Determinants of Plasma 25-Hydroxyvitamin D Concentrations among Breast Cancer Survivors in Korea
Abstract
1. Introduction
2. Methods
2.1. Study Participants
2.2. Demographic and Clinical Factors
2.3. Dietary Assessment
2.4. Measurement of Plasma 25(OH)D Concentration
2.5. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Conflicts of Interest
Funding
References
- Colston, K.; Berger, U.; Coombes, R.C. Possible role for vitamin D in controlling breast cancer cell proliferation. Lancet 1989, 333, 188–191. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Vitamin D intake, blood 25 (OH) D levels, and breast cancer risk or mortality: A meta-analysis. Br. J. Cancer 2014, 110, 2772–2784. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.M.; Sandler, D.P.; Taylor, J.A.; Weinberg, C.R. Serum Vitamin D and Risk of Breast Cancer within Five Years. Environ. Health Perspect. 2017, 125, 077004. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 2004, 79, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Madsen, M.W.; Lukas, J.; Binderup, L.; Bartek, J. Inhibitory effects of 1alpha,25-dihydroxyvitamin D(3) on the G(1)-S phase-controlling machinery. Mol. Endocrinol. 2001, 15, 1370–1380. [Google Scholar] [PubMed]
- Ruggiero, M.; Pacini, S.; Aterini, S.; Fallai, C.; Ruggiero, C.; Pacini, P. Vitamin D receptor gene polymorphism is associated with metastatic breast cancer. Oncol. Res. 1998, 10, 43–46. [Google Scholar] [PubMed]
- Flanagan, L.; Packman, K.; Juba, B.; O’Neill, S.; Tenniswood, M.; Welsh, J. Efficacy of Vitamin D compounds to modulate estrogen receptor negative breast cancer growth and invasion. J. Steroid Biochem. Mol. Biol. 2003, 84, 181–192. [Google Scholar] [CrossRef]
- Abbas, S.; Linseisen, J.; Slanger, T.; Kropp, S.; Mutschelknauss, E.J.; Flesch-Janys, D.; Chang-Claude, J. Serum 25-hydroxyvitamin D and risk of post-menopausal breast cancer-results of a large case-control study. Carcinogenesis 2008, 29, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Shinki, T.; Jin, C.H.; Ohyama, Y.; Noshiro, M.; Okuda, K.; Suda, T. Regulation of messenger ribonucleic acid expression of 1 alpha,25-dihydroxyvitamin D3-24-hydroxylase in rat osteoblasts. Endocrinology 1994, 134, 1794–1799. [Google Scholar] [CrossRef] [PubMed]
- Colston, K.W.; Hansen, C.M. Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocr. Relat. Cancer 2002, 9, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, K.A.; Giovannucci, E.; Liu, Y.; Malspeis, S.; Eliassen, A.H.; Wu, K.; Holmes, M.D.; Laden, F.; Feskanich, D. Determinants of plasma 25-hydroxyvitamin D and development of prediction models in three US cohorts. Br. J. Nutr. 2012, 108, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Soininen, S.; Eloranta, A.M.; Lindi, V.; Venalainen, T.; Zaproudina, N.; Mahonen, A.; Lakka, T.A. Determinants of serum 25-hydroxyvitamin D concentration in Finnish children: The Physical Activity and Nutrition in Children (PANIC) study. Br. J. Nutr. 2016, 115, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.R.; Gerlovin, H.; Bethea, T.N.; Bertrand, K.A.; Holick, M.F.; Ruiz-Narvaez, E.N.; Wise, L.A.; Haddad, S.A.; Adams-Campbell, L.L.; Kaufman, H.W.; et al. Predicted 25-hydroxyvitamin D in relation to incidence of breast cancer in a large cohort of African American women. Breast Cancer Res. 2016, 18, 86. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nashimoto, M.; Hori, Y.; Yamamoto, M. Serum 25-hydroxyvitamin D concentrations and related dietary factors in peri-and postmenopausal Japanese women. Am. J. Clin. Nutr. 2000, 71, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nashimoto, M.; Hori, Y.; Muto, K.; Yamamoto, M. Serum 25-hydroxyvitamin D levels in active women of middle and advanced age in a rural community in Japan. Nutrition 1999, 15, 870–873. [Google Scholar] [CrossRef]
- Nakamura, K.; Nashimoto, M.; Matsuyama, S.; Yamamoto, M. Low serum concentrations of 25-hydroxyvitamin D in young adult Japanese women: A cross sectional study. Nutrition 2001, 17, 921–925. [Google Scholar] [CrossRef]
- Xu, C.; Perera, R.A.; Chan, Y.H.; Fang, V.J.; Ng, S.; Ip, D.K.; Kam, A.M.; Leung, G.M.; Peiris, J.S.; Cowling, B.J. Determinants of serum 25-hydroxyvitamin D in Hong Kong. Br. J. Nutr. 2015, 114, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Hwang, E.; Moon, H.-G.; Noh, D.-Y.; Lee, J.E. Dietary Intake Status among Korean Female Breast Cancer Survivors. Korean J. Community Nutr. 2014, 19, 163–175. [Google Scholar] [CrossRef][Green Version]
- Lynch, B.M.; Dunstan, D.W.; Healy, G.N.; Winkler, E.; Eakin, E.; Owen, N. Objectively measured physical activity and sedentary time of breast cancer survivors, and associations with adiposity: Findings from NHANES (2003–2006). Cancer Causes Control 2010, 21, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Murphy, S.; Shumay, D.; Kakai, H. Dietary changes among cancer survivors. Eur. J. Cancer Care 2001, 10, 12–20. [Google Scholar] [CrossRef]
- Velentzis, L.S.; Keshtgar, M.R.; Woodside, J.V.; Leathem, A.J.; Titcomb, A.; Perkins, K.A.; Mazurowska, M.; Anderson, V.; Wardell, K.; Cantwell, M.M. Significant changes in dietary intake and supplement use after breast cancer diagnosis in a UK multicentre study. Breast Cancer Res. Treat. 2011, 128, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Kwan, M.L.; Ergas, I.J.; Roh, J.M.; Cheng, T.-Y.D.; Hong, C.-C.; McCann, S.E.; Tang, L.; Davis, W.; Liu, S. Association of serum level of vitamin D at diagnosis with breast cancer survival: A case-cohort analysis in the pathways study. JAMA Oncol. 2017, 3, 351–357. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Almeida-Filho, B.; Vespoli, H.D.L.; Pessoa, E.C.; Machado, M.; Nahas-Neto, J.; Nahas, E.A.P. Vitamin D deficiency is associated with poor breast cancer prognostic features in postmenopausal women. J. Steroid Biochem. Mol. Boil. 2017, 174, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Dani, S.U.; Dietrich, D.; Hochstrasser, A.; Klingbiel, D.; Mark, M.T.; Riesen, W.F.; Ruhstaller, T.; Templeton, A.J.; Thürlimann, B. Vitamin D levels in Swiss breast cancer survivors. Swiss Med. Wkly. 2018, 148, w14576. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Willett, W. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Altman, D.G.; Bland, J.M. Measurement in medicine: The analysis of method comparison studies. Statistician 1983, 32, 307–317. [Google Scholar] [CrossRef]
- World Health Organization. Prevention and Management of Osteoporosis: Report of a WHO Scientific Group; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Millen, A.E.; Wactawski-Wende, J.; Pettinger, M.; Melamed, M.L.; Tylavsky, F.A.; Liu, S.; Robbins, J.; LaCroix, A.Z.; LeBoff, M.S.; Jackson, R.D. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: The Women’s Health Initiative Calcium plus Vitamin D clinical trial. Am. J. Clin. Nutr. 2010, 91, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L.; Sorensen, B.; Hollis, B.W.; Ambs, A.; Ulrich, C.M.; McTiernan, A.; Bernstein, L.; Wayne, S.; Gilliland, F.; Baumgartner, K.; et al. Vitamin D insufficiency in a multiethnic cohort of breast cancer survivors. Am. J. Clin. Nutr. 2008, 88, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Arunabh, S.; Pollack, S.; Yeh, J.; Aloia, J.F. Body fat content and 25-hydroxyvitamin D levels in healthy women. J. Clin. Endocrinol. Metab. 2003, 88, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Bolland, M.J.; Grey, A.B.; Ames, R.W.; Mason, B.H.; Horne, A.M.; Gamble, G.D.; Reid, I.R. The effects of seasonal variation of 25-hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency. Am. J. Clin. Nutr. 2007, 86, 959–964. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Weinstein, S.J.; Freedman, D.M.; Helzlsouer, K.; Flanders, W.D.; Koenig, K.; Kolonel, L.; Laden, F.; Le Marchand, L.; Purdue, M.; et al. Correlates of circulating 25-hydroxyvitamin D: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am. J. Epidemiol. 2010, 172, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, T.; Kaaks, R.; Teucher, B.; Hirche, F.; Dierkes, J.; Weikert, C.; Katzke, V.; Boeing, H.; Stangl, G.I.; Buijsse, B. Dietary, lifestyle, and genetic determinants of vitamin D status: A cross-sectional analysis from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Germany study. Eur. J. Nutr. 2014, 53, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Sohl, E.; Heymans, M.W.; de Jongh, R.T.; den Heijer, M.; Visser, M.; Merlijn, T.; Lips, P.; van Schoor, N.M. Prediction of vitamin D deficiency by simple patient characteristics. Am. J. Clin. Nutr. 2014, 99, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, E.T.; Alberts, D.S.; Foote, J.A.; Green, S.B.; Hollis, B.W.; Yu, Z.; Martínez, M.E. Vitamin D insufficiency in southern Arizona. Am. J. Clin. Nutr. 2008, 87, 608–613. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jacques, P.F.; Felson, D.T.; Tucker, K.L.; Mahnken, B.; Wilson, P.; Rosenberg, I.H.; Rush, D. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am. J. Clin. Nutr. 1997, 66, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, R.M.; Snijder, M.B.; Dekker, J.M.; Stehouwer, C.D.; Bouter, L.M.; Heine, R.J.; Lips, P. Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: The Hoorn Study. Am. J. Clin. Nutr. 2007, 85, 755–761. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Stevens, V.L.; Patel, R.; Jacobs, E.J.; Bain, E.B.; Horst, R.L.; Gapstur, S.M.; Thun, M.J.; Calle, E.E. Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: A nested case control study in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res. 2009, 11, R64. [Google Scholar] [CrossRef] [PubMed]
- Schneuer, F.J.; Roberts, C.L.; Guilbert, C.; Simpson, J.M.; Algert, C.S.; Khambalia, A.Z.; Tasevski, V.; Ashton, A.W.; Morris, J.M.; Nassar, N. Effects of maternal serum 25-hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population. Am. J. Clin. Nutr. 2014, 99, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Mithal, A.; Wahl, D.A.; Bonjour, J.-P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.M.; Cahoon, E.K.; Rajaraman, P.; Major, J.M.; Doody, M.M.; Alexander, B.H.; Hoffbeck, R.W.; Kimlin, M.G.; Graubard, B.I.; Linet, M.S. Sunlight and other determinants of circulating 25-hydroxyvitamin D levels in black and white participants in a nationwide US study. Am. J. Epidemiol. 2013, 177, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Meigs, J.B.; Pittas, A.G.; Economos, C.D.; McKeown, N.M.; Booth, S.L.; Jacques, P.F. Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham Offspring Study. Am. J. Clin. Nutr. 2010, 91, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Need, A.G.; Morris, H.A.; Horowitz, M.; Nordin, C. Effects of skin thickness, age, body fat, and sunlight on serum 25-hydroxyvitamin D. Am. J. Clin. Nutr. 1993, 58, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Bhattoa, H.; Bettembuk, P.; Ganacharya, S.; Balogh, A. Prevalence and seasonal variation of hypovitaminosis D and its relationship to bone metabolism in community dwelling postmenopausal Hungarian women. Osteoporos. Int. 2004, 15, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Brustad, M.; Alsaker, E.; Engelsen, O.; Aksnes, L.; Lund, E. Vitamin D status of middle-aged women at 65–71 N in relation to dietary intake and exposure to ultraviolet radiation. Public Health Nutr. 2004, 7, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Youl, P.H.; Janda, M.; Kimlin, M. Vitamin D and sun protection: The impact of mixed public health messages in Australia. Int. J. Cancer 2009, 124, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Linos, E.; Keiser, E.; Kanzler, M.; Sainani, K.L.; Lee, W.; Vittinghoff, E.; Chren, M.M.; Tang, J.Y. Sun protective behaviors and vitamin D levels in the US population: NHANES 2003–2006. Cancer Causes Control 2012, 23, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Liu, Y.; Rimm, E.B.; Hollis, B.W.; Fuchs, C.S.; Stampfer, M.J.; Willett, W.C. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl. Cancer Inst. 2006, 98, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Jaceldo-Siegl, K.; Fraser, G.E. Determinants of serum 25 hydroxyvitamin D levels in a nationwide cohort of blacks and non-Hispanic whites. Cancer Causes Control 2010, 21, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.; Martin, R.M.; Fraser, W.D.; Lewis, S.; Donovan, J.; Hamdy, F.; Neal, D.E.; Lane, J.A.; Metcalfe, C. Predictors of 25-hydroxyvitamin D and its association with risk factors for prostate cancer: Evidence from the prostate testing for cancer and treatment study. Cancer Causes Control 2012, 23, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.N.; Yu, K.; Horst, R.L.; Hayes, R.B.; Purdue, M.P. Long-term variation in serum 25-hydroxyvitamin D concentration among participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol. Biomark. Prev. 2010, 19, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Batai, K.; Murphy, A.B.; Shah, E.; Ruden, M.; Newsome, J.; Agate, S.; Dixon, M.A.; Chen, H.Y.; Deane, L.A.; Hollowell, C.M. Common vitamin D pathway gene variants reveal contrasting effects on serum vitamin D levels in African Americans and European Americans. Hum. Genet. 2014, 133, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
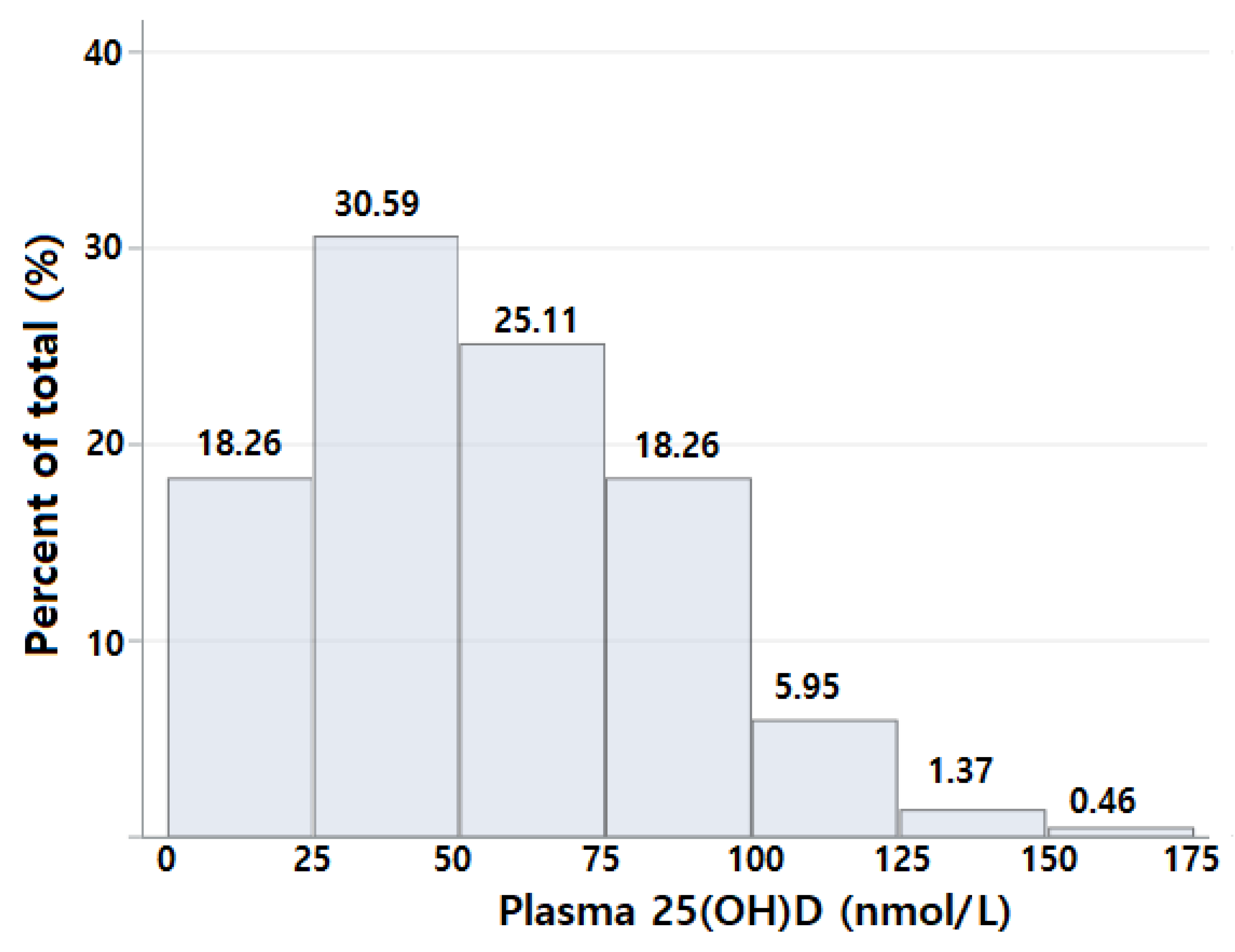
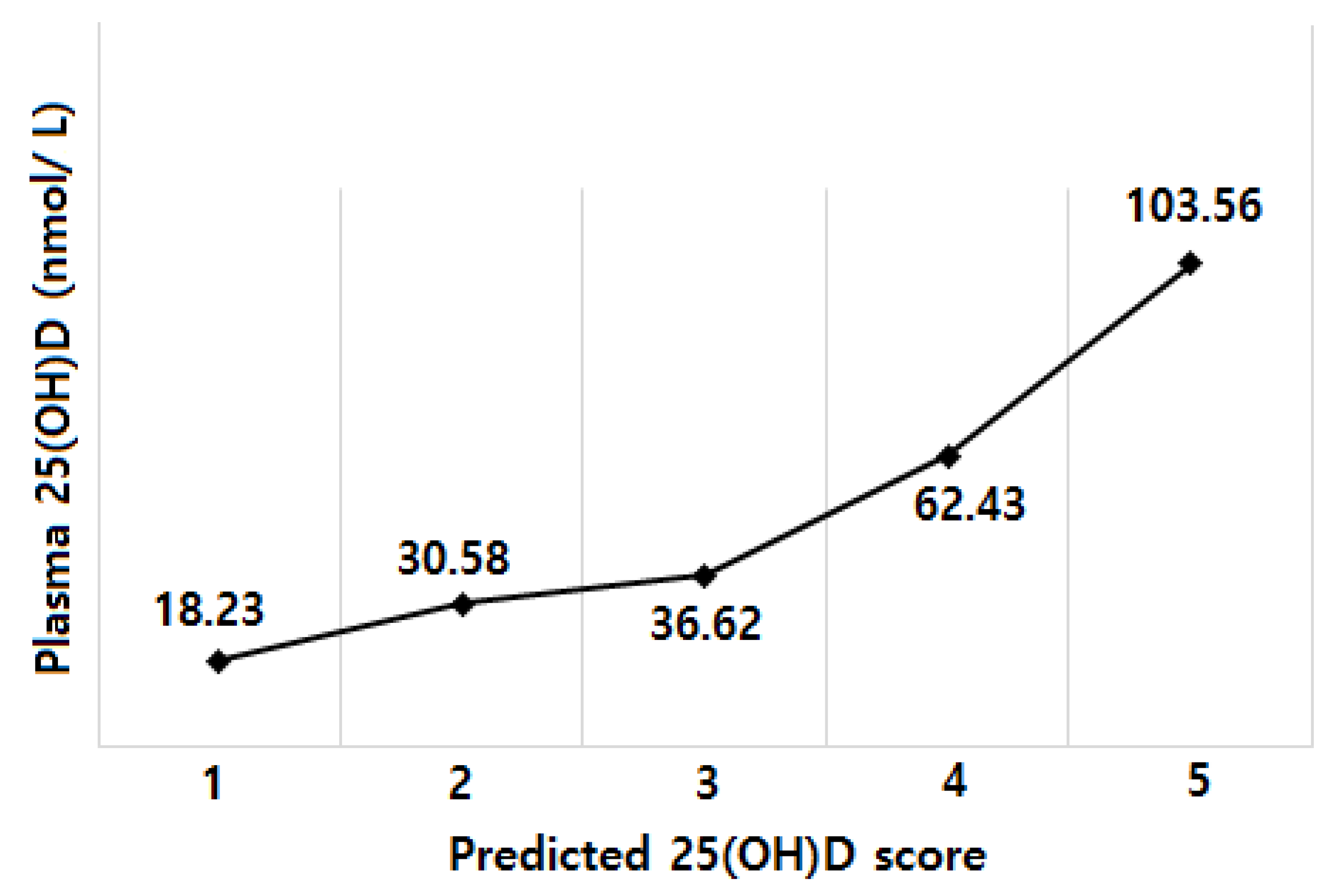
| Variables | All (n = 219) | Plasma 25(OH)D Concentrations | |
|---|---|---|---|
| <50 nmol/L (n = 107) | ≥50 nmol/L (n = 112) | ||
| Age at diagnosis (years) | 48 (43–53) | 48 (42–53) | 48 (44–52) |
| Body mass index at diagnosis (kg/m2) | 23.37 (21.63–25.74) | 24.14 (21.91–26.37) | 25.29 (21.03–25.29) |
| Energy intake (kcal/day) | 1695.25 (1410.58–2039.44) | 1684.84 (1428.35–1979.86) | 1737.09 (1379.82–2055.63) |
| Physical activity (MET-hours/week) | 49.95 (11.70–49.95) | 26.20 (10.00–47.15) | 28.41 (15.90–52.35) |
| Total vitamin D intake (µg/day) | 5.01 (0.06–15.56) | 2.21 (0.00–7.00) | 10.01 (1.76–24.05) |
| Supplementary vitamin D intake (µg/day) | 2.5 (0.00–10.00) | 0.00 (0.00–5.00) | 10.00 (0.00–25.00) |
| Use of any supplement | |||
| Yes | 156 (71.23) | 62 (57.94) | 94 (83.93) |
| No | 57 (26.03) | 42 (39.25) | 15 (13.39) |
| Time since surgery | |||
| 6 month–<1 year | 2 (0.91) | 1 (0.93) | 1 (0.89) |
| 1 year–<3 years | 144 (65.75) | 64 (59.81) | 80 (71.43) |
| 3 year–<5years | 35 (15.98) | 16 (14.95) | 19 (16.96) |
| 5 years and more | 38 (17.35) | 26 (24.30) | 12 (10.71) |
| AJCC a stage | |||
| I | 102 (46.58) | 51 (47.66) | 51 (45.54) |
| II | 86 (39.27) | 39 (36.45) | 47 (41.96) |
| III | 31 (14.16) | 17 (15.89) | 14 (12.50) |
| Season of the blood draw | |||
| Spring | 48 (21.92) | 28 (26.17) | 20 (17.86) |
| Summer | 64 (29.22) | 32 (29.91) | 32 (28.57) |
| Fall | 58 (26.48) | 24 (22.43) | 34 (30.36) |
| Winter | 49 (22.37) | 23 (21.50) | 26 (21.50) |
| Menopausal status at the diagnosis | |||
| Yes | 151 (68.95) | 72 (67.29) | 79 (70.54) |
| No | 68 (31.05) | 35 (32.71) | 33 (29.46) |
| Alcohol intake | |||
| Never | 94 (43.32) | 51 (48.11) | 43 (38.74) |
| Ever | 123 (56.68) | 55 (51.89) | 68 (61.26) |
| Smoking status | |||
| Never | 175 (79.91) | 91 (85.05) | 84 (75.00) |
| Former b | 22 (10.05) | 3 (2.80) | 19 (16.96) |
| Education level | |||
| High school or less | 149 (68.35) | 74 (69.81) | 75 (66.96) |
| College or more | 69 (31.65) | 32 (30.19) | 37 (33.04) |
| Marital status | |||
| Married or cohabitation | 172 (79.26) | 84 (80.00) | 88 (78.57) |
| Unmarried or divorced or widowed | 45 (20.74) | 21 (20.00) | 24 (21.43) |
| Parity number | |||
| None | 37 (16.89) | 14 (13.08) | 23 (20.54) |
| 1 | 128 (58.45) | 65 (60.75) | 63 (56.25) |
| 2 | 42 (19.18) | 25 (23.36) | 17 (15.18) |
| 3 and more | 10 (4.57) | 2 (1.87) | 8 (1.87) |
| Centre | |||
| 1 | 79 (36.07) | 17 (15.89) | 62 (55.36) |
| 2 | 70 (31.96) | 41 (38.32) | 29 (25.89) |
| 3 | 70 (31.96) | 49 (45.79) | 21 (18.75) |
| Determinants a | Difference in 25(OH)D (nmol/L; β) | p Value |
|---|---|---|
| Age at the diagnosis (per 1 year) | −0.001 | 0.83 |
| Time since diagnosis (per 1 month) | −0.005 | 0.01 |
| Supplementary vitamin D intake (per 10 ug/day) | 0.06 | 0.10 |
| Season of the blood draw | ||
| Spring (referent) | 0 | |
| Summer | 0.35 | 0.02 |
| Fall | 0.32 | 0.005 |
| Winter | 0.26 | 0.03 |
| Smoking status | ||
| Never (referent) | 0 | |
| Former b | 0.28 | 0.03 |
| Use of any supplement | ||
| Yes (referent) | 0 | |
| No | −0.35 | 0.0007 |
| Parity number | ||
| None | −0.10 | 0.63 |
| 1 (referent) | 0 | |
| 2 | −0.04 | 0.70 |
| 3 or more | −0.30 | 0.02 |
| Centre | ||
| 1 (referent) | 0 | |
| 2 | −0.30 | 0.02 |
| 3 | −0.36 | 0.002 |
| Determinants a | Number of Case/Total | OR (95% CI) |
|---|---|---|
| Age at the diagnosis (per 1 year) | 107/219 | 0.99 (0.94, 1.04) |
| Supplementary vitamin D intake (per 10 µg/day) | 107/219 | 0.44 (0.29, 0.66) |
| Body mass index at the diagnosis (per 1 kg/m2) | 107/219 | 1.16 (1.03, 1.31) |
| Time since diagnosis (per 1 month) | 107/219 | 1.02 (1.01, 1.04) |
| Smoking status | ||
| Never (referent) | 91/175 | 1.00 |
| Former b | 3/22 | 0.09 (0.02, 0.38) |
| Use of any supplement | ||
| Yes (referent) | 62/156 | 1.00 |
| No | 42/57 | 2.88 (1.24, 6.65) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, W.-K.; Kim, Z.; Youn, H.J.; Cho, J.; Lee, J.E. Determinants of Plasma 25-Hydroxyvitamin D Concentrations among Breast Cancer Survivors in Korea. Nutrients 2018, 10, 380. https://doi.org/10.3390/nu10030380
Shin W-K, Kim Z, Youn HJ, Cho J, Lee JE. Determinants of Plasma 25-Hydroxyvitamin D Concentrations among Breast Cancer Survivors in Korea. Nutrients. 2018; 10(3):380. https://doi.org/10.3390/nu10030380
Chicago/Turabian StyleShin, Woo-Kyoung, Zisun Kim, Hyun Jo Youn, Jihyoung Cho, and Jung Eun Lee. 2018. "Determinants of Plasma 25-Hydroxyvitamin D Concentrations among Breast Cancer Survivors in Korea" Nutrients 10, no. 3: 380. https://doi.org/10.3390/nu10030380
APA StyleShin, W.-K., Kim, Z., Youn, H. J., Cho, J., & Lee, J. E. (2018). Determinants of Plasma 25-Hydroxyvitamin D Concentrations among Breast Cancer Survivors in Korea. Nutrients, 10(3), 380. https://doi.org/10.3390/nu10030380





