Effects of Whey Protein Hydrolysate Ingestion on Postprandial Aminoacidemia Compared with a Free Amino Acid Mixture in Young Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
2.3. Experimental Procedures
2.4. Test Solutions
2.5. Blood Analyses
2.6. Statistics
3. Results
3.1. Subject Characteristics
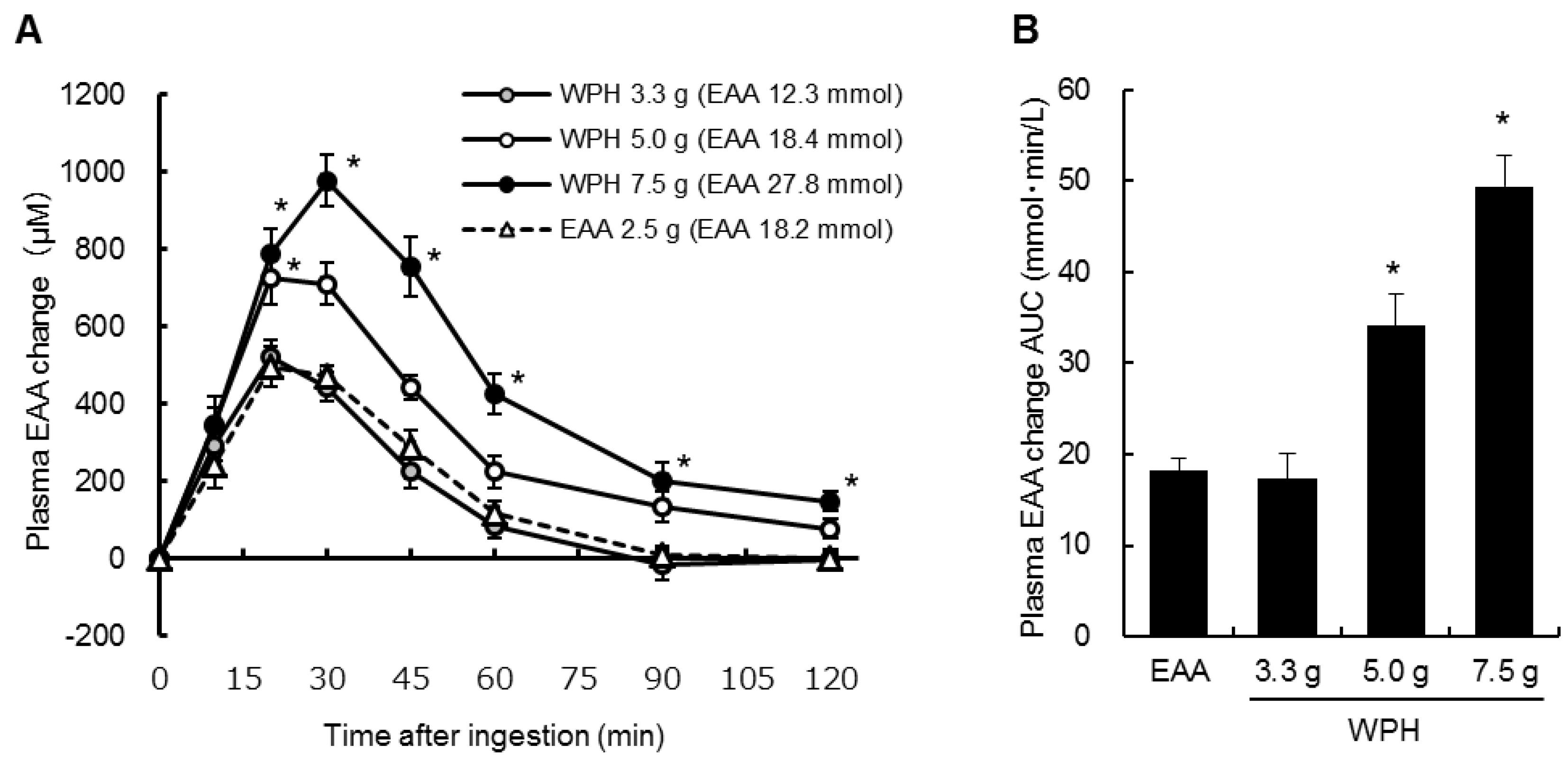
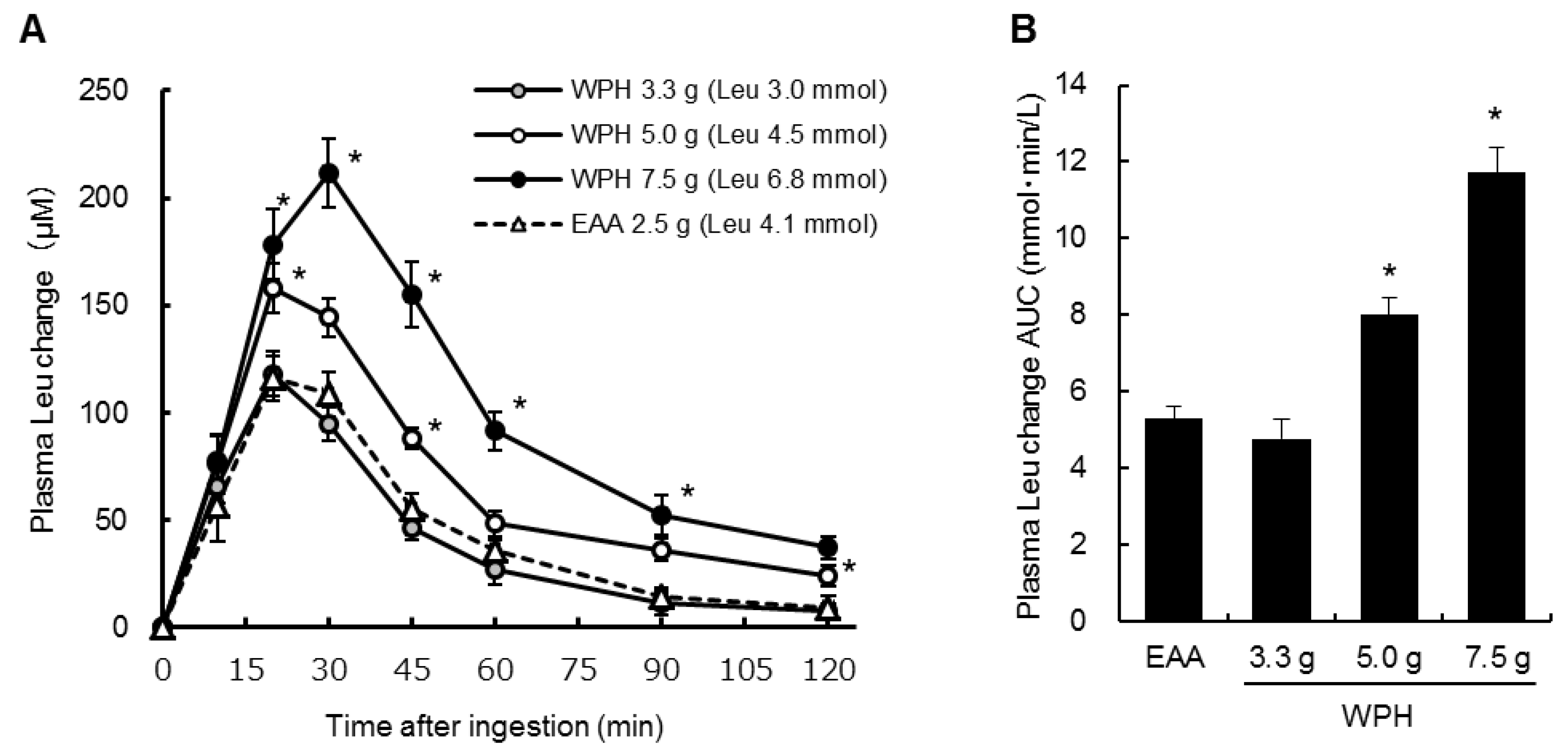
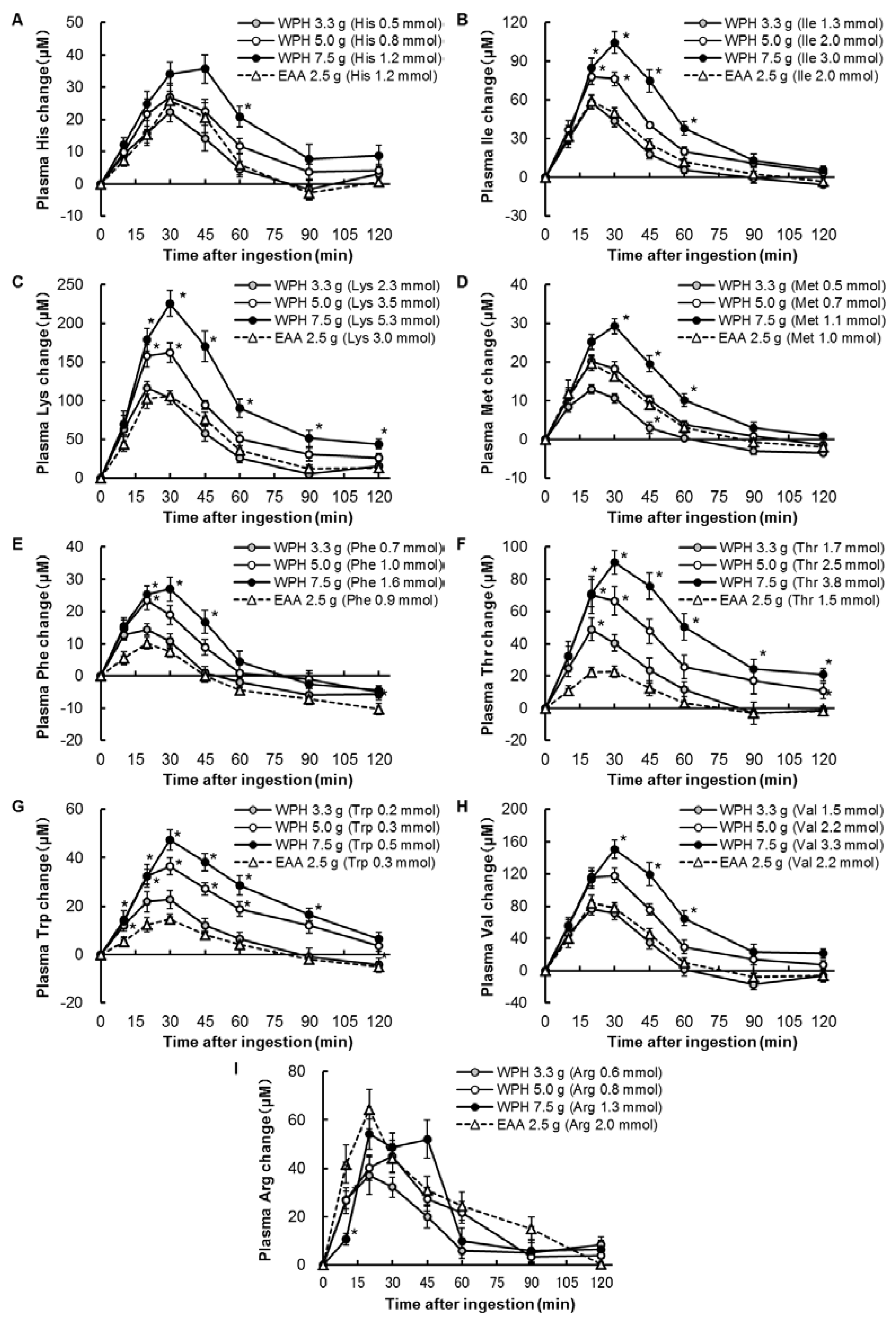
3.2. Plasma Amino Acids
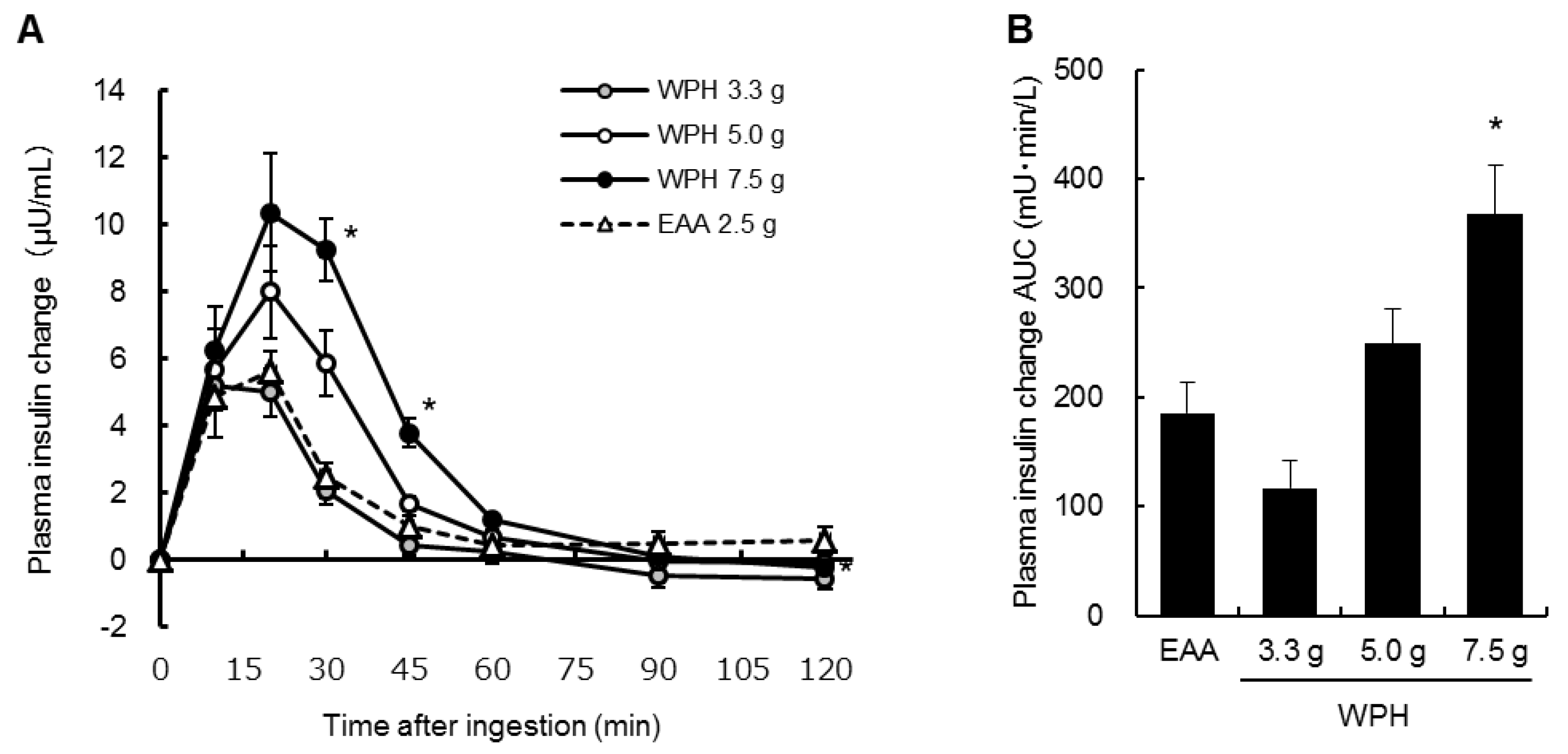
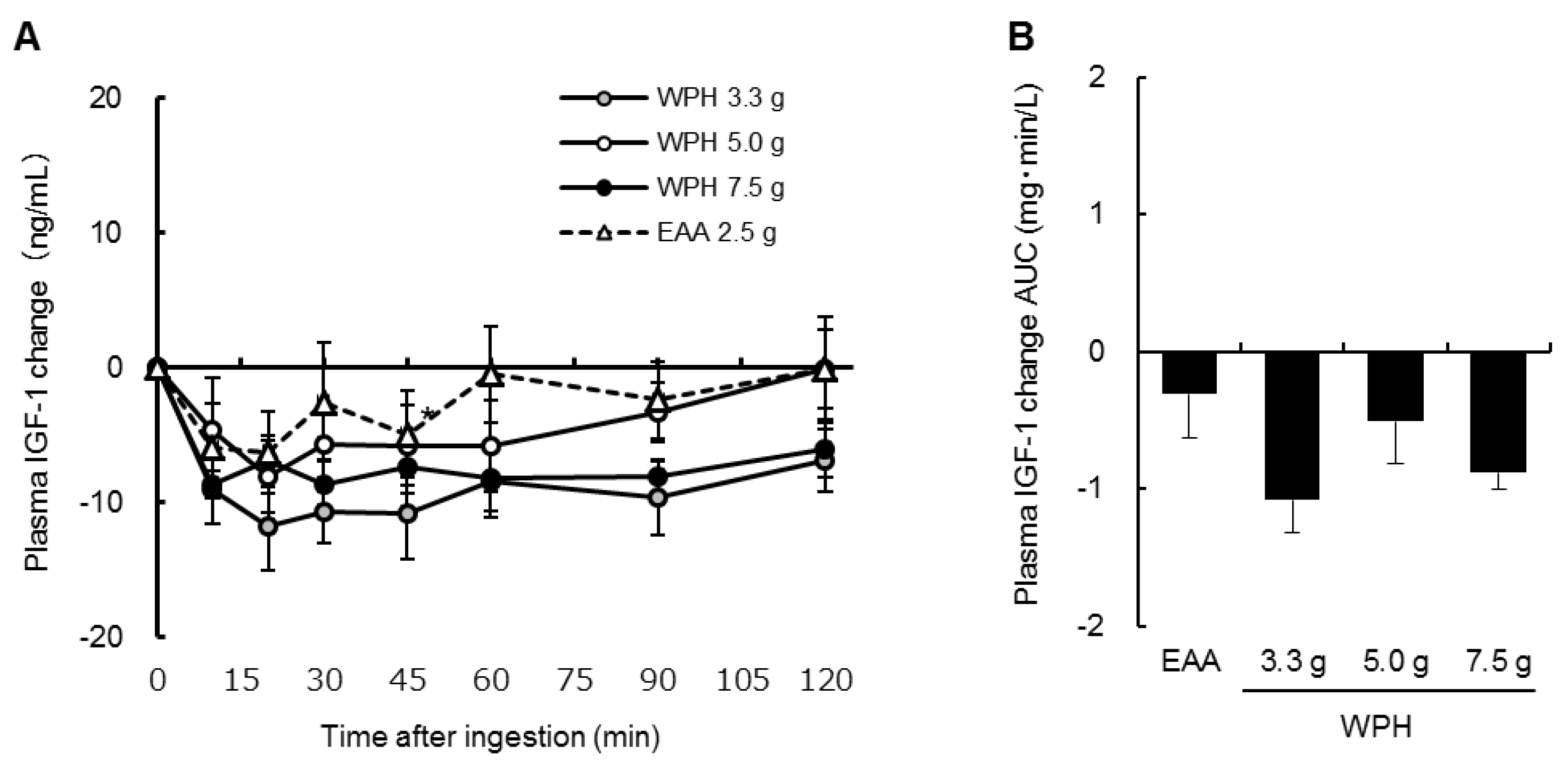
3.3. Plasma Insulin, Glucose and IGF-1
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rennie, M.J. Exercise- and nutrient-controlled mechanisms involved in maintenance of the musculoskeletal mass. Biochem. Soc. Trans. 2007, 35, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Wolfe, R.R. Is there a maximal anabolic response to protein intake with a meal? Clin. Nutr. 2013, 32, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Glynn, E.L.; Fry, C.S.; Drummond, M.J.; Dreyer, H.C.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R533–R540. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.J.; Tipton, K.D. Protein and amino acid metabolism during and after exercise and the effects of nutrition. Annu. Rev. Nutr. 2000, 20, 457–483. [Google Scholar] [CrossRef] [PubMed]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Bohe, J.; Low, A.; Wolfe, R.R.; Rennie, M.J. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: A dose-response study. J. Physiol. 2003, 552, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Kanda, A.; Tagawa, R.; Sanbongi, C.; Ikegami, S.; Itoh, H. Post-exercise muscle protein synthesis in rats after ingestion of acidified bovine milk compared with skim milk. Nutrients 2017, 9, 1071. [Google Scholar] [CrossRef] [PubMed]
- West, D.W.; Burd, N.A.; Coffey, V.G.; Baker, S.K.; Burke, L.M.; Hawley, J.A.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am. J. Clin. Nutr. 2011, 94, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Crombach, N.; Gijsen, A.P.; Walrand, S.; Fauquant, J.; Kies, A.K.; Lemosquet, S.; Saris, W.H.; Boirie, Y.; van Loon, L.J. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am. J. Clin. Nutr. 2009, 90, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrere, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [PubMed]
- Reitelseder, S.; Agergaard, J.; Doessing, S.; Helmark, I.C.; Lund, P.; Kristensen, N.B.; Frystyk, J.; Flyvbjerg, A.; Schjerling, P.; van Hall, G.; et al. Whey and casein labeled with l-[1-13c]leucine and muscle protein synthesis: Effect of resistance exercise and protein ingestion. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E231–E242. [Google Scholar] [CrossRef] [PubMed]
- Morifuji, M.; Ishizaka, M.; Baba, S.; Fukuda, K.; Matsumoto, H.; Koga, J.; Kanegae, M.; Higuchi, M. Comparison of different sources and degrees of hydrolysis of dietary protein: Effect on plasma amino acids, dipeptides, and insulin responses in human subjects. J. Agric. Food Chem. 2010, 58, 8788–8797. [Google Scholar] [CrossRef] [PubMed]
- Rerat, A.; Nunes, C.S.; Mendy, F.; Roger, L. Amino acid absorption and production of pancreatic hormones in non-anaesthetized pigs after duodenal infusions of a milk enzymic hydrolysate or of free amino acids. Br. J. Nutr. 1988, 60, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Monchi, M.; Rerat, A.A. Comparison of net protein utilization of milk protein mild enzymatic hydrolysates and free amino acid mixtures with a close pattern in the rat. JPEN J. Parenter. Enteral. Nutr. 1993, 17, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Kanda, A.; Nakayama, K.; Fukasawa, T.; Koga, J.; Kanegae, M.; Kawanaka, K.; Higuchi, M. Post-exercise whey protein hydrolysate supplementation induces a greater increase in muscle protein synthesis than its constituent amino acid content. Br. J. Nutr. 2013, 110, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.; Smith, K.; Babraj, J.; Leese, G.; Waddell, T.; Atherton, P.; Wackerhage, H.; Taylor, P.M.; Rennie, M.J. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005, 19, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Ferrando, A.A.; Phillips, S.M.; Doyle, D., Jr.; Wolfe, R.R. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am. J. Physiol. 1999, 276, E628–E634. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M. Methodology for determining degree of hydrolysis of proteins in hydrolysates: A review. J. AOAC Int. 2010, 93, 1515–1522. [Google Scholar] [PubMed]
- Cohen, S.A.; Michaud, D.P. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-n-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal. Biochem. 1993, 211, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Grimble, G.K. Mechanisms of peptide and amino acid transport and their regulation. Nestle Nutr. Workshop Ser. Clin. Perform. Programme 2000, 3, 63–84; discussion 84–68. [Google Scholar] [PubMed]
- Grimble, G.K. The significance of peptides in clinical nutrition. Annu. Rev. Nutr. 1994, 14, 419–447. [Google Scholar] [CrossRef] [PubMed]
- Adibi, S.A. Intestinal transport of dipeptides in man: Relative importance of hydrolysis and intact absorption. J. Clin. Investig. 1971, 50, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Adibi, S.A.; Morse, E.L.; Masilamani, S.S.; Amin, P.M. Evidence for two different modes of tripeptide disappearance in human intestine. Uptake by peptide carrier systems and hydrolysis by peptide hydrolases. J. Clin. Investig. 1975, 56, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.; Burrin, D.G. Measuring splanchnic amino acid metabolism in vivo using stable isotopic tracers. J. Anim. Sci. 2006, 84 (Suppl. 13), E60–E72. [Google Scholar] [CrossRef] [PubMed]
- Fouillet, H.; Gaudichon, C.; Bos, C.; Mariotti, F.; Tome, D. Contribution of plasma proteins to splanchnic and total anabolic utilization of dietary nitrogen in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E88–E97. [Google Scholar] [CrossRef] [PubMed]
- Manninen, A.H. Protein hydrolysates in sports nutrition. Nutr. Metab. 2009, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Power, O.; Hallihan, A.; Jakeman, P. Human insulinotropic response to oral ingestion of native and hydrolysed whey protein. Amino Acids 2009, 37, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Grimble, G.K.; Rees, R.G.; Keohane, P.P.; Cartwright, T.; Desreumaux, M.; Silk, D.B. Effect of peptide chain length on absorption of egg protein hydrolysates in the normal human jejunum. Gastroenterology 1987, 92, 136–142. [Google Scholar] [CrossRef]
- Farup, J.; Rahbek, S.K.; Storm, A.C.; Klitgaard, S.; Jorgensen, H.; Bibby, B.M.; Serena, A.; Vissing, K. Effect of degree of hydrolysis of whey protein on in vivo plasma amino acid appearance in humans. Springerplus 2016, 5, 382. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 2003, 78, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Garlick, P.J. The role of leucine in the regulation of protein metabolism. J. Nutr. 2005, 135, 1553s–1556s. [Google Scholar] [CrossRef] [PubMed]
- Crozier, S.J.; Kimball, S.R.; Emmert, S.W.; Anthony, J.C.; Jefferson, L.S. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J. Nutr. 2005, 135, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med. 2014, 44 (Suppl. 1), S71–S77. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Williams, B.D.; Fleming, R.Y.; Wolfe, R.R. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes 1999, 48, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R.; Stellingwerff, T.; Breuille, D.; Offord, E.A.; Baker, S.K.; et al. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: A double-blind, randomized trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef] [PubMed]
| Sequence | n | Period | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| 1 | 3 | A | B | C | D |
| 2 | 3 | B | D | A | C |
| 3 | 3 | C | A | D | B |
| 4 | 3 | D | C | B | A |
| WPH | EAA | |||
|---|---|---|---|---|
| Protein | 3.3 g | 5.0 g | 7.5 g | 2.5 g |
| Amino acid content (mmol) | ||||
| His | 0.5 | 0.8 | 1.2 | 1.2 |
| Ile | 1.3 | 2.0 | 3.0 | 2.0 |
| Leu | 3.0 | 4.5 | 6.8 | 4.1 |
| Lys | 2.3 | 3.5 | 5.3 | 3.0 |
| Met | 0.5 | 0.7 | 1.1 | 1.0 |
| Phe | 0.7 | 1.0 | 1.6 | 0.9 |
| Thr | 1.7 | 2.5 | 3.8 | 1.5 |
| Trp | 0.2 | 0.3 | 0.5 | 0.3 |
| Val | 1.5 | 2.2 | 3.3 | 2.2 |
| Arg | 0.6 | 0.8 | 1.3 | 2.0 |
| EAA | 12.3 | 18.4 | 27.8 | 18.2 |
| Other nutrients (g) | ||||
| Carbohydrates | 0.5 | 0.8 | 1.1 | 0.0 |
| Fat | 0.0 | 0.0 | 0.0 | 0.0 |
| Time after Ingestion | |||||||
|---|---|---|---|---|---|---|---|
| 10 min | 20 min | 30 min | 45 min | 60 min | 90 min | 120 min | |
| ΔGlucose (mg/dL) | |||||||
| WPH 7.5 g | 0.9 ± 0.7 | 2.9 ± 1.3 | 1.5 ± 1.1 | −2.2 ± 1.9 | −2.5 ± 1.0 | −0.4 ± 1.2 | 0.9 ± 1.0 |
| WPH 5.0 g | 1.8 ± 1.0 | 1.9 ± 1.4 | 1.7 ± 1.2 | −0.7 ± 1.8 | −0.4 ± 1.1 | 0.1 ± 1.0 | 0.4 ± 1.0 |
| WPH 3.3 g | 1.2 ± 0.8 | 0.3 ± 1.0 | −1.2 ± 1.4 | −2.3 ± 1.1 | −1.5 ± 1.1 | −1.1 ± 0.8 | −1.8 ± 0.8 |
| EAA | 2.5 ± 1.0 | 0.5 ± 1.2 | −0.3 ± 1.1 | −0.4 ± 1.2 | −0.5 ± 0.9 | −0.5 ± 0.8 | −0.1 ± 1.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, K.; Sanbongi, C.; Ikegami, S. Effects of Whey Protein Hydrolysate Ingestion on Postprandial Aminoacidemia Compared with a Free Amino Acid Mixture in Young Men. Nutrients 2018, 10, 507. https://doi.org/10.3390/nu10040507
Nakayama K, Sanbongi C, Ikegami S. Effects of Whey Protein Hydrolysate Ingestion on Postprandial Aminoacidemia Compared with a Free Amino Acid Mixture in Young Men. Nutrients. 2018; 10(4):507. https://doi.org/10.3390/nu10040507
Chicago/Turabian StyleNakayama, Kyosuke, Chiaki Sanbongi, and Shuji Ikegami. 2018. "Effects of Whey Protein Hydrolysate Ingestion on Postprandial Aminoacidemia Compared with a Free Amino Acid Mixture in Young Men" Nutrients 10, no. 4: 507. https://doi.org/10.3390/nu10040507




