Beneficial Effects of Different Flavonoids on Vascular and Renal Function in L-NAME Hypertensive Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Groups
2.3. Experimental Procedures
2.3.1. Measurement of Blood Pressure and Samples Extraction
2.3.2. Vascular Reactivity Study
2.4. Analytical Procedures
2.5. Histopathological Analysis
2.6. Statistical Methods
3. Results
3.1. Blood Pressure and Urinary Variables
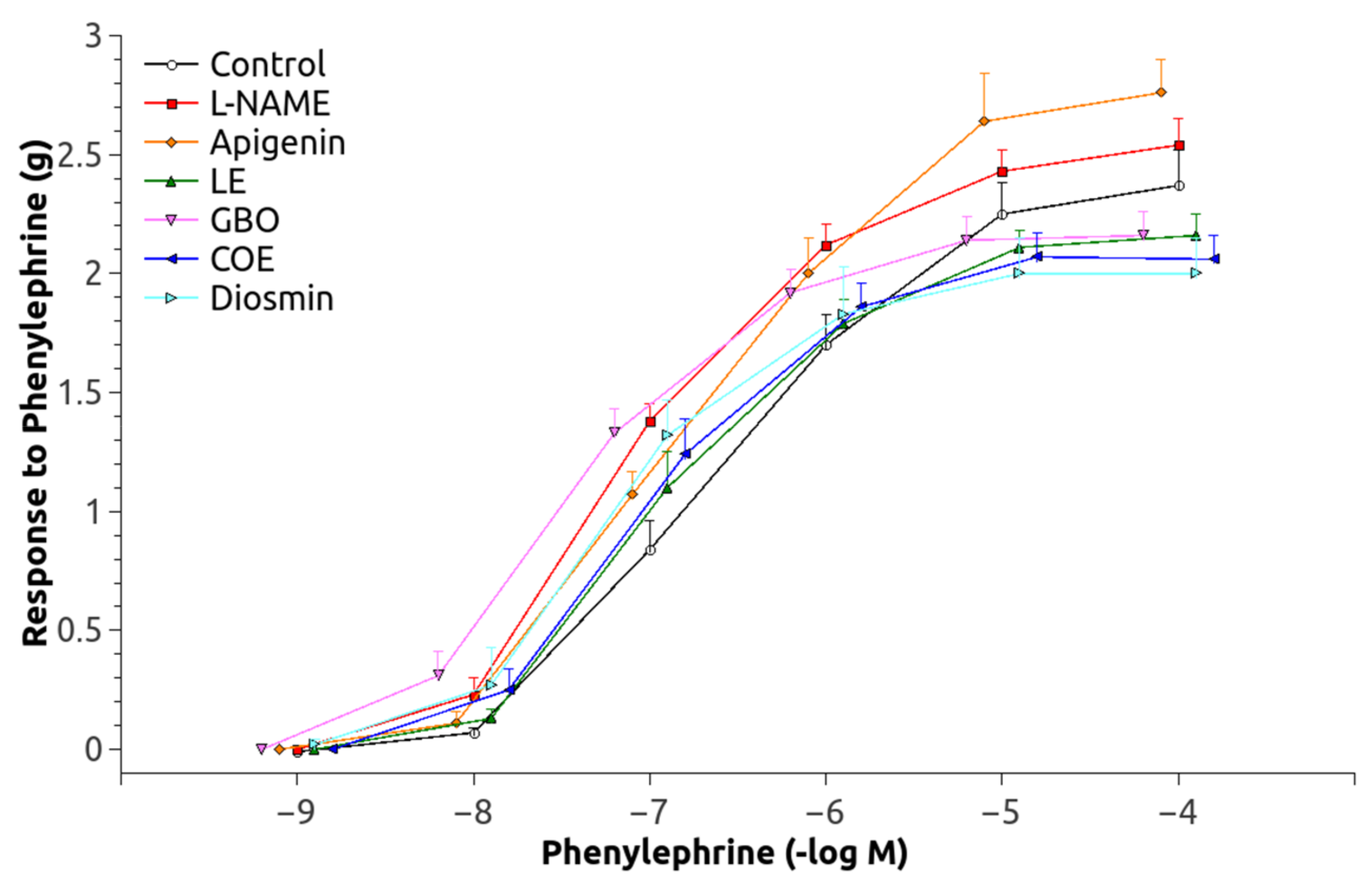
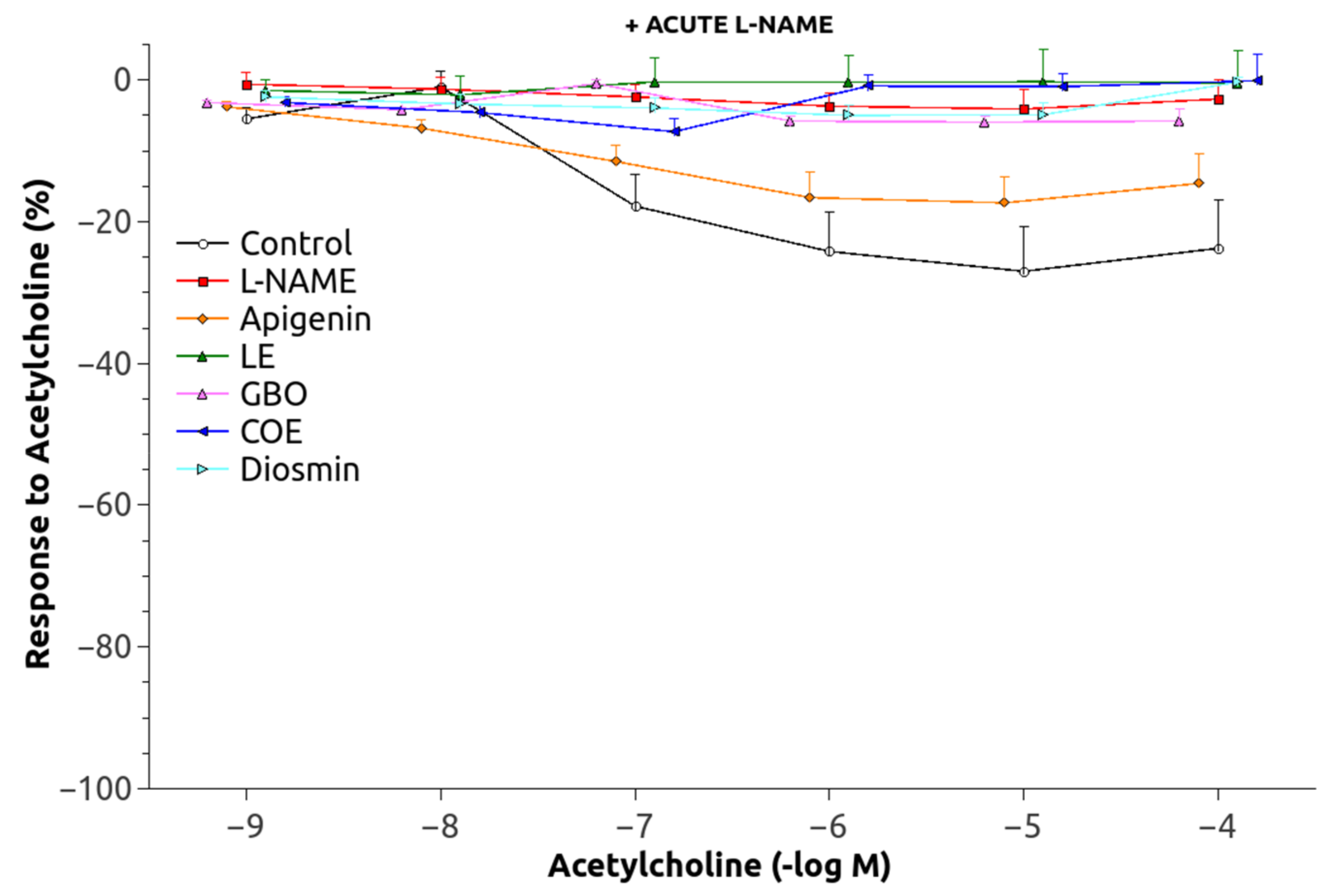
3.2. Vascular Function
3.3. Effect of Flavonoid Extracts on Oxidative Stress Status
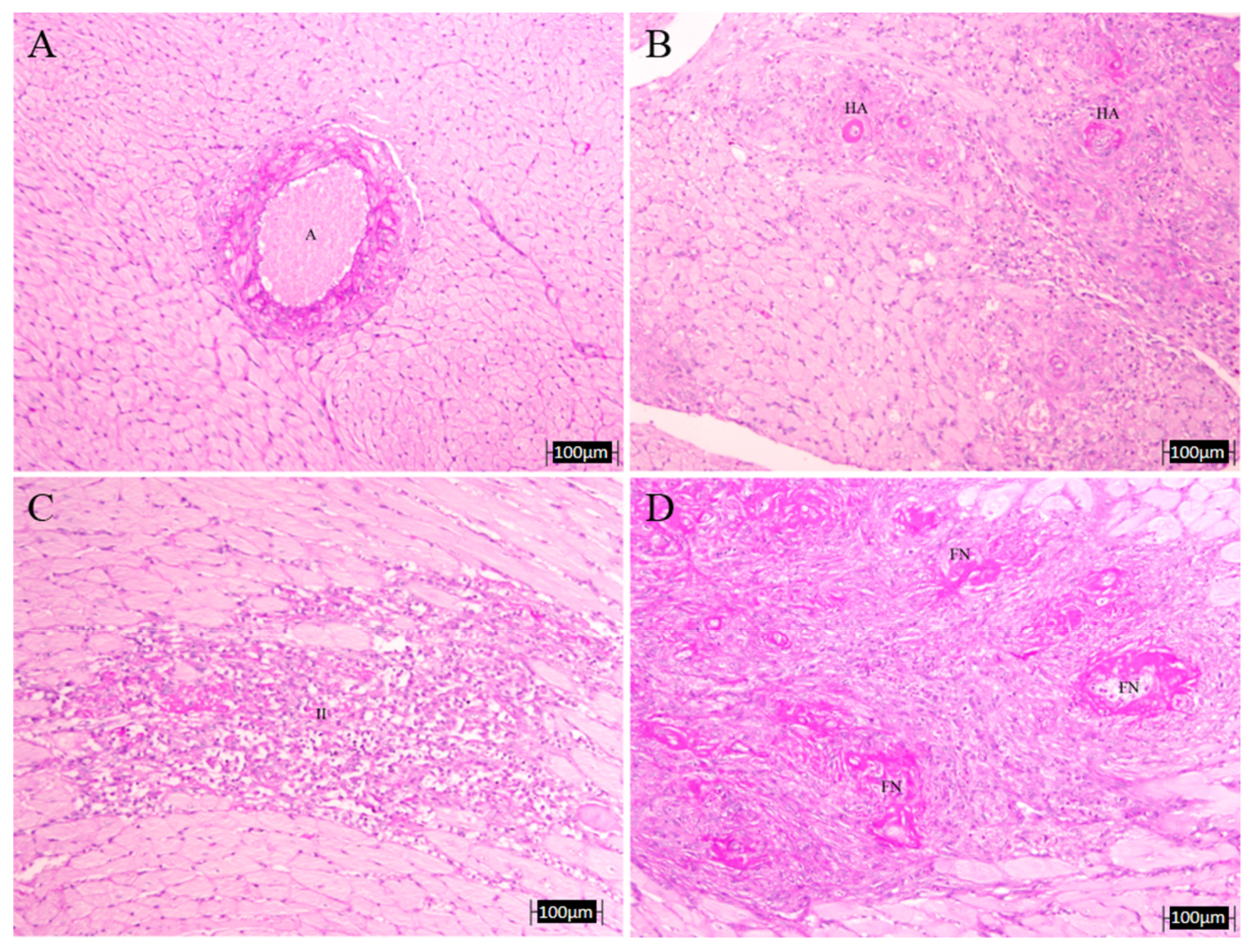
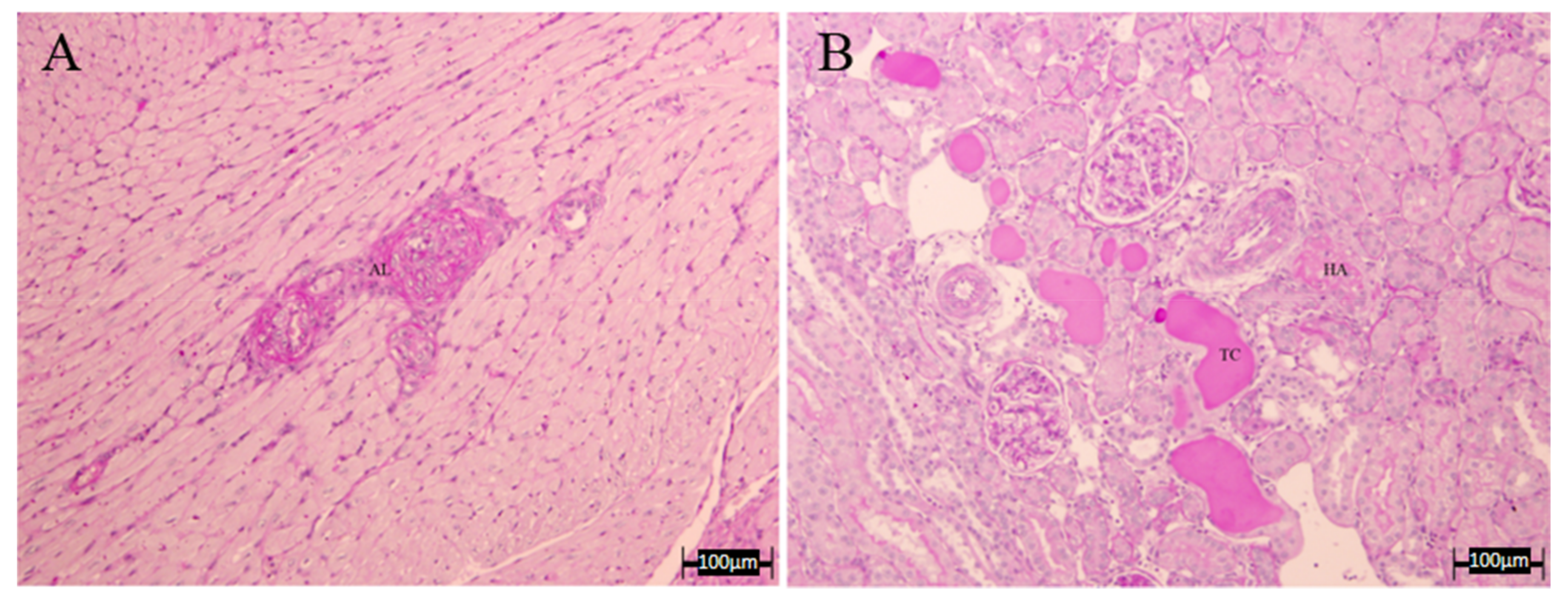
3.4. Histopathology Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duarte, J.; Francisco, V.; Perez-Vizcaino, F. Modulation of nitric oxide by flavonoids. Food Funct. 2014, 5, 1653–1668. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.M.; Steluti, J.; Fisberg, R.M.; Marchioni, D.M. Association between Polyphenol Intake and Hypertension in Adults and Older Adults: A Population-Based Study in Brazil. PLoS ONE 2016, 11, e0165791. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C. Anthocyanins in Cardiovascular Disease. Adv. Nutr. 2011, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Rhone, M.; Lyons, T.J. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.; Miguel, M.; Muguerza, B.; Aleixandre, A. Effect of cocoa polyphenol extract in spontaneously hypertensive rats. Food Funct. 2011, 2, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Habauzit, V.; Morand, C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Ther. Adv. Chronic Dis. 2012, 3, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the Cardiovascular System in Health and in Aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef] [PubMed]
- Almeida Rezende, B.; Pereira, A.C.; Cortes, S.F.; Lemos, V.S. Vascular effects of flavonoids. Curr. Med. Chem. 2016, 23, 87–102. [Google Scholar] [CrossRef] [PubMed]
- López-Sepúlveda, R.; Jiménez, R.; Romero, M.; Zarzuelo, M.J.; Sánchez, M.; Gómez-Guzmán, M.; Vargas, F.; O’Valle, F.; Zarzuelo, A.; Pérez-Vizcaíno, F.; et al. Wine Polyphenols Improve Endothelial Function in Large Vessels of Female Spontaneously Hypertensive Rats. Hypertension 2008, 51, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Cid, J.; Maeso, R.; Rodrigo, E.; Muñoz-García, R.; Ruilope, L.M.; Lahera, V.; CAchofeiro, V. Renal and vascular consequences of the chronic nitric oxide synthase inhibition. Effects of antihypertensive drugs. Am. J. Hypertens. 1996, 9, 1077–1083. [Google Scholar] [CrossRef]
- Fortepiani, L.A.; Rodrigo, E.; Ortíz, M.C.; CAchofeiro, V.; Atucha, N.M.; Ruilope, L.M.; Lahera, V.; García-Estañ, J. Pressure natriuresis in nitric oxide-deficient hypertensive rats: Effect of antihypertensive treatments. J. Am. Soc. Nephrol. 1999, 10, 21–27. [Google Scholar] [PubMed]
- García-Estañ, J.; Ortiz, M.C.; O’Valle, F.; Alcaraz, A.; Navarro, E.G.; Vargas, F.; Evangelista, S.; Atucha, N.M. Effects of angiotensin-converting-enzyme inhibitors in combination with diuretics on blood pressure and renal injury in nitric oxide-deficiency-induced hypertension in rats. Clin. Sci. 2006, 110, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Pechánová, O.; Rezzani, R.; Babál, P.; Bernátová, I.; Andriantsitohaina, R. Beneficial effects of Provinols: Cardiovascular system and kidney. Physiol. Res. 2006, 55 (Suppl. 1), S1–S30. [Google Scholar]
- Bunbupha, S.; PrAchaney, P.; Kukongviriyapan, U.; Kukongviriyapan, V.; Welbat, J.U.; Pakdeechote, P. Asiatic acid alleviates cardiovascular remodelling in rats with L-NAME-induced hypertension. Clin. Exp. Pharmacol. Physiol. 2015, 42, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Berkban, T.; Boonprom, P.; Bunbupha, S.; Welbat, J.U.; Kukongviriyapan, U.; Kukongviriyapan, V.; Pakdeechote, P.; PrAchaney, P. Ellagic acid prevents L-NAME-induced hypertension via restoration of eNOS and p47phox expression in rats. Nutrients 2015, 7, 5265–5280. [Google Scholar] [CrossRef] [PubMed]
- Bernátová, I.; Kopincová, J.; Púzserová, A.; Janega, P.; Babál, P. Chronic low-dose L-NAME treatment increases nitric oxide production and vasorelaxation in normotensive rats. Physiol. Res. 2007, 56, S17–S24. [Google Scholar] [PubMed]
- Alcaraz, A.; Hernández, D.; Iyú, D.; Mota, R.; Atucha, N.M.; Ortiz, A.J.; García-Estañ, J.; Ortiz, M.C. Effects of chronic L-NAME on nitrotyrosine expression and renal vascular reactivity in rats with chronic bile-duct ligation. Clin. Sci. 2008, 115, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.Y.; Qian, L.B.; Zhu, L.G.; Liang, H.T.; Tan, Y.N.; Lu, H.T.; Lu, J.F.; Wang, H.P.; Xia, Q. Betulinic acid ameliorates endothelium-dependent relaxation in L-NAME-induced hypertensive rats by reducing oxidative stress. Eur. J. Pharm. Sci. 2011, 44, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Benavente-Garcı́a, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, A.; Iyú, D.; Atucha, N.M.; García-Estañ, J.; Ortiz, M.C. Vitamin E supplementation reverses renal altered vascular reactivity in chronic bile duct-ligated rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1486–R1493. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, I.; Banegas, I.; Wangensteen, R.; Quesada, A.; Jiménez, R.; Gómez-Morales, M.; O’Valle, F.; Duarte, J.; Vargas, F. Influence of thyroid state on cardiac and renal capillary density and glomerular morphology in rats. J. Endocrinol. 2013, 216, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Perez-Abud, R.; Rodríguez-Gómez, I.; Villarejo, A.B.; Moreno, J.M.; Wangensteen, R.; Tassi, M.; O’Valle, F.; Osuna, A.; Vargas, F. Salt sensitivity in experimental thyroid disorders in rats. Am. J. Physiol. Endocrinol. MeTable 2011, 301, E281–E287. [Google Scholar] [CrossRef] [PubMed][Green Version]
- ImageJ 1.47. Available online: http://rsb.info.nih.gov/ij/ (accessed on 1 June 2017).
- Morley, J.E.; Flood, J.F. Competitive antagonism of nitric oxide synthetase causes weight loss in mice. Life Sci. 1992, 51, 1285–1289. [Google Scholar] [CrossRef]
- Ke, J.-Y.; Kliewer, K.L.; Hamad, E.M.; Cole, R.M.; Powell, K.A.; Andridge, R.R.; Straka, S.R.; Yee, L.D.; Belury, M.A. The flavonoid, naringenin, decreases adipose tissue mass and attenuates ovariectomy-associated metabolic disturbances in mice. Nutr. Metab. 2015, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Litterio, M.C.; Jaggers, G.; Celep, G.S.; Adamo, A.M.; Costa, M.A.; Oteiza, P.I.; Fraga, C.G.; Galleano, M. Blood pressure-lowering effect of dietary (−)-epicatechin administration in L-NAME-treated rats is associated with restored nitric oxide levels. Free Radic. Biol. Med. 2012, 53, 1894–1902. [Google Scholar] [CrossRef] [PubMed]
- Mali, V.R.; Mohan, V.; Bodhankar, S.L. Antihypertensive and cardioprotective effects of the Lagenaria siceraria fruit in NG-nitro-l-arginine methyl ester (L-NAME) induced hypertensive rats. Pharm. Biol. 2012, 50, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130 (Suppl. 8S), 2073S–2078S. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Park, Y.S.; Kim, T.J.; Fang, L.H.; Ahn, H.Y.; Hong, J.T.; Kim, Y.; Lee, C.K.; Yun, Y.P. Endothelium-dependent vasorelaxant and antiproliferative effects of apigenin. Gen. Pharmacol. 2000, 35, 341–347. [Google Scholar] [CrossRef]
- Ajay, M.; Gilani, A.U.; Mustafa, M.R. Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. Life Sci. 2003, 74, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, L.; Pons, Z.; Margalef, M.; Arola-Arnal, A.; Muguerza, B. Regulation of vascular endothelial genes by dietary flavonoids: Structure-expression relationship studies and the role of the transcription factor KLF-2. J. Nutr. Biochem. 2015, 26, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ferrándiz, M.L.; Alcaraz, M.J. Anti-inflammatory activity and inhibition of arAchidonic acid metabolism by flavonoids. Agents Actions 1991, 32, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.R.; Liu, D. Flavonoids influence epigenetic-modifying enzyme activity: Structure-function relationships and the therapeutic potential for cancer. Curr. Med. Chem. 2010, 17, 1756–1768. [Google Scholar] [CrossRef] [PubMed]
- Busch, C.; Burkard, M.; Leischner, C.; Lauer, U.M.; Frank, J.; Venturelli, S. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin. Epigenet. 2015, 7, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.C.; Manriquez, M.C.; Romero, J.C.; Juncos, L.A. Antioxidants block angiotensin II-induced increases in blood pressure and endothelin. Hypertension 2001, 38, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Singhal, U.; Hossain, M.M.; Islam, N.; Rizvi, I. The Role of the Endogenous Antioxidant Enzymes and Malondialdehyde in Essential Hypertension. J. Clin. Diagn. Res. 2013, 7, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S. The role of nitric oxide in hypertension and renal disease progression. Nephrol. Dial. Transplant. 2001, 16, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Kristek, F.; Gerova, M.; Devat, L.; Varga, I. Cardiac hypertrophy and vascular remodelling in NO-deficient hypertension. Endothelium 1995, 3, S94. [Google Scholar]
- Numaguchi, K.; Egashira, K.; Takemoto, M.; Kadokami, T.; Shimokawa, H.; Sueishi, K.; Takeshita, A. Chronic inhibition of nitric oxide synthesis causes coronary microvascular remodeling in rats. Hypertension 1995, 26, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Jiménez, R.; O’Valle, F.; Galisteo, M.; Pérez-Palencia, R.; Vargas, F.; Pérez-Vizcaíno, F.; Zarzuelo, A.; Tamargo, J. Protective effects of the flavonoid quercetin in chronic nitric oxide deficient rats. J. Hypertens. 2002, 20, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, R.; Duarte, J.; Pérez-Vizcaíno, F. Epicatechin: Endothelial Function and Blood Pressure. J. Agric. Food Chem. 2012, 60, 8823–8830. [Google Scholar] [CrossRef] [PubMed]




| Body Weight (g) | Hematocrit (%) | Food Intake (g/24 h) | Water Intake (mL/24 h) | Diuresis (mL/24 h) | Natriuresis (mEq/24 h) | Sodium Balance (mEq/24 h/100 g) | |
|---|---|---|---|---|---|---|---|
| C | 429.73 ± 6.36 | 47.38 ± 0.47 | 23.05 ± 0.68 | 35 ± 0.54 | 12.83 ± 1.46 | 0.41 ± 0.08 | 0.15 ± 0.05 |
| L-N | 345.73 ± 22.29 * | 47.20 ± 1.20 | 21.1 ± 1.21 | 31.25 ± 4.7 | 7.85 ± 1.69 | 0.22 ± 0.06 | 0.43 ± 0.11 * |
| A | 426.90 ± 11.46 | 50.67 ± 2.38 | 20.83 ± 0.63 | 30 ± 0.22 | 17.37 ± 3.85 | 0.47 ± 0.02 | 0.04 ± 0.05 † |
| LE | 404.72 ± 16.96 | 49.83 ± 1.21 | 18.6 ± 0.49 * | 25 ± 1.67 * | 13.45 ± 2.79 | 0.33 ± 0.08 | 0.15 ± 0.11 |
| GBO | 392.65 ± 14.98 | 48.75 ± 3.35 | 16.1 ± 1.49 * | 16.88 ± 2.48 * | 10.65 ± 1.87 | 0.26 ± 0.08 | 0.17 ± 0.11 |
| COE | 399.38 ± 20.57 | 49.40 ± 1.47 | 16.58 ± 0.9 * | 31.24 ± 5.6 | 21.84 ± 4.52 | 0.24 ± 0.06 | 0.19 ± 0.04 |
| Phenylephrine | Acetylcholine | SNP | |||
|---|---|---|---|---|---|
| Group | pEC50 (mol/L) | Maximal Contraction (g) | Maximal Relaxation (%) | After Acute L-NAME | Maximal Relaxation (%) |
| C | −6.45 ± 0.086 | 2.37 ± 0.15 | 82.70 ± 2.50 | 27.38 ± 6.54 | 99.38 ± 1.62 |
| L-N | −6.83 ± 0.064 * | 2.54 ± 0.12 | 19.88 ± 4.14 * | 4.55 ± 2.70 * | 89.71 ± 2.03 * |
| A | −6.56 ± 0.09 † | 2.76 ± 0.37 | 35.87 ± 6.89 * | 18.47 ± 3.92 † | 96.14 ± 1.73 |
| LE | −6.77 ± 0.12 | 2.16 ± 0.10 | 31.57 ± 8.23 * | 5.74 ± 3.93 * | 91.65 ± 3.12 |
| GBO | −7.22 ± 0.18 * | 2.18 ± 0.49 | 21.96 ± 5.32 * | 7.67 ± 1.02 * | 84.74 ± 2.93 *,† |
| COE | −7.08 ± 0.14 * | 2.09 ± 0.02 | 29.63 ± 3.99 * | 13.65 ± 2.40 † | 90.50 ± 1.57 * |
| D | −7.00 ± 0.14 * | 2.01 ± 0.34 | 13.78 ± 4.77 * | 5.23 ± 1.81 * | 91.59 ± 3.13 |
| Plasma TBARS (nmol/mL) | Kidney TBARS (nmol/mg prot) | Urine TBARS (nmol/mg prot/24 h) | Plasma Nitrite (µg/mL) | Urinary Excretion of Nitrite (µg/24 h) | Urinary Protein Excretion (mg/24 h/kg bw) | |
|---|---|---|---|---|---|---|
| C | 1.5 ± 0.3 | 1.5 ± 0.1 | 576 ± 65 | 1.25 ± 0.18 | 11.83 ± 1.31 | 367.58 ± 11.19 |
| L-N | 1.8 ± 0.2 | 2.0 ± 0.3 * | 437 ± 37 | 1.02 ± 0.05 | 6.39 ± 0.46 * | 468.60 ± 39.52 * |
| A | 1.7 ± 0.3 | 1.6 ± 0.2 | 512 ± 46 | 1.26 ± 0.09 | 13.40 ± 1.82 † | 360.64 ± 40.15 |
| LE | 1.6 ± 0.3 | 1.1 ± 0.3 | 488 ± 69 | 0.88 ± 0.04 | 11.02 ± 1.63 | 373.50 ± 61.90 |
| GBO | 1.3 ± 0.2 | 1.6 ± 0.0 | 458 ± 51 | 1.02 ± 0.02 | 9.05 ± 1.64 | 426.51 ± 18.81 * |
| COE | 1.3 ± 0.2 | 1.4 ± 0.3 | 482 ± 108 | 0.99 ± 0.11 | 14.12 ± 4.97 | 498.51 ± 67.17 |
| D | 1.4 ± 0.1 | 1.5 ± 0.1 | 546 ± 78 | 1.11 ± 0.12 | 12.02 ± 1.03 † | 420.13 ± 75.24 |
| # Infarcts | HA | FN | LWR | IVS | TAT | AOT | |
|---|---|---|---|---|---|---|---|
| C | 0.0 | 0.0 | 0.0 | 3.01 ± 0.58 | 2.41 ± 0.03 | 131.9 ± 3.1 | 110.7 ± 7.9 |
| L-N | 4.33 ± 3.84 | 0.67 ± 0.33 | 0.67 ± 0.33 | 2.22 ± 0.95 | 3.20 ± 0.10 * | 176.3 ± 4.2 * | 159.1 ± 6.5 * |
| A | 0.67 ± 0.67 | 0.33 ± 0.33 | 0.0 | 2.39 ± 0.44 | 2.62 ± 0.25 | 178.0 ± 14.8 * | 112.1 ± 5.9 † |
| LE | 3.33 ± 3.33 | 0.0 | 0.0 | 2.97 ± 0.10 | 2.71 ± 0.16 | 141.4 ± 2.4 *,† | 137.7 ± 3.7 *,† |
| GBO | 3.67 ± 1.86 | 0.33 ± 0.33 | 0.33 ± 0.33 | 1.99 ± 0.40 | 2.42 ± 0.22 † | 157.5 ± 8.5 * | 144.5 ± 9.6 * |
| COE | 2.67 ± 1.45 | 0.0 | 0.0 | 2.49 ± 0.06 | 3.11 ± 0.36 | 155.9 ± 5.4 *,† | 135.2 ± 5.4 *,† |
| D | 4.00 ± 4.00 | 0.33 ± 0.33 | 0.33 ± 0.33 | 2.74 ± 0.94 | 2.22 ± 0.08 † | 164.3 ± 7.2 * | 116 ± 4.4 † |
| LWR | HA | TC | Combined Vascular Damage | |
|---|---|---|---|---|
| C | 1.80 ± 0.05 | 0 | 0 | 0 |
| L-N | 1.72 ± 0.32 | 0.67 ± 0.33 | 0.33 ± 0.33 | 0.67 ± 0.33 |
| A | 1.85 ± 0.27 | 0 | 0.33 ± 0.33 | 0 |
| LE | 1.07 ± 0.19 | 0.17 ± 0.17 | 0.50 ± 0.29 | 0 |
| GBO | 2.97 ± 1.07 | 0.33 ± 0.33 | 0.37 ± 0.33 | 0.33 ± 0.33 |
| COE | 1.63 ± 0.45 | 0.33 ± 0.33 | 1.00 ± 0.00 | 0 |
| D | 1.53 ± 0.09 | 0.33 ± 0.33 | 0.67 ± 0.33 | 0.33 ± 0.33 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paredes, M.D.; Romecín, P.; Atucha, N.M.; O’Valle, F.; Castillo, J.; Ortiz, M.C.; García-Estañ, J. Beneficial Effects of Different Flavonoids on Vascular and Renal Function in L-NAME Hypertensive Rats. Nutrients 2018, 10, 484. https://doi.org/10.3390/nu10040484
Paredes MD, Romecín P, Atucha NM, O’Valle F, Castillo J, Ortiz MC, García-Estañ J. Beneficial Effects of Different Flavonoids on Vascular and Renal Function in L-NAME Hypertensive Rats. Nutrients. 2018; 10(4):484. https://doi.org/10.3390/nu10040484
Chicago/Turabian StyleParedes, M. Dolores, Paola Romecín, Noemí M. Atucha, Francisco O’Valle, Julián Castillo, M. Clara Ortiz, and Joaquín García-Estañ. 2018. "Beneficial Effects of Different Flavonoids on Vascular and Renal Function in L-NAME Hypertensive Rats" Nutrients 10, no. 4: 484. https://doi.org/10.3390/nu10040484
APA StyleParedes, M. D., Romecín, P., Atucha, N. M., O’Valle, F., Castillo, J., Ortiz, M. C., & García-Estañ, J. (2018). Beneficial Effects of Different Flavonoids on Vascular and Renal Function in L-NAME Hypertensive Rats. Nutrients, 10(4), 484. https://doi.org/10.3390/nu10040484






