Influence of Blue Mussel (Mytilus edulis) Intake on Disease Activity in Female Patients with Rheumatoid Arthritis: The MIRA Randomized Cross-Over Dietary Intervention
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
2.3. Dietary Intervention
2.4. Clinical Response Variables and Participant Monitoring
2.5. Statistical Methods
3. Results
3.1. Influence of Intervention on Disease Activity
3.2. Influence of Intervention on Quality of Life and Disability
3.3. Influence of Intervention on Blood Lipids, Hemoglobin, White Blood Cell and Platelet Counts and Body Weight
3.4. Habitual Dietary Intake of Participants
3.5. Compliance, Drop-Out and Adverse Events
4. Discussion
4.1. Study Limitations
4.2. Study Strengths
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef]
- Altawil, R.; Saevarsdottir, S.; Wedrén, S.; Alfredsson, L.; Klareskog, L.; Lampa, J. Remaining pain in early rheumatoid arthritis patients treated with methotrexate. Arthritis Care Res. 2016, 68, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.L.; Forslind, K.; Hafstrom, I. Comparing five year out-come in two cohorts of patients with early rheumatoid arthritis—A barfot study. Open Rheumatol. J. 2015, 9, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Druce, K.L.; Bhattacharya, Y.; Jones, G.T.; Macfarlane, G.J.; Basu, N. Most patients who reach disease remission following anti-TNF therapy continue to report fatigue: Results from the British society for rheumatology biologics register for rheumatoid arthritis. Rheumatology 2016, 55, 1786–1790. [Google Scholar] [CrossRef] [PubMed]
- Feldthusen, C.; Grimby-Ekman, A.; Forsblad-d’Elia, H.; Jacobsson, L.; Mannerkorpi, K. Explanatory factors and predictors of fatigue in persons with rheumatoid arthritis: A longitudinal study. J. Rehabil. Med. 2016, 48, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Cramp, F.; Hewlett, S.; Almeida, C.; Kirwan, J.R.; Choy, E.H.; Chalder, T.; Pollock, J.; Christensen, R. Non-pharmacological interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst. Rev. 2013, CD008322. [Google Scholar] [CrossRef] [PubMed]
- Hagen, K.B.; Byfuglien, M.G.; Falzon, L.; Olsen, S.U.; Smedslund, G. Dietary interventions for rheumatoid arthritis. Cochrane Database Syst. Rev. 2009, CD006400. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Diet, Nutrition and the Prevention of Chronic Diseases; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Albers, R.; Bourdet-Sicard, R.; Braun, D.; Calder, P.C.; Herz, U.; Lambert, C.; Lenoir-Wijnkoop, I.; Meheust, A.; Ouwehand, A.; Phothirath, P.; et al. Monitoring immune modulation by nutrition in the general population: Identifying and substantiating effects on human health. Br. J. Nutr. 2013, 110, S1–S30. [Google Scholar] [CrossRef] [PubMed]
- Canter, P.H.; Wider, B.; Ernst, E. The antioxidant vitamins A, C, E and selenium in the treatment of arthritis: A systematic review of randomized clinical trials. Rheumatology 2007, 46, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Han, F.; Lin, X.; Tang, C.; Ye, J.; Cai, X. The association between serum selenium levels with rheumatoid arthritis. Biol. Trace Elem. Res. 2016, 172, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Calder, P.C. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br. J. Nutr. 2012, 107 (Suppl. 2), S171–S184. [Google Scholar] [CrossRef] [PubMed]
- Gioxari, A.; Kaliora, A.C.; Marantidou, F.; Panagiotakos, D.P. Intake of omega-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition 2018, 45, 114–124.e4. [Google Scholar] [CrossRef] [PubMed]
- Proudman, S.M.; Cleland, L.G.; Metcalf, R.G.; Sullivan, T.R.; Spargo, L.D.; James, M.J. Plasma n-3 fatty acids and clinical outcomes in recent-onset rheumatoid arthritis. Br. J. Nutr. 2015, 114, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Lourdudoss, C.; Wolk, A.; Nise, L.; Alfredsson, L.; Vollenhoven, R.V. Are dietary vitamin d, omega-3 fatty acids and folate associated with treatment results in patients with early rheumatoid arthritis? Data from a swedish population-based prospective study. BMJ Open 2017, 7, e016154. [Google Scholar] [CrossRef] [PubMed]
- Adam, O.; Beringer, C.; Kless, T.; Lemmen, C.; Adam, A.; Wiseman, M.; Adam, P.; Klimmek, R.; Forth, W. Anti-inflammatory effects of a low arachidonic acid diet and fish oil in patients with rheumatoid arthritis. Rheumatol. Int. 2003, 23, 27–36. [Google Scholar] [PubMed]
- McPhee, S.; Hodges, L.D.; Wright, P.F.; Wynne, P.M.; Kalafatis, N.; Macrides, T.A. Prophylactic and therapeutic effects of Mytilus edulis fatty acids on adjuvant-induced arthritis in male Wistar rats. Prostaglandins Leukot. Essent. Fatty Acids 2010, 82, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, G.; Zhang, X.; Xing, G.; Hu, X.; Yang, L.; Li, D. Lipid extract from hard-shelled mussel (Mytilus coruscus) improves clinical conditions of patients with rheumatoid arthritis: A randomized controlled trial. Nutrients 2015, 7, 625–645. [Google Scholar] [CrossRef] [PubMed]
- Treschow, A.P.; Hodges, L.D.; Wright, P.F.; Wynne, P.M.; Kalafatis, N.; Macrides, T.A. Novel anti-inflammatory omega-3 pufas from the New Zealand green-lipped mussel, Perna canaliculus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 147, 645–656. [Google Scholar] [CrossRef] [PubMed]
- McPhee, S.; Hodges, L.D.; Wright, P.F.; Wynne, P.M.; Kalafatis, N.; Harney, D.W.; Macrides, T.A. Anti-cyclooxygenase effects of lipid extracts from the New Zealand green-lipped mussel, Perna canaliculus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.H.; Lee, S.H.; Jeon, Y.J.; Lee, D.S. Mussel (Mytilus coruscus) water extract containing taurine prevents LPS-induced inflammatory responses in zebrafish model. Adv. Exp. Med. Biol. 2017, 975, 931–942. [Google Scholar] [PubMed]
- Kim, Y.S.; Ahn, C.B.; Je, J.Y. Anti-inflammatory action of high molecular weight Mytilus edulis hydrolysates fraction in LPS-induced RAW264.7 macrophage via NF-kappab and MAPK pathways. Food Chem. 2016, 202, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, H.M.; Langkilde, A.M.; Undeland, I.; Sandberg, A.S. Herring (Clupea harengus) intake influences lipoproteins but not inflammatory and oxidation markers in overweight men. Br. J. Nutr. 2009, 101, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Das-score NL Home of the Das: Disease Activity Score in Rheumatoid Arthritis. 2017. Available online: http://www.Das28.Nl/das28/dascalculators/dasculators.Html (accessed on 28 June 2016).
- Van Gestel, A.M.; Haagsma, C.J.; van Riel, P.L. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998, 41, 1845–1850. [Google Scholar] [CrossRef]
- Van Gestel, A.M.; Prevoo, M.L.; van’t Hof, M.A.; van Rijswijk, M.H.; van de Putte, L.B.; van Riel, P.L. Development and validation of the European League against Rheumatism Response Criteria for rheumatoid arthritis. Comparison with the preliminary American college of rheumatology and the World Health Organization/International League against Rheumatism Criteria. Arthritis Rheum. 1996, 39, 34–40. [Google Scholar] [PubMed]
- Ekdahl, C.; Eberhardt, K.; Andersson, S.I.; Svensson, B. Assessing disability in patients with rheumatoid arthritis. Scand. J. Rheumatol. 1988, 17, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.; Karlsson, J.; Ware, J.E., Jr. The Swedish SF-36 health survey—I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc. Sci. Med. 1995, 41, 1349–1358. [Google Scholar] [CrossRef]
- Sullivan, M.; Karlsson, J. The Swedish SF-36 health survey III. Evaluation of criterion-based validity: Results from normative population. J. Clin. Epidemiol. 1998, 51, 1105–1113. [Google Scholar] [CrossRef]
- Persson, L.O.; Karlsson, J.; Bengtsson, C.; Steen, B.; Sullivan, M. The Swedish SF-36 health survey II. Evaluation of clinical validity: Results from population studies of elderly and women in gothenborg. J. Clin. Epidemiol. 1998, 51, 1095–1103. [Google Scholar] [CrossRef]
- Matsui, T.; Kuga, Y.; Kaneko, A.; Nishino, J.; Eto, Y.; Chiba, N.; Yasuda, M.; Saisho, K.; Shimada, K.; Tohma, S. Disease activity score 28 (DAS28) using C-reactive protein underestimates disease activity and overestimates EULAR response criteria compared with DAS28 using erythrocyte sedimentation rate in a large observational cohort of rheumatoid arthritis patients in Japan. Ann. Rheum Dis. 2007, 66, 1221–1226. [Google Scholar] [PubMed]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H. Could n-3 polyunsaturated fatty acids reduce pathological pain by direct actions on the nervous system? Prostaglandins Leukot. Essent. Fatty Acids 2003, 68, 219–224. [Google Scholar] [CrossRef]
- Goldberg, R.J.; Katz, J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 2007, 129, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Lourdudoss, C.; Di Giuseppe, D.; Wolk, A.; Westerlind, H.; Klareskog, L.; Alfredsson, L.; van Vollenhoven, R.F.; Lampa, J. Dietary intake of polyunsaturated fatty acids and pain in spite of inflammatory control among methotrexate treated early rheumatoid arthritis patients. Arthritis Care Res. 2017, 28, 23245. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.A.; Ives, S.J.; Narkowicz, C.; Jones, G. Plasma glutathione peroxidase (GSH-PX) concentration is elevated in rheumatoid arthritis: A case-control study. Clin. Rheumatol. 2012, 31, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Deacon, G.; Kettle, C.; Hayes, D.; Dennis, C.; Tucci, J. Omega 3 polyunsaturated fatty acids and the treatment of depression. Crit. Rev. Food Sci. Nutr. 2017, 57, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Amcoff, E.; Edberg, A.; Barbieri, H.E.; Lindroos, A.K.; Nälsén, C.; Pearson, M.; Lemming, E.W. Riksmaten vuxna 2010–2011 Livsmedels-och Näringsintag Bland Vuxna I Sverige; Livsmedelsverket: Uppsala, Sweden, 2013. [Google Scholar]
- Salminen, E.; Heikkila, S.; Poussa, T.; Lagstrom, H.; Saario, R.; Salminen, S. Female patients tend to alter their diet following the diagnosis of rheumatoid arthritis and breast cancer. Prev. Med. 2002, 34, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Elkan, A.C.; Hakansson, N.; Frostegard, J.; Cederholm, T.; Hafstrom, I. Rheumatoid cachexia is associated with dyslipidemia and low levels of atheroprotective natural antibodies against phosphorylcholine but not with dietary fat in patients with rheumatoid arthritis: A cross-sectional study. Arthritis Res. Ther. 2009, 11, R37. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L. Omega-3 polyunsaturated fatty acids in the treatment of hypertriglyceridaemia. Int. J. Cardiol. 2013, 170, S16–S20. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanihaghjo, A.; Kolahi, S.; Seifirad, S.; Rashtchizadeh, N.; Argani, H.; Hajialilo, M.; Khabazi, A.; Alizadeh, S.; Bahreini, E. Effect of fish oil supplements on serum paraoxonase activity in female patients with rheumatoid arthritis: A double-blind randomized controlled trial. Arch. Iran. Med. 2012, 15, 549–552. [Google Scholar] [PubMed]
- Dawczynski, C.; Schubert, R.; Hein, G.; Muller, A.; Eidner, T.; Vogelsang, H.; Basu, S.; Jahreis, G. Long-term moderate intervention with n-3 long-chain pufa-supplemented dairy products: Effects on pathophysiological biomarkers in patients with rheumatoid arthritis. Br. J. Nutr. 2009, 101, 1517–1526. [Google Scholar] [CrossRef] [PubMed]


| Baseline Characteristics | Median (Q1, Q3) a n = 23 | Range n = 23 | Median (Q1, Q3) a n = 30 | Range n = 30 |
|---|---|---|---|---|
| Age (year) | 55 (46, 63) | (32–66) | 57 (51, 63) | (32–66) |
| Body mass index (kg/m2) | 25.1 (23.2, 28.5) | (19.1–37.3) | 25.4(23.1, 28.4) | (19.1–38.9) |
| Weight (kg) | 68 (63, 83) | (51–90) | 69 (62, 82) | (51–103) |
| Waist circumference | 85 (80, 93) | (72–118) | 84 (80, 93) | (72–118) |
| Hip circumference | 101 (97, 110) | (85–122) | 101 (97, 110) | (85–130) |
| Blood pressure systolic (mmHg) | 125(120,140) | (100–160) | 128(120,140) | (100–165) |
| Blood pressure diastolic (mmHg) | 80 (80, 90) | (60–105) | 80 (80, 90) | (60–105) |
| Serum cholesterol (mmol/L) | 5.3 (4.7, 6.2) | (3.4–7.4) | 5.4 (4.7, 6.3) | (3.4–8.0) |
| Serum low density lipoprotein (mmol/L) | 3.2 (2.8, 4.1) | (2.2–4.9) | 3.3 (2.7, 4.1) | (1.4–5.3) |
| Serum high density lipoprotein (mmol/L) | 1.8 (1.4, 2.1) | (1.0–3.0) | 1.9 (1.6, 2.3) | (1.0–3.0) |
| Serum triacylglycerides (mmol/L) | 0.98 (0.61, 1.50) | (0.46–2.60) | 0.94 (0.70, 1.58) | (0.46–2.60) |
| Apolipoprotein A1 | 1.7 (1.5, 2.0) | (1.2–2.4) | 1.9(1.7, 2.0) | (1.2–2.4) |
| Apolipoprotein B | 1.0 (0.8, 1.2) | (0.8–1.5) | 1.0 (0.8, 1.2) | (0.8–1.2) |
| White blood cell count (×109/L) | 5.1 (4.3, 6.2) | (2.8–12.1) | 5.7 (4.6, 7.2) | (2.8–12.8) |
| Platelet count (×109/L) | 269 (213, 322) | (168–559) | 270 (214, 327) | (168–559) |
| Hemoglobin (g/L) | 133(129, 140) | (116–145) | 132(127, 139) | (114–151) |
| DAS28 b,c | 3.9 (3.5, 4.5) | (3.1–5.3) | 4.0 (3.5, 4.6) | (3.1–6.1) |
| DAS28-CRP c | 3.6 (3.2, 4.2) | (2.0–4.8) | 3.7 (3.2, 4.2) | (2.0–6.4) |
| Erythrocyte sedimentation rate (mm/1 h) | 12.5 (5.75, 25) | (2–45) | 14.0 (5.5, 23) | (2–45) |
| C-reactive protein (mg/L) | 2 (1, 3) | (0–14) | 2 (1, 3) | (0–39) |
| Swollen joints 28 (no) | 2 (1, 2) | (0–7) | 2 (1, 3) | (0–7) |
| Tender joints 28 (no) | 4 (2, 8) | (0–21) | 5 (2, 9) | (0–21) |
| VAS global health (mm) d | 54 (39, 66) | (22–78) | 54 (41, 67) | (22–87) |
| VAS Pain (mm) | 42(30, 64) | (7–82) | 46(32, 64) | (7–93) |
| VAS Fatigue (mm) | 64 (49, 75) | (16–90) | 64 (48, 75) | (10–95) |
| Disability (HAQ) c,e | 1.13 (0.59, 1.25) | (0–2) | 1.13 (0.62, 1.25) f | (0–2) |
| SF-36 MCS (total score) | 48 (39, 51) | (27–59) | 48 (39, 52) | (27–62) |
| Vitality c | 25 (13, 44) | (6–56) | 31 (13, 44) | (6–63) |
| Social functioning c | 63 (50, 75) | (12–100) | 63 (50, 75) | (13–100) |
| Role limitations due to emotional problems c | 83 (50, 100) | (0–100) | 79 (50, 100) | (0–100) |
| Mental health c | 60 (50, 70) | (35–85) | 60 (53, 73) | (35–90) |
| SF-36 PCS (total score) | 35 (30, 40) | (27–56) | 35 (30, 41) | (25–56) |
| Physical functioning c | 45 (30, 65) | (25–100) | 45 (29, 65) | (15–100) |
| Role limitations due to physical health c | 56 (31, 75) | (0–100) | 56 (31, 70) | (0–100) |
| Bodily pain c | 41 (31,51) | (12–72) | 41 (31,51) | (12–72) |
| General health c | 35 (30, 55) | (10–72) | 35 (30, 55) | (10–72) |
| Diagnosis: | n = 23 | % | n = 30 | % |
|---|---|---|---|---|
| Seropositive | 13 | 57% | 18 | 60% |
| Seronegative | 10 | 43% | 12 | 40% |
| Rheumatic drug treatment: | ||||
| Analgesic: | 16 | 70% | 20 | 67% |
| NSAIDs n (%) | 15 | 65% | 18 | 60% |
| DMARD (methotrexate), n (%) | 14 | 61% | 18 | 60% |
| Anti-TNF n (%) | 5 | 22% | 8 | 27% |
| Monoclonal antibody (MabThera) n (%) | 4 | 17% | 5 | 17% |
| Sulfasalazine: n (%) | 4 | 17% | 4 | 13% |
| Glucocorticosteroid n (%) | 2 | 9% | 6 | 29% |
| Nutrients Provided Daily: | Blue Mussels | Meat | Blue Mussels % RDI | Meat % RDI |
|---|---|---|---|---|
| Energy (kJ) | 254 | 225 | 3% | 3% |
| Energy (kcal) | 61 | 54 | 3% | 3% |
| Protein (g) | 9.5 | 11 | 12% | 14% |
| Fat (g) | 1.5 | 1.2 | 2% | 2% |
| Carbohydrates (g) | 2.2 | 0.1 | 1% | 0% |
| Saturated fatty acids (g) | 0.24 | 0.40 | 1% | 2% |
| Mono unsaturated fatty acids (g) | 0.14 | 0.50 | 0% | 2% |
| Poly unsaturated fatty acids (g) | 0.47 | 0.22 | 3% | 1% |
| EPA (fatty acid 20:5) (g) | 0.18 | 0.00 | ||
| DHA (fatty acid 22:6) (g) | 0.16 | 0.01 | ||
| Iron (mg) | 2.64 | 0.17 | 18% | 1% |
| Calcium (mg) | 25.6 | 3.39 | 3% | 0% |
| Zinc (mg) | 1.54 | 0.50 | 22% | 7% |
| Selenium (µg) | 33.2 | 5.8 | 66% | 12% |
| Iodine (µg) | 117 | 1.34 | 78% | 1% |
| Retinol EQ | 33.5 | 4.99 | 5% | 1% |
| Vitamin D (µg) | 0.01 | 0.24 | 0% | 2% |
| Vitamin B12 (µg) | 12.1 | 0.13 | 603% | 6% |
| Control Diet | Blue Mussel Diet | ||||||
|---|---|---|---|---|---|---|---|
| Before a | After a | Δ a | Before a | After a | Δ a | pb | |
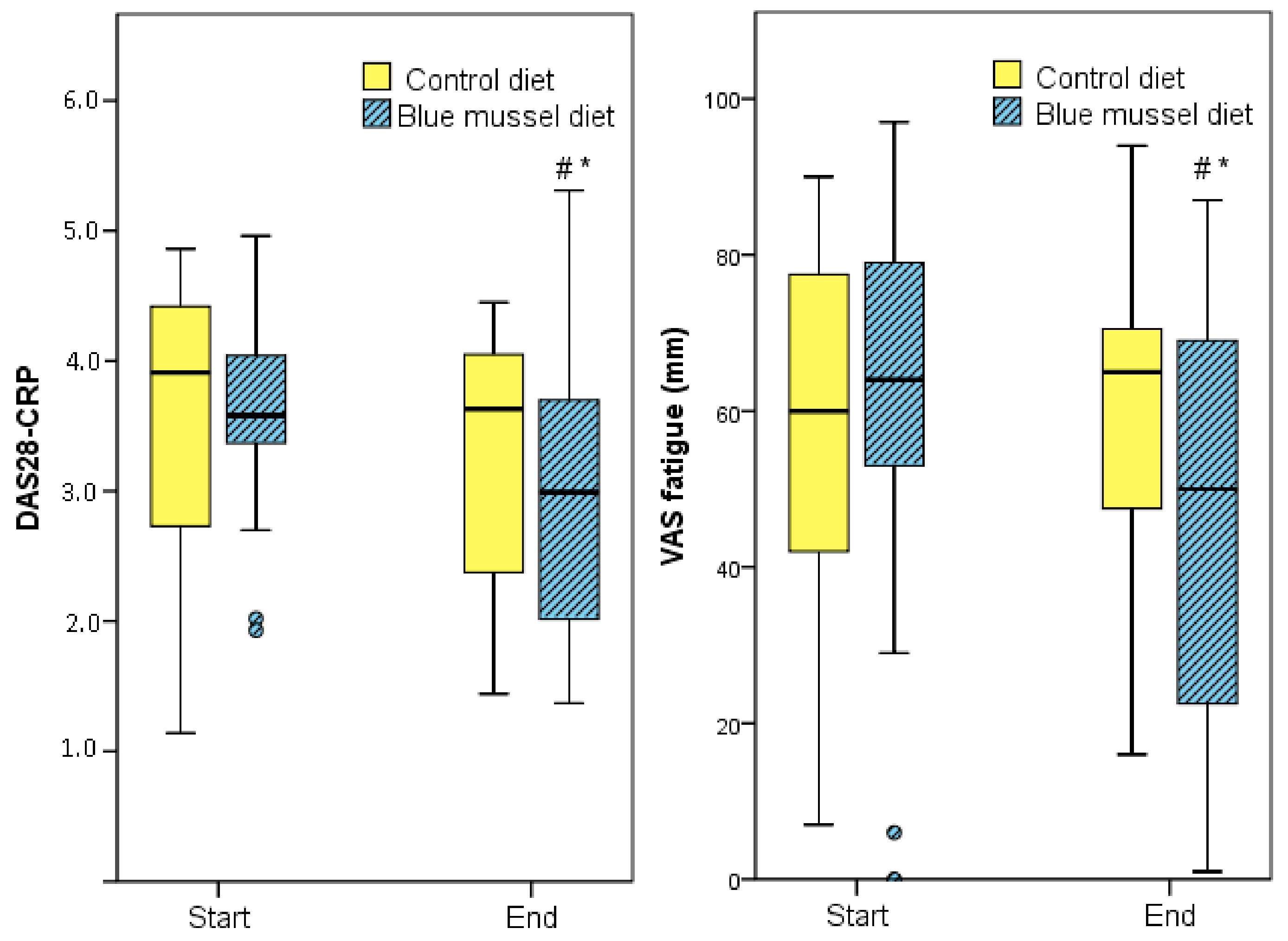
| DAS28 c,d | 3.81 (3.16, 3.73) | 3.77 (2.69, 4.22) | −0.15 (−0.72, 0.21) | 3.75 (3.15, 4.53) | 3.40 (2.41, 3.73) | −0.72 (−1.5, −0.06) f | 0.200 |
| SA | 3.95 (3.35, 4.53) | 3.88 (2.83, 4.23) | −0.16 (−0.74, 0.22) | 3.96 (3.14, 4.53) | 3.40 (2.52, 3.76) | −0.81 (−1.5, −0.08) e | 0.023 * |
| DAS28-CRP d | 3.91 (2.50, 4.54) | 3.63 (2.36, 4.21) | −0.19 (−0.83, 0.17) | 3.58 (3.35, 4.06) | 2.99 (1.88, 3.72) | −0.50 (−1.69, −0.02) | 0.048 * |
| SA | 3.92 (2.86, 4.56) | 3.5 (2.7, 4.2) | −0.18 (−0.81, 0.11) | 3.61 (3.35, 4.16) | 3.0 (2.2, 3.7) | −0.50 (−1.42, −0.01) | 0.008 * |
| ESR (mm/1 h) | 12.0 (5.0, 25.0) | 8.0 (6.0, 25.0) | 0.0 (−3.0, 2.0) | 12.5 (5.0, 25.5) c | 9.0 (4.8, 19.3) c | −1.0 (−5.5, 1.0) e | 0.952 |
| SA | 14.0 (5.0, 25.0) | 8.0 (6.8, 25.0) | 0.0 (−3.0, 2.0) | 13.0 (5.0, 25.0) | 9.0 (5.0, 19.3) | −0.5 (−5.3, 1.0) | 0.289 |
| CRP (mg/L) | 2.0 (0.0, 3.0) | 2.0 (0.0, 3.0) | 0.0 (−1.0, 1.0) | 2.0 (1.0, 5.0) | 1.0 (1.0, 3.0) | −1.0 (−1.0, 1.0) | 0.106 |
| SA | 2.0 (0.0, 4.8) | 2.0 (0.8, 4.3) | −0.2 (−0.8, 0.11) | 2.5 (1.0, 5.3) | 2.0 (0.8, 3.0) | −1.0 (−1.5, 1.0) | 0.080 |
| SJ 28 (no) | 2 (1, 3) | 2 (0, 4) | 0 (−2, 1) | 2 (1, 3) | 1 (0, 2) | −1 (−3, 1) | 0.363 |
| SA | 2 (1, 3) | 1.5 (0, 4) | 0 (−2, 1) | 2 (1, 3) | 1 (0, 2.2) | 0 (−3, 1) | 0.192 |
| TJ 28 (no) | 3 (1, 12) | 4 (0, 7) | −1 (−4, 1) | 4 (2, 7) | 2 (1, 5) | −2 (−5, 0) | 0.075 |
| SA | 3.5 (1, 12) | 4 (0.8, 6.3) | −1 (−4, 1) | 4.5 (2, 8) | 3 (0.8, 5.0) | −1 (−5, 0) | 0.087 |
| VAS GH (mm) | 51 (31, 65) | 40 (22, 57) | −2 (−15, 18) | 57 (37, 69) | 30 (11, 58) | −11 (−35, 5) | 0.041 * |
| SA | 51 (31, 65) | 52 (34, 67) | 1 (−9, 19) | 56 (41, 69) | 39 (14, 60) | −9 (−33, 5) | 0.005 * |
| VAS pain (mm) | 25 (15, 54) | 46 (19, 68) | −5 (−12, 8) | 51 (30, 72) | 25 (15, 54) | −15 (−25, 3) | 0.048 * |
| SA | 47 (32, 65) | 54 (25, 71) | −2 (−10, 9) | 51 (32, 72) | 37 (15, 54) | −12 (−25, 3) | 0.004 * |
| VAS fatigue (mm) | 60 (39, 78) | 65 (47, 72) | −12 (−18, 19) | 64 (49, 82) | 50 (20, 69) | −12 (−37, 10) | 0.021 * |
| SA | 58 (38, 77) | 65 (48, 73) | −1 (−14, 19) | 63 (49, 78) | 52 (28, 71) | −5 (−25, 9) | 0.084 |
| Control Diet | Blue Mussel Diet | ||||||
|---|---|---|---|---|---|---|---|
| Before a | After a | Δ a | Before a | After a | Δ a | pb | |
| SF-36 MCS (total score) | 48 (41, 51) | 46 (41, 54) | 0 (−6, 7) | 43 (38, 52) | 50 (47, 56) | 6 (2, 13) | 0.005 ** |
| Vitality c | 31 (19, 44) | 38 (19, 50) | 6 (−13, 19) | 31 (13, 44) | 50 (31, 63) | 13 (4, 25) | 0.012 * |
| Social functioning c | 63 (50, 88) | 63 (50, 88) | 0 (−13, 13) | 63 (38, 75) | 75 (50, 100) | 13 (0, 38) | 0.04 * |
| RL emotional problems c | 75 (50, 100) | 75 (50, 100) | 0 (−17, 17) | 75 (50, 100) | 79 (75, 100) | 8 (0, 27) | 0.145 |
| Mental health c | 60 (55, 75) | 65 (55, 80) | 0 (−5, 15) | 65 (50, 70) | 70 (60, 85) | 10 (0, 20) | 0.008 ** |
| SF-36 PCS (total score) | 38 (33, 44) | 37 (33, 44) | 0 (−2, 4) | 35 (27, 41) | 38 (32, 48) | 3 (−3, 7) | 0.503 |
| Physical functioning c | 50 (35, 74) | 50 (35, 65) | 0 (−5, 5) | 45 (30,65) | 50 (35,75) | 5 (−5, 15) | 0.299 |
| RL physical health c | 63 (44, 81) | 50 (50, 88) | 6 (−13, 13) | 44 (31, 75) | 69 (50, 88) | 13 (−6, 27) | 0.442 |
| Bodily pain c | 41 (22, 62) | 51 (31, 62) | 15 (−9, 28) | 41 (31, 51) | 42 (41, 62) | 10 (0, 21) | 0.599 |
| General health c | 35 (30, 57) | 35 (25, 52) | −5 (−10, 10) | 35 (30, 55) | 47 (25, 67) | 3 (−5, 17) | 0.03 * |
| HAQ d | 1.13 (0.44,1.28) | 1.00 (0.50,1.38) | 0 (−0.25,0.16) | 1.06 (0.47,1.25) | 1.00 (0.13,1.28) | −0.13 (−0.38,0.00) | 0.088 |
| Control Diet | Blue Mussel Diet | ||||||
|---|---|---|---|---|---|---|---|
| Before a | After a | Δ a | Before a | After a | Δ a | pb | |
| BMI (kg/m2) | 25.6 (23.6,27.8) | 25.1 (23.6,28.1) | 0.00 (−0.35,0.35) | 24.8 (22.9,28.5) | 25.1 (23.1,28.3) | 0.08 (−0.38,0.40) | 0.852 |
| Cholesterol c | 5.4 (4.8, 6.2) | 5.4 (4.5, 6.3) | −0.2 (−0.4, 0.2) | 5.3 (4.6, 6.2) | 5.2 (4.8, 6.2) | 0.1 (−0.3, 0.3) | 0.131 |
| LDLc | 3.3 (3.0, 4.1) | 3.3 (2.5, 4.1) | −0.1 (−0.2, 0.1) | 3.1 (2.6, 4.1) | 3.2 (2.7, 4.2) | 0.0 (−0.2, 0.3) | 0.359 |
| HDLc | 1.8 (1.4, 2.2) | 1.7 (1.4, 2.4) | −0.1 (−0.2, 0.1) | 1.9 (1.3, 2.2) | 1.8 (1.4, 2.2) | 0.0 (−0.2, 0.1) | 0.336 |
| TAG c | 1.00 (0.72, 1.50) | 0.85 (0.67, 1.40) | −0.09 (−0.21,0.10) | 1.00 (0.71, 1.40) | 0.99 (0.71, 1.50) | −0.02 (−0.20,0.11) | 0.263 |
| Apo A1 c | 1.7 (1.6, 2.0) | 1.8 (1.4, 2.0) | 0.0 (−0.2, 0.1) | 1.7 (1.4, 2.1) | 1.8 (1.5, 2.0) | 0.0 (−0.1, 0.1) | 0.486 |
| Apo Bc | 0.99 (0.85, 1.20) | 0.96 (0.81, 1.20) | 0.0 (−0.1, 0.1) | 1.00 (0.82, 1.20) | 0.92 (0.83, 1.30) | 0.0 (−0.1, 0.1) | 0.432 |
| WBC (*109/L) | 5.0 (4.4, 6.0) | 6.0 (4.6, 6.6) | 0.3 (−0.5, 0.0) | 5.3 (4.1, 6.2) | 5.1 (4.6, 6.2) | 0.2 (−0.2, 0.5) | 0.076 |
| PC (*109/L) | 271 (224, 317) | 277 (229, 309) | 5 (−18, 28) | 269 (236, 322) | 282 (228, 331) | 10 (−20, 27) | 0.513 |
| Hb (g/L) | 136 (128, 143) | 137 (127, 143) | 0 (−3, 5) | 131 (129, 140) | 135 (125, 140) | 1 (−6, 6) | 0.088 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindqvist, H.M.; Gjertsson, I.; Eneljung, T.; Winkvist, A. Influence of Blue Mussel (Mytilus edulis) Intake on Disease Activity in Female Patients with Rheumatoid Arthritis: The MIRA Randomized Cross-Over Dietary Intervention. Nutrients 2018, 10, 481. https://doi.org/10.3390/nu10040481
Lindqvist HM, Gjertsson I, Eneljung T, Winkvist A. Influence of Blue Mussel (Mytilus edulis) Intake on Disease Activity in Female Patients with Rheumatoid Arthritis: The MIRA Randomized Cross-Over Dietary Intervention. Nutrients. 2018; 10(4):481. https://doi.org/10.3390/nu10040481
Chicago/Turabian StyleLindqvist, Helen M., Inger Gjertsson, Tove Eneljung, and Anna Winkvist. 2018. "Influence of Blue Mussel (Mytilus edulis) Intake on Disease Activity in Female Patients with Rheumatoid Arthritis: The MIRA Randomized Cross-Over Dietary Intervention" Nutrients 10, no. 4: 481. https://doi.org/10.3390/nu10040481
APA StyleLindqvist, H. M., Gjertsson, I., Eneljung, T., & Winkvist, A. (2018). Influence of Blue Mussel (Mytilus edulis) Intake on Disease Activity in Female Patients with Rheumatoid Arthritis: The MIRA Randomized Cross-Over Dietary Intervention. Nutrients, 10(4), 481. https://doi.org/10.3390/nu10040481





