamin D and the prevention of preeclampsia: A systematic review. Pak. J. Pharm. Sci. 2015, 28, 1015–1021. [Google Scholar] [PubMed]
- Harvey, N.C.; Holroyd, C.; Ntani, G.; Javaid, K.; Cooper, P.; Moon, R.; Cole, Z.; Tinati, T.; Godfrey, K.; Dennison, E.; et al. Vitamin D supplementation in pregnancy: A systematic review. Health Technol. Assess. 2014, 18, 1–190. [Google Scholar] [CrossRef] [PubMed]
- Christesen, H.T.; Falkenberg, T.; Lamont, R.F.; Jorgensen, J.S. The impact of vitamin D on pregnancy: A systematic review. Acta Obstet. Gynecol. Scand. 2012, 91, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Thorne-Lyman, A.; Fawzi, W.W. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2012, 26, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Nassar, N.; Halligan, G.H.; Roberts, C.L.; Morris, J.M.; Ashton, A.W. Systematic review of first-trimester vitamin D normative levels and outcomes of pregnancy. Am. J. Obstet. Gynecol. 2011, 205, 208.e1–208.e7. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [CrossRef] [PubMed]
- Scientific Advisory Committee on Nutrition. Report on Vitamin D and Health. Available online: https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report (accessed on 24 May 2017).
- March, K.M.; Chen, N.N.; Karakochuk, C.D.; Shand, A.W.; Innis, S.M.; von Dadelszen, P.; Barr, S.I.; Lyon, M.R.; Whiting, S.J.; Weiler, H.A.; et al. Maternal vitamin D(3) supplementation at 50 mug/d protects against low serum 25-hydroxyvitamin D in infants at 8 wk of age: A randomized controlled trial of 3 doses of vitamin D beginning in gestation and continued in lactation. Am. J. Clin. Nutr. 2015, 102, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Harvey, N.C.; Bishop, N.J.; Kennedy, S.; Papageorghiou, A.T.; Schoenmakers, I.; Fraser, R.; Gandhi, S.V.; Carr, A.; D’Angelo, S.; et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): A multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016, 4, 393–402. [Google Scholar] [CrossRef]
| Author | Year | Design | n | Gestational Age | Outcome | Significant Association |
|---|---|---|---|---|---|---|
| Abedi [55] | 2014 | Case-control | 118 | Delivery | Risk of PE was higher at 25(OH)D concentrations <25 nmol/L (OR 24.04, 95% CI: 2.1, 274.8) | Yes |
| Achkar [40] | 2015 | Nested case-control | 2144 | <20 weeks | Risk of PE was higher at 25(OH)D concentrations <30 nmol/L compared to >50 nmol/L (OR 2.23, 95% CI: 1.29, 3.83) | Yes |
| Al-Shaikh [63] | 2016 | Cross-sectional | 1000 | Delivery | PIH was not seen at 25(OH)D concentrations ≥75 nmol/L but frequency of PIH was not significant | No |
| Álvarez-Fernández [44] | 2015 | Retrospective cohort | 257 | 9–12 & 20–41 weeks | Risk of late onset PE was higher at 25(OH)D concentrations <50 nmol/L (OR 4.6, 95% CI: 1.4, 15) | Yes |
| Anderson [52] | 2015 | Case-control | 48 | First trimester | 25(OH)D concentrations did not differ between preeclamptic/hypertensive and normotensive women | No |
| Bärebring [35] | 2016 | Prospective cohort | 2000 | First & third trimester | An increase in 25(OH)D of ≥30 nmol/L was associated with lower odds of PE (OR 0.22, 95% CI: 0.084, 0.581) but not PIH alone | Yes |
| Bodnar [58] | 2014 | Case-cohort | 3703 | ≤26 weeks | Risk of severe PE was lower at 25(OH)D concentrations >50 nmol/L (RR 0.65, 95% CI: 0.43, 0.98) | Yes |
| Bomba-Opon [43] | 2014 | Prospective cohort | 289 | First trimester | 25(OH)D concentrations were not related to early biomarkers of PE | No |
| Burris [61] | 2014 | Prospective cohort | 1591 | 16.4–36.9 weeks | Higher 25(OH)D concentrations were associated with a greater risk of hypertension (OR 1.32 for per 25 nmol/L increment, 95% CI: 1.01, 1.72) | Yes |
| Gidlöf [49] | 2015 | Nested case-control | 159 | 12 weeks | 25(OH)D concentrations <50 nmol/L was not associated with PE | No |
| Hossain [38] | 2011 | Prospective cohort | 75 | Delivery | Women with 25(OH)D concentrations in the lowest vs. highest tertile were more likely to develop hypertension (OR 3.38, 95% CI: 0.40, 28.37) and/or PE (OR 2.28, 95% CI: 0.35, 23.28); ≤50 nmol/L was identified as the risk threshold | Yes |
| Kiely [39] | 2016 | Prospective cohort | 1768 | 15 weeks | Risk of PE and small for gestational age combined was lower at 25(OH)D concentrations ≥75 nmol/L (OR 0.64, 95% CI: 0.43, 0.96) | Yes |
| Lechtermann [51] | 2014 | Nested case-control | 63 | Delivery | Mean ± SD summertime 25(OH)D status was lower in women with than without PE (45 ± 43 vs. 123 ± 73 nmol/L) | Yes |
| Mohaghegh [56] | 2015 | Case-control | 91 | Delivery | Mean ± SD 25(OH)D status was lower in women with than without PE (38 ± 34 vs. 58 ± 38 nmol/L) | Yes |
| Pena [54] | 2015 | Cross-sectional | 179 | Delivery | Preeclamptic mothers had a higher rate of 25(OH)D <50 nmol/L than those without PE (50% vs. 23%) | Yes |
| Ringrose [64] | 2011 | Case-control | 187 | Delivery | Hypertensive women had lower mean ± SD 25(OH)D concentrations compared with controls (62 ± 26 vs. 70 ± 29 nmol/L) in the univariate analysis but not when controlled for BMI | No |
| Robinson [53] | 2013 | Case-control | 80 | Diagnosis (28+ weeks) | Median 25(OH)D concentrations were lower in women with EOSPE than in controls (42 vs. 83 nmol/L) | Yes |
| Schneuer [50] | 2014 | Nested case-control | 5109 | 10–14 weeks | 25(OH)D status was not a predictor of PE | No |
| Scholl [41] | 2013 | Prospective cohort | 1141 | <20 weeks | Women with secondary hyperparathyroidism had a >2-fold increased risk of PE when 25(OH)D concentrations were <50 nmol/L (95% CI: 1.23-, 6.41-fold) | Yes |
| Shand [37] | 2010 | Prospective cohort | 221 | 10–20 weeks | 25(OH)D status was not related to PE | No |
| Singla [59] | 2015 | Case-control | 174 | NS (mean 35–36 weeks) | Mean ± SD 25(OH)D status was lower in women with than without PE (24 ± 12 vs. 37 ± 17 nmol/L) | Yes |
| Wei [36] | 2012 | Prospective cohort | 697 | 12–18 & 24–26 weeks | 25(OH)D concentrations <50 nmol/L at 24–26 weeks were associated with increased risk of PE (OR 3.24, 95% CI: 1.37, 7.69) | Yes |
| Wei [42] | 2013 | Prospective cohort | 697 | 12–18 & 24–26 weeks | PlGF levels were lower in women with 25(OH)D concentrations <50 nmol/L | Yes |
| Wetta [58] | 2014 | Nested case-control | 300 | 15–21 weeks | 25(OH)D status in early pregnancy was not related to PE at <37 weeks’ gestation | No |
| Woodham [45] | 2011 | Nested case-control | 164 | 15–20 weeks | For each 10 nmol/L increase in 25(OH)D, risk of severe PE decreased by 38% (95% CI: 0.51, 0.76) | Yes |
| Xu [47] | 2014 | Nested case-control | 200 | ≥24 weeks | Risk of PE quadrupled when 25(OH)D concentrations were <37.5 nmol/L (OR 4.2, 95% CI: 1.4, 12.8) | Yes |
| Ullah [57] | 2013 | Case-control | 188 | >20 weeks | Risk of eclampsia and PE was higher at 25(OH)D concentrations <75 nmol/L (OR 5.14, 95% CI: 1.98, 13.37 and OR 3.9, 95% CI: 1.18, 12.87, respectively) | Yes |
| Zabul [46] | 2015 | Nested case-control | 74 | Late gestation | 25(OH)D status did not significantly differ between preeclamptic and non-preeclamptic women | No |
| Author | Year | Design | n | Gestational Age | Outcome | Significant Association |
|---|---|---|---|---|---|---|
| Abedi [55] | 2014 | Case-control | 118 | Delivery | Vitamin D supplement use did not differ between preeclamptic and non-preeclamptic women | No |
| Anderson [52] | 2015 | Case-control | 48 | First trimester | Dietary vitamin D intake did not differ between hypertensive and normotensive women | No |
| Haugen [60] | 2009 | Prospective cohort | 23,423 | 15, 22 & 30 weeks | Women taking a vitamin D supplement (400–600 IU/day) had a reduced risk of PE compared to non-users (OR 0.73, 95% CI: 0.58, 0.92) | Yes |
| Kazemian [65] | 2013 | Case-control | 263 | 21–35 weeks | Vitamin D intake was not associated with risk of gestational hypertension | No |
| Oken [62] | 2007 | Prospective cohort | 1718 | First trimester | Women with higher vitamin D intakes had an increased risk of gestational hypertension (OR 1.11 per 100 IU, 95% CI: 1.01, 1.21) | Yes |
| Ringrose [64] | 2011 | Case-control | 187 | Delivery | Vitamin D intake (diet + supplements) did not differ between hypertensive and normotensive women | No |
| Author | Year | Design of Studies | Number of Studies | Sample Size | Meta-Analysis (Yes/No) | Outcome | Association (Yes/No) |
|---|---|---|---|---|---|---|---|
| De-Regil [28] | 2016 | Randomised controlled trials | 2 | 219 | Yes | Relative to placebo, a ‘trend’ in the risk reduction of PE was seen among gravidae consuming supplemental vitamin D 1 (8.9% vs. 15.5%; average risk ratio 0.52; 95% CI: 0.25, 1.05) | Yes |
| De-Regil [28] | 2016 | Randomised controlled trials | 3 | 1114 | Yes | Combined supplementation of vitamin D 1 plus calcium resulted in a reduced risk of PE (5% vs. 9%; average risk ratio 0.51; 95% CI: 0.32, 0.80) | Yes |
| Arain [66] | 2015 | Intervention & observational | 7 | 26,924 | No | Risk of PE may be increased at lower levels of 25(OH)D (range < 37.5–75 nmol/L), but the relationship between vitamin D and PE is conflicted by large heterogeneity between studies | Yes |
| Harvey [67] | 2014 | Intervention & observational | 12 | 642 | Yes | Meta-analysis of 4 observational studies found the risk of PE did not increase with decreased vitamin D status 1 (pooled OR 0.75, 95% CI: 0.48, 1.19) | No |
| Aghajafari [26] | 2013 | Observational | 9 | 3191 | Yes | PE was significantly associated with 25(OH)D concentrations <50 nmol/L (pooled OR 1.79, 95% CI: 1.25, 2.58) | Yes |
| Hyppönen [30] | 2013 | Randomised trials | 4 | 5982 | Yes | Women receiving supplemental vitamin D 1 had a reduced risk of PE compared to controls (pooled OR 0.66, 95% CI: 0.52, 0.83) | Yes |
| Hyppönen [30] | 2013 | Prospective observational | 6 | 6864 | Yes | Mothers with higher serum 25(OH)D status 1 had a reduced risk of PE (pooled OR 0.52, 95% CI: 0.30, 0.89) | Yes |
| Hyppönen [30] | 2013 | Prospective observational | 2 | 77,165 | Yes | Mothers receiving supplemental vitamin D 1 in early pregnancy had lower odds of developing PE (pooled OR 0.81, 95% CI: 0.75, 0.87) | Yes |
| Tabesh [27] | 2013 | Observational | 15 | 3007 | Yes | Eight studies (2485 women) were included in the meta-analysis, for which PE was significantly correlated with 25(OH)D concentrations <50 nmol/L but not <38 nmol/L | Yes |
| Wei [29] | 2013 | Observational | 8 | 2273 | Yes | Risk of PE was increased at 25(OH)D concentrations <50 nmol/L (OR 2.09, 95% CI: 1.50, 2.90) | Yes |
| Christesen [68] | 2012 | Observational | 9 | 24,704 | No | Risk of PE was inversely associated with a vitamin D intake of ≥400–600 IU/day and/or status ≥37.5–80 nmol/L in studies where the number PE cases exceeded 40 but no association was found in studies with <40 cases of PE | Yes |
| Thorne-Lyman & Fawzi [69] | 2012 | Intervention & observational | 7 | NS | Yes | Pooled analysis of 2 studies, (>25,000 women), found no difference PE risk when stratified by highest and lowest categories of total vitamin D intake 1 (OR 0.95, 95% CI: 0.86, 1.06) | No |
| Nassar [70] | 2011 | Nested case-control | 2 | 435 | No | Sufficient evidence was not available to firmly established an association between first trimester 25(OH)D status and PE risk | No |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Callaghan, K.M.; Kiely, M. Systematic Review of Vitamin D and Hypertensive Disorders of Pregnancy. Nutrients 2018, 10, 294. https://doi.org/10.3390/nu10030294
O’Callaghan KM, Kiely M. Systematic Review of Vitamin D and Hypertensive Disorders of Pregnancy. Nutrients. 2018; 10(3):294. https://doi.org/10.3390/nu10030294
Chicago/Turabian StyleO’Callaghan, Karen M., and Mairead Kiely. 2018. "Systematic Review of Vitamin D and Hypertensive Disorders of Pregnancy" Nutrients 10, no. 3: 294. https://doi.org/10.3390/nu10030294





