Effects of Dietary Fatty Acids in Pancreatic Beta Cell Metabolism, Implications in Homeostasis
Abstract
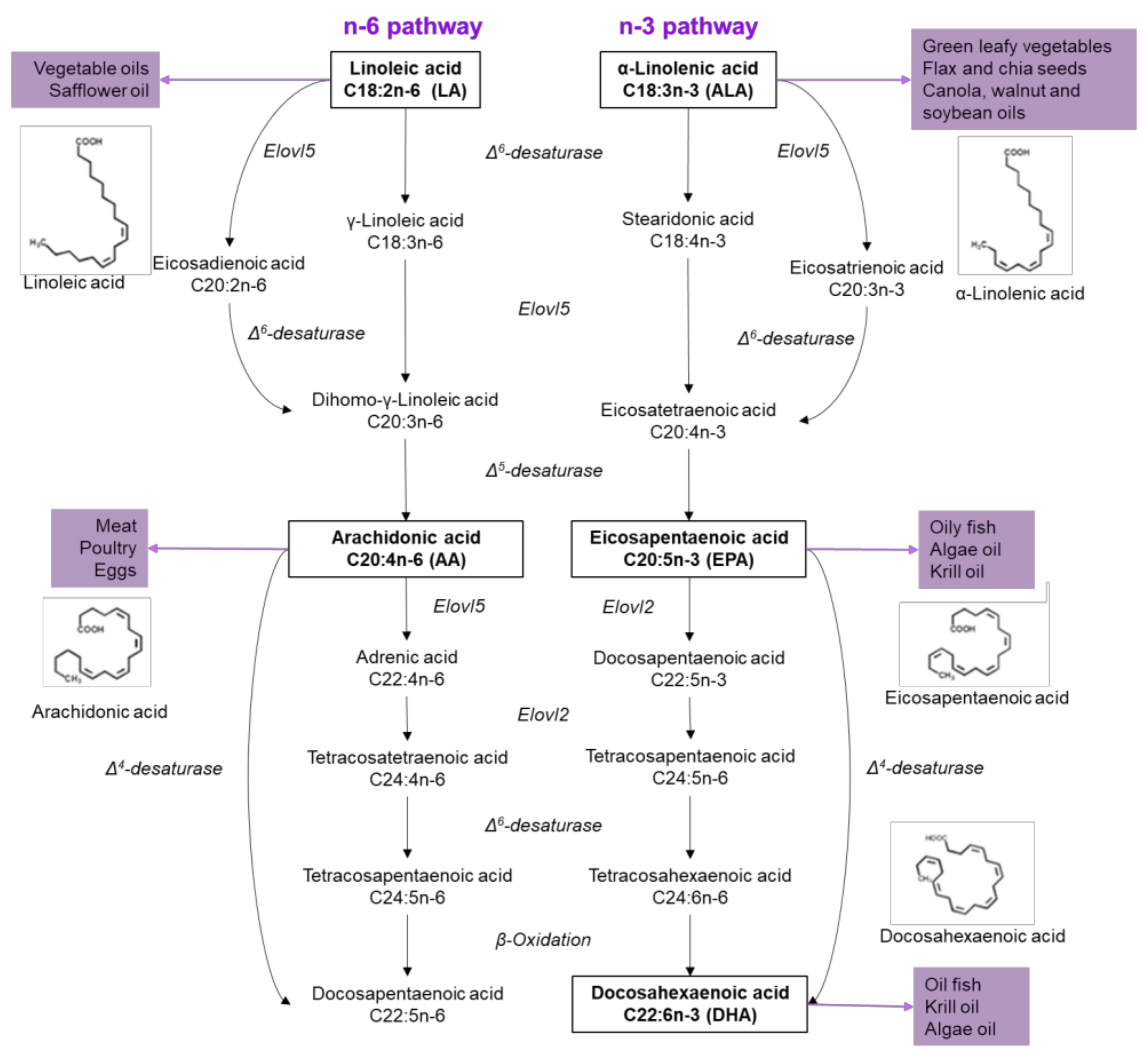
:1. Introduction
2. Fatty Acids Properties
3. Fatty Acid Metabolism in β-Cells
4. Effects of Saturated Fatty Acids on β-Cells
5. Effects of Unsaturated Fatty Acids on β-Cells
6. Final Considerations
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Poudyal, H.; Panchal, S.K.; Ward, L.C.; Brown, L. Effects of ALA, EPA and DHA in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J. Nutr. Biochem. 2013, 24, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.M.; Ma, D.W. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 2009, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Top 10 Causes of Death. Available online: www.who.int/mediacentre/facsheet/fs310/en/ (accessed on 30 November 2017).
- IDF. Diabetes Atlas. Available online: www.diabetesatlas.org (accessed on 4 December 2017).
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar]
- Konner, A.C.; Janoschek, R.; Plum, L.; Jordan, S.D.; Rother, E.; Ma, X.; Xu, C.; Enriori, P.; Hampel, B.; Barsh, G.S.; et al. Insulin action in agrp-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007, 5, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Rorsman, P.; Renstrom, E. Insulin granule dynamics in pancreatic beta cells. Diabetologia 2003, 46, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Scheuner, D.; Kaufman, R.J. The unfolded protein response: A pathway that links insulin demand with beta-cell failure and diabetes. Endocr. Rev. 2008, 29, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Schuit, F.C.; In’t Veld, P.A.; Pipeleers, D.G. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc. Natl. Acad. Sci. USA 1988, 85, 3865–3869. [Google Scholar] [CrossRef] [PubMed]
- Bratanova-Tochkova, T.K.; Cheng, H.; Daniel, S.; Gunawardana, S.; Liu, Y.J.; Mulvaney-Musa, J.; Schermerhorn, T.; Straub, S.G.; Yajima, H.; Sharp, G.W. Triggering and augmentation mechanisms, granule pools, and biphasic insulin secretion. Diabetes 2002, 51, S83–S90. [Google Scholar] [CrossRef] [PubMed]
- Remedi, M.S.; Emfinger, C. Pancreatic beta-cell identity in diabetes. Diabetes Obes. Metab. 2016, 18, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Capiz, A.; Martínez-Navarro, I.; Guevara-Olaya, L.; Díaz-Molina, R.; Mas-Oliva, J.; Rivero, I.; García-González, V. Modulation of amyloidogenesis controlled by the c-terminal domain of amylin, shows new functions on hepatocyte cholesterol metabolism. Unpublished work, 2018.
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.G.; Gromada, J.; Urano, F. Endoplasmic reticulum stress and pancreatic beta-cell death. Trends Endocrinol. Metab. 2011, 22, 266–274. [Google Scholar] [PubMed]
- Diaz-Villanueva, J.F.; Diaz-Molina, R.; Garcia-Gonzalez, V. Protein folding and mechanisms of proteostasis. Int. J. Mol. Sci. 2015, 16, 17193–17230. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Zeng, H.; Zhang, Y.; Jungries, R.; Chung, P.; Plesken, H.; Sabatini, D.D.; Ron, D. Diabetes mellitus and exocrine pancreatic dysfunction in perk/mice reveals a role for translational control in secretory cell survival. Mol. Cell 2001, 7, 1153–1163. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, J.; Tong, Y.; Hu, X.; Lv, Q.; Tong, N. Protective role of ppardelta in lipoapoptosis of pancreatic beta cells. Lipids 2016, 51, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Murru, E.; Lisai, S.; Sirigu, A.; Piras, A.; Collu, M.; Batetta, B.; Gambelli, L.; Banni, S. Dietary triacylglycerols with palmitic acid in the sn-2 position modulate levels of N-acylethanolamides in rat tissues. PLoS ONE 2015, 10, e0120424. [Google Scholar] [CrossRef] [PubMed]
- Sette, S.; Le Donne, C.; Piccinelli, R.; Arcella, D.; Turrini, A.; Leclercq, C.; Group, I.-S.S. The third Italian national food consumption survey, Inran-Scai 2005-06—Part 1: Nutrient intakes in Italy. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic acid: Physiological role, metabolism and nutritional implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Zhao, L.; Hwang, D.H. Modulation of pattern recognition receptor-mediated inflammation and risk of chronic diseases by dietary fatty acids. Nutr. Rev. 2010, 68, 38–61. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Panchal, S.K.; Diwan, V.; Brown, L. Omega-3 fatty acids and metabolic syndrome: Effects and emerging mechanisms of action. Prog. Lipid Res. 2011, 50, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Parker-Barnes, J.M.; Das, T.; Bobik, E.; Leonard, A.E.; Thurmond, J.M.; Chaung, L.T.; Huang, Y.S.; Mukerji, P. Identification and characterization of an enzyme involved in the elongation of n-6 and n-3 polyunsaturated fatty acids. Proc. Natl. Acad. Sci. USA 2000, 97, 8284–8289. [Google Scholar] [CrossRef] [PubMed]
- Portolesi, R.; Powell, B.C.; Gibson, R.A. Competition between 24:5n-3 and ALA for delta 6 desaturase may limit the accumulation of DHA in hepg2 cell membranes. J. Lipid Res. 2007, 48, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Sarda, P.; Nessmann, C.; Boulot, P.; Leger, C.L.; Descomps, B. Delta6- and delta5-desaturase activities in the human fetal liver: Kinetic aspects. J. Lipid Res. 1998, 39, 1825–1832. [Google Scholar] [PubMed]
- Vermunt, S.H.; Mensink, R.P.; Simonis, M.M.; Hornstra, G. Effects of dietary alpha-linolenic acid on the conversion and oxidation of 13c-alpha-linolenic acid. Lipids 2000, 35, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Menard, C.R.; Likhodii, S.S.; Brenna, J.T.; Crawford, M.A. Carbon recycling into de novo lipogenesis is a major pathway in neonatal metabolism of linoleate and alpha-linolenate. Prostaglandins Leukot. Essent. Fatty Acids 1999, 60, 387–392. [Google Scholar] [CrossRef]
- Emmison, N.; Gallagher, P.A.; Coleman, R.A. Linoleic and linolenic acids are selectively secreted in triacylglycerol by hepatocytes from neonatal rats. Am. J. Physiol. 1995, 269, R80–R86. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Ryan, M.A.; Nadeau, C.R.; Bazinet, R.P.; Musa-Veloso, K.; McCloy, U. Why is carbon from some polyunsaturates extensively recycled into lipid synthesis? Lipids 2003, 38, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Edmond, J. Ketone bodies as precursors of sterols and fatty acids in the developing rat. J. Biol. Chem. 1974, 249, 72–80. [Google Scholar] [PubMed]
- Malaisse, W.J.; Best, L.; Kawazu, S.; Malaisse-Lagae, F.; Sener, A. The stimulus-secretion coupling of glucose-induced insulin release: Fuel metabolism in islets deprived of exogenous nutrient. Arch. Biochem. Biophys. 1983, 224, 102–110. [Google Scholar] [CrossRef]
- Ashcroft, F.M.; Rohm, M.; Clark, A.; Brereton, M.F. Is type 2 diabetes a glycogen storage disease of pancreatic beta cells? Cell Metab. 2017, 26, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Yaney, G.C.; Corkey, B.E. Fatty acid metabolism and insulin secretion in pancreatic beta cells. Diabetologia 2003, 46, 1297–1312. [Google Scholar] [CrossRef] [PubMed]
- Fatehi-Hassanabad, Z.; Chan, C.B. Transcriptional regulation of lipid metabolism by fatty acids: A key determinant of pancreatic beta-cell function. Nutr. Metab. 2005, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Deeney, J.T.; Gromada, J.; Hoy, M.; Olsen, H.L.; Rhodes, C.J.; Prentki, M.; Berggren, P.O.; Corkey, B.E. Acute stimulation with long chain acyl-coa enhances exocytosis in insulin-secreting cells (hit t-15 and nmri beta-cells). J. Biol. Chem. 2000, 275, 9363–9368. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.J.; Madiraju, M.S.; Delghingaro-Augusto, V.; Peyot, M.L.; Prentki, M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes 2006, 55, S16–S23. [Google Scholar] [CrossRef] [PubMed]
- Cimen, I.; Kocaturk, B.; Koyuncu, S.; Tufanli, O.; Onat, U.I.; Yildirim, A.D.; Apaydin, O.; Demirsoy, S.; Aykut, Z.G.; Nguyen, U.T.; et al. Prevention of atherosclerosis by bioactive palmitoleate through suppression of organelle stress and inflammasome activation. Sci. Transl. Med. 2016, 8, 358ra126. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A.; Civelek, V.N.; Kamp, F.; Tornheim, K.; Corkey, B.E. Changes in internal pH caused by movement of fatty acids into and out of clonal pancreatic beta-cells (hit). J. Biol. Chem. 1994, 269, 20852–20856. [Google Scholar] [PubMed]
- Noushmehr, H.; D’Amico, E.; Farilla, L.; Hui, H.; Wawrowsky, K.A.; Mlynarski, W.; Doria, A.; Abumrad, N.A.; Perfetti, R. Fatty acid translocase (fat/cd36) is localized on insulin-containing granules in human pancreatic beta-cells and mediates fatty acid effects on insulin secretion. Diabetes 2005, 54, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, R.; Yang, T.; Xu, N.; Chen, J.; Gao, Y.; Stetler, R.A. Fatty acid transporting proteins: Roles in brain development, aging, and stroke. Prostaglandins Leukot. Essent. Fatty Acids 2017. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-function of cd36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.; Guy, E.; Coburn, C.; Knapp, F.F., Jr.; Beets, A.L.; Abumrad, N.A.; Silverstein, R.L. The impact of overexpression and deficiency of fatty acid translocase (fat)/cd36. Mol. Cell Biochem. 2002, 239, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Wallin, T.; Ma, Z.; Ogata, H.; Jorgensen, I.H.; Iezzi, M.; Wang, H.; Wollheim, C.B.; Bjorklund, A. Facilitation of fatty acid uptake by cd36 in insulin-producing cells reduces fatty-acid-induced insulin secretion and glucose regulation of fatty acid oxidation. Biochim. Biophys. Acta 2010, 1801, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, C.P.; Tadayyon, M.; Andrews, J.L.; Benson, W.G.; Chambers, J.K.; Eilert, M.M.; Ellis, C.; Elshourbagy, N.A.; Goetz, A.S.; Minnick, D.T.; et al. The orphan g protein-coupled receptor gpr40 is activated by medium and long chain fatty acids. J. Biol. Chem. 2003, 278, 11303–11311. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Kawamata, Y.; Harada, M.; Kobayashi, M.; Fujii, R.; Fukusumi, S.; Ogi, K.; Hosoya, M.; Tanaka, Y.; Uejima, H.; et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through gpr40. Nature 2003, 422, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Yabuki, C.; Maruyama, M.; Abiru, A.; Komatsu, H.; Negoro, N.; Tsujihata, Y.; Takeuchi, K.; Habata, Y.; Mori, M. Fasiglifam (tak-875) has dual potentiating mechanisms via galphaq-gpr40/ffar1 signaling branches on glucose-dependent insulin secretion. Pharmacol. Res. Perspect. 2016, 4, e00237. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, H.; Smith, D.M.; Bergsten, P.; Sargsyan, E. Ffar1 is involved in both the acute and chronic effects of palmitate on insulin secretion. Endocrinology 2013, 154, 4078–4088. [Google Scholar] [CrossRef] [PubMed]
- Steneberg, P.; Rubins, N.; Bartoov-Shifman, R.; Walker, M.D.; Edlund, H. The FFA receptor gpr40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 2005, 1, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.H. Lipotoxicity in the pathogenesis of obesity-dependent niddm. Genetic and clinical implications. Diabetes 1995, 44, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Eitel, K.; Staiger, H.; Brendel, M.D.; Brandhorst, D.; Bretzel, R.G.; Haring, H.U.; Kellerer, M. Different role of saturated and unsaturated fatty acids in beta-cell apoptosis. Biochem. Biophys. Res. Commun. 2002, 299, 853–856. [Google Scholar] [CrossRef]
- Bollheimer, L.C.; Skelly, R.H.; Chester, M.W.; McGarry, J.D.; Rhodes, C.J. Chronic exposure to free fatty acid reduces pancreatic beta cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J. Clin. Investig. 1998, 101, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Brun, T.; Assimacopoulos-Jeannet, F.; Corkey, B.E.; Prentki, M. Long-chain fatty acids inhibit acetyl-coa carboxylase gene expression in the pancreatic beta-cell line ins-1. Diabetes 1997, 46, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Gremlich, S.; Bonny, C.; Waeber, G.; Thorens, B. Fatty acids decrease idx-1 expression in rat pancreatic islets and reduce glut2, glucokinase, insulin, and somatostatin levels. J. Biol. Chem. 1997, 272, 30261–30269. [Google Scholar] [CrossRef] [PubMed]
- Assimacopoulos-Jeannet, F.; Thumelin, S.; Roche, E.; Esser, V.; McGarry, J.D.; Prentki, M. Fatty acids rapidly induce the carnitine palmitoyltransferase i gene in the pancreatic beta-cell line ins-1. J. Biol. Chem. 1997, 272, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Habener, J.F.; Stoffers, D.A. A newly discovered role of transcription factors involved in pancreas development and the pathogenesis of diabetes mellitus. Proc. Assoc. Am. Phys. 1998, 110, 12–21. [Google Scholar] [PubMed]
- Stoffers, D.A.; Thomas, M.K.; Habener, J.F. Homeodomain protein idx-1: A master regulator of pancreas development and insulin gene expression. Trends Endocrinol. Metab. 1997, 8, 145–151. [Google Scholar] [CrossRef]
- Brissova, M.; Shiota, M.; Nicholson, W.E.; Gannon, M.; Knobel, S.M.; Piston, D.W.; Wright, C.V.; Powers, A.C. Reduction in pancreatic transcription factor pdx-1 impairs glucose-stimulated insulin secretion. J. Biol. Chem. 2002, 277, 11225–11232. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, W.M.; Frayling, T.M.; Ellard, S.; Evans, J.C.; Allen, L.I.; Bulman, M.P.; Ayres, S.; Shepherd, M.; Clark, P.; Millward, A.; et al. Missense mutations in the insulin promoter factor-1 gene predispose to type 2 diabetes. J. Clin. Investig. 1999, 104, R33–R39. [Google Scholar] [CrossRef] [PubMed]
- Maedler, K.; Spinas, G.A.; Dyntar, D.; Moritz, W.; Kaiser, N.; Donath, M.Y. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes 2001, 50, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Reed, J.C. Mitochondrial control of cell death. Nat. Med. 2000, 6, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Woldegiorgis, G.; Yousufzai, S.Y.; Shrago, E. Studies on the interaction of palmitoyl coenzyme a with the adenine nucleotide translocase. J. Biol. Chem. 1982, 257, 14783–14787. [Google Scholar] [PubMed]
- Moffitt, J.H.; Fielding, B.A.; Evershed, R.; Berstan, R.; Currie, J.M.; Clark, A. Adverse physicochemical properties of tripalmitin in beta cells lead to morphological changes and lipotoxicity in vitro. Diabetologia 2005, 48, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Preston, A.M.; Gurisik, E.; Bartley, C.; Laybutt, D.R.; Biden, T.J. Reduced endoplasmic reticulum (er)-to-golgi protein trafficking contributes to er stress in lipotoxic mouse beta cells by promoting protein overload. Diabetologia 2009, 52, 2369–2373. [Google Scholar] [CrossRef] [PubMed]
- Volmer, R.; van der Ploeg, K.; Ron, D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc. Natl. Acad. Sci. USA 2013, 110, 4628–4633. [Google Scholar] [CrossRef] [PubMed]
- Karaskov, E.; Scott, C.; Zhang, L.; Teodoro, T.; Ravazzola, M.; Volchuk, A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to ins-1 pancreatic beta-cell apoptosis. Endocrinology 2006, 147, 3398–3407. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of bip and er stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Scheuner, D.; Vander Mierde, D.; Song, B.; Flamez, D.; Creemers, J.W.; Tsukamoto, K.; Ribick, M.; Schuit, F.C.; Kaufman, R.J. Control of mrna translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat. Med. 2005, 11, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Cunha, D.A.; Hekerman, P.; Ladriere, L.; Bazarra-Castro, A.; Ortis, F.; Wakeham, M.C.; Moore, F.; Rasschaert, J.; Cardozo, A.K.; Bellomo, E.; et al. Initiation and execution of lipotoxic er stress in pancreatic beta-cells. J. Cell Sci. 2008, 121, 2308–2318. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jawahar, S.; Qian, Y.; Duong, Q.; Chan, G.; Parker, A.; Meyer, J.M.; Moore, K.J.; Chayen, S.; Gross, D.J.; et al. Missense polymorphism in the human carboxypeptidase e gene alters enzymatic activity. Hum. Mutat. 2001, 18, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Naggert, J.K.; Fricker, L.D.; Varlamov, O.; Nishina, P.M.; Rouille, Y.; Steiner, D.F.; Carroll, R.J.; Paigen, B.J.; Leiter, E.H. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase e mutation which reduces enzyme activity. Nat. Genet. 1995, 10, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, K.D.; Alejandro, E.U.; Luciani, D.S.; Kalynyak, T.B.; Hu, X.; Li, H.; Lin, Y.; Townsend, R.R.; Polonsky, K.S.; Johnson, J.D. Carboxypeptidase e mediates palmitate-induced beta-cell er stress and apoptosis. Proc. Natl. Acad. Sci. USA 2008, 105, 8452–8457. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.D. Proteomic identification of carboxypeptidase e connects lipid-induced beta-cell apoptosis and dysfunction in type 2 diabetes. Cell Cycle 2009, 8, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Alsters, S.I.; Goldstone, A.P.; Buxton, J.L.; Zekavati, A.; Sosinsky, A.; Yiorkas, A.M.; Holder, S.; Klaber, R.E.; Bridges, N.; van Haelst, M.M.; et al. Truncating homozygous mutation of carboxypeptidase e (CPE) in a morbidly obese female with type 2 diabetes mellitus, intellectual disability and hypogonadotrophic hypogonadism. PLoS ONE 2015, 10, e0131417. [Google Scholar] [CrossRef] [PubMed]
- Hagman, D.K.; Hays, L.B.; Parazzoli, S.D.; Poitout, V. Palmitate inhibits insulin gene expression by altering pdx-1 nuclear localization and reducing mafa expression in isolated rat islets of langerhans. J. Biol. Chem. 2005, 280, 32413–32418. [Google Scholar] [CrossRef] [PubMed]
- Marshak, S.; Benshushan, E.; Shoshkes, M.; Havin, L.; Cerasi, E.; Melloul, D. Functional conservation of regulatory elements in the pdx-1 gene: Pdx-1 and hepatocyte nuclear factor 3beta transcription factors mediate beta-cell-specific expression. Mol. Cell Biol. 2000, 20, 7583–7590. [Google Scholar] [CrossRef] [PubMed]
- Wendland, E.; Farmer, A.; Glasziou, P.; Neil, A. Effect of alpha linolenic acid on cardiovascular risk markers: A systematic review. Heart 2006, 92, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Djousse, L.; Hunt, S.C.; Tang, W.; Eckfeldt, J.H.; Province, M.A.; Ellison, R.C. Dietary linolenic acid and fasting glucose and insulin: The national heart, lung, and blood institute family heart study. Obesity 2006, 14, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, T.; Yatsuya, H.; Toyoshima, H.; Sasaki, S.; Li, Y.; Otsuka, R.; Wada, K.; Hotta, Y.; Mitsuhashi, H.; Matsushita, K.; et al. Higher dietary intake of alpha-linolenic acid is associated with lower insulin resistance in middle-aged Japanese. Prev. Med. 2010, 50, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Sarbolouki, S.; Javanbakht, M.H.; Derakhshanian, H.; Hosseinzadeh, P.; Zareei, M.; Hashemi, S.B.; Dorosty, A.R.; Eshraghian, M.R.; Djalali, M. Eicosapentaenoic acid improves insulin sensitivity and blood sugar in overweight type 2 diabetes mellitus patients: A double-blind randomised clinical trial. Singap. Med. J. 2013, 54, 387–390. [Google Scholar] [CrossRef]
- Liu, X.; Xue, Y.; Liu, C.; Lou, Q.; Wang, J.; Yanagita, T.; Xue, C.; Wang, Y. Eicosapentaenoic acid-enriched phospholipid ameliorates insulin resistance and lipid metabolism in diet-induced-obese mice. Lipids Health Dis. 2013, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Murakawa, Y.; Yokoyama, J.; Tajima, N.; Ikeda, Y.; Nobukata, H.; Ishikawa, T.; Shibutani, Y. Effect of highly purified eicosapentaenoic acid ethyl ester on insulin resistance and hypertension in dahl salt-sensitive rats. Metabolism 1999, 48, 1089–1095. [Google Scholar] [CrossRef]
- Shimura, T.; Miura, T.; Usami, M.; Ishihara, E.; Tanigawa, K.; Ishida, H.; Seino, Y. Docosahexanoic acid (DHA) improved glucose and lipid metabolism in KK-ay mice with genetic non-insulin-dependent diabetes mellitus (NIDDM). Biol. Pharm. Bull. 1997, 20, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Lupi, R.; Dotta, F.; Marselli, L.; Del Guerra, S.; Masini, M.; Santangelo, C.; Patane, G.; Boggi, U.; Piro, S.; Anello, M.; et al. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: Evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and bcl-2 regulated. Diabetes 2002, 51, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.G.; Dhayal, S. Unsaturated fatty acids as cytoprotective agents in the pancreatic beta-cell. Prostaglandins Leukot. Essent. Fatty Acids 2010, 82, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, R.G.; Huang, S.; Namgaladze, D.; Jandali, O.; Shao, T.; Sama, S.; Brune, B.; Hwang, D.H. Docosahexaenoic acid and palmitic acid reciprocally modulate monocyte activation in part through endoplasmic reticulum stress. J. Nutr. Biochem. 2016, 32, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Amyot, J.; Semache, M.; Ferdaoussi, M.; Fontes, G.; Poitout, V. Lipopolysaccharides impair insulin gene expression in isolated islets of langerhans via toll-like receptor-4 and nf-kappab signalling. PLoS ONE 2012, 7, e36200. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; Qiu, Y.; Mu, Y.; Zhang, X.J.; Liu, L.; Hou, X.H.; Zhang, L.; Xu, X.N.; Ji, A.L.; Cao, R.; et al. A high ratio of dietary n-3/n-6 polyunsaturated fatty acids improves obesity-linked inflammation and insulin resistance through suppressing activation of tlr4 in SD rats. Nutr. Res. 2013, 33, 849–858. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Montaño, P.; García-González, V. Effects of Dietary Fatty Acids in Pancreatic Beta Cell Metabolism, Implications in Homeostasis. Nutrients 2018, 10, 393. https://doi.org/10.3390/nu10040393
Acosta-Montaño P, García-González V. Effects of Dietary Fatty Acids in Pancreatic Beta Cell Metabolism, Implications in Homeostasis. Nutrients. 2018; 10(4):393. https://doi.org/10.3390/nu10040393
Chicago/Turabian StyleAcosta-Montaño, Paloma, and Víctor García-González. 2018. "Effects of Dietary Fatty Acids in Pancreatic Beta Cell Metabolism, Implications in Homeostasis" Nutrients 10, no. 4: 393. https://doi.org/10.3390/nu10040393




