Anti-Inflammatory Effects of Angelica sinensis (Oliv.) Diels Water Extract on RAW 264.7 Induced with Lipopolysaccharide
Abstract
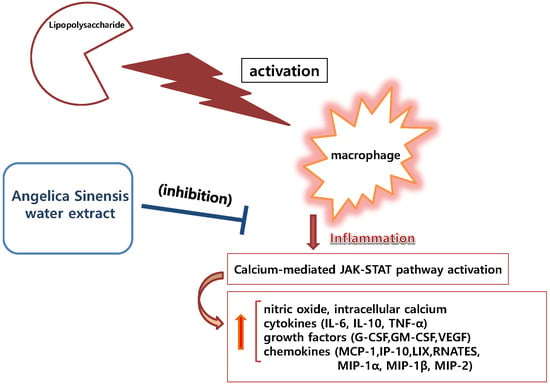
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of ASW
2.3. Total Flavonoid Content
2.4. Cell Viability
2.5. NO Concentration
2.6. Multiplex Cytokine Assay
2.7. Intracellular Calcium Assay
2.8. Real Time RT-PCR Assay
2.9. Statistical Analysis
3. Results
3.1. Determination of the Total Flavonoid Content of ASW
3.2. Effects of ASW on Cell Viability
3.3. Effects of ASW on NO Production
3.4. Effect of ASW on Cytokine Production
3.5. Effect of ASW on Intracellular Calcium Release
3.6. Effect of ASW on mRNA Expression
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NO | Nitric oxide |
| ASW | Angelica sinensis root water extract |
| LPS | Lipopolysaccharide |
| JAK | Janus kinase |
| STAT | Signal transducers and activators of transcription |
| RE | rutin equivalents |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| G-CSF | Granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte macrophage colony-stimulating factor |
| LIX | Lipopolysaccharide-induced CXC chemokine |
| MCP | Monocyte chemotactic activating factor |
| MIP | Macrophage inflammatory protein |
| RANTES | Regulated on activation, normal T cell expressed and secreted |
| VEGF | Vascular endothelial growth factor |
| IP-10 | Interferon gamma-induced protein 10 |
| LIF | Leukemia inhibitory factor |
| CHOP | C/EBP homologous protein |
| FAS | First apoptosis signal receptor |
| NOS2 | Nitric oxide synthase 2 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 |
| ER | Endoplasmic reticulum |
| KC | Keratinocyte-derived chemokine |
References
- Hu, Z.; Murakami, T.; Suzuki, K.; Tamura, H.; Kuwahara-Arai, K.; Iba, T.; Nagaoka, I. Antimicrobial Cathelicidin Peptide LL-37 Inhibits the LPS/ATP-Induced Pyroptosis of Macrophages by Dual Mechanism. PLoS ONE 2014, 9, e85765. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.W.; Hong, Y.H.; Chen, M.L.; Lin, B.F. Inhibitory Effects of Angelica Sinensis Ethyl Acetate Extract and Major Compounds on NF-kappaB Trans-Activation Activity and LPS-Induced Inflammation. J. Ethnopharmacol. 2010, 129, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Vlase, L.; Raita, O.; Hanganu, D.; Păltinean, R.; Dezsi, Ş.; Gheldiu, A.; Oprean, R.; Crişan, G. Comparative Studies on Antioxidant Activity and Polyphenolic Content of Lycium Barbarum L. and Lycium Chinense Mill, Leaves. Pak. J. Pharm. Sci. 2015, 28, 1511–1515. [Google Scholar] [PubMed]
- Lee, J.Y.; Park, W. Anti-Inflammatory Effect of Wogonin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Molecules 2015, 20, 6888–6900. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, W. Anti-Inflammatory Effect of Quercetin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Molecules 2016, 21, 450. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, W. Anti-Inflammatory Effect of Myristicin on RAW 264.7 Macrophages Stimulated with Polyinosinic-Polycytidylic Acid. Molecules 2011, 16, 7132–7142. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, W. Anti-Inflammatory Effect of Chrysin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Biotechnol. Bioprocess Eng. 2015, 20, 1026–1034. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, J.Y.; Han, H.S.; Kim, Y.J.; Kim, H.J.; Kim, Y.S.; Kim, H.M.; Ko, S.G.; An, H.J.; Lee, Y.J.; et al. Immunomodulatory Effects of Liriope Platyphylla Water Extract on Lipopolysaccharide-Activated Mouse Macrophage. Nutrients 2012, 4, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, W. Anti-inflammatory effects of oroxylin A on RAW 264.7 mouse macrophages induced with polyinosinic-polycytidylic acid. Exp. Ther. Med. 2016, 12, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, W. Evidence-guided optimization of herbal formula Cheong-Hwa-Bo-Um-Tang using multiplex cytokine profiling. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 72–80. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, Y.; Park, S.K.; Kim, H.; Bae, H.; Kim, H.M.; Ko, S.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-Inflammatory Effects of Scutellaria Baicalensis Water Extract on LPS-Activated RAW 264.7 Macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Yuk, S.; Lim, E.; Lee, J.Y.; Lee, Y.; Kim, Y.; Lee, T.H.; Park, S.K.; Bae, H.; Kim, H.M.; Ko, S. Antiinflammatory Effects of Epimedium Brevicornum Water Extract on lipopolysaccharide-activated RAW264. 7 Macrophages. Phytother. Res. 2010, 24, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.W.; Chiou, W.F.; Chao, S.H.; Lee, M.H.; Chen, C.C.; Tsai, Y.C. Ligustilide Prevents LPS-Induced iNOS Expression in RAW 264.7 Macrophages by Preventing ROS Production and Down-Regulating the MAPK, NF-kappaB and AP-1 Signaling Pathways. Int. Immunopharmacol. 2011, 11, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Mori, M.; Akira, S.; Gotoh, T. C/EBP Homologous Protein (CHOP) is Crucial for the Induction of Caspase-11 and the Pathogenesis of Lipopolysaccharide-Induced Inflammation. J. Immunol. 2006, 176, 6245–6253. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, T.; Terada, K.; Oyadomari, S.; Mori, M. Hsp70-DnaJ Chaperone Pair Prevents Nitric Oxide-and CHOP-Induced Apoptosis by Inhibiting Translocation of Bax to Mitochondria. Cell Death Differ. 2004, 11, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Seimon, T.; Timmins, J.; Li, G.; Lim, W. Macrophage Apoptosis in Advanced Atherosclerosis. Ann. N. Y. Acad. Sci. 2009, 1173, E40–E45. [Google Scholar] [CrossRef] [PubMed]
- Timmins, J.M.; Ozcan, L.; Seimon, T.A.; Li, G.; Malagelada, C.; Backs, J.; Backs, T.; Bassel-Duby, R.; Olson, E.N.; Anderson, M.E.; et al. Calcium/calmodulin-Dependent Protein Kinase II Links ER Stress with Fas and Mitochondrial Apoptosis Pathways. J. Clin. Investig. 2009, 119, 2925–2941. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Q.; Ihsan, A.; Huang, L.; Dai, M.; Hao, H.; Cheng, G.; Liu, Z.; Wang, Y.; Yuan, Z. JAK/STAT Pathway Plays a Critical Role in the Proinflammatory Gene Expression and Apoptosis of RAW264.7 Cells Induced by Trichothecenes as DON and T-2 Toxin. Toxicol. Sci. 2012, 127, 412–424. [Google Scholar] [CrossRef] [PubMed]






| Name 1 | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| STAT1 | TGAGATGTCCCGGATAGTGG | CGCCAGAGAGAAATTCGTGT |
| STAT3 | GTCTGCAGAGT TCAAGCACCT | TCCTCAGTCACGATCAAGGAG |
| CHOP | CGCTGTTTTCCCTTGCTG | TCCTCATACCAGGCTTCCA |
| JAK2 | TTGGTTTTGAATTATGGTGTCTGT | TCCAAATTTTACAAATTCTTGAACC |
| FAS | CGCTGTTTTCCCTTGCTG | CCTTGAGTATGAACTCTTAACTGTGAG |
| c-Fos | AGAGCGGGAATGGTGAAGA | TCTTCCTCTTCAGGAGATAGCTG |
| NOS2 | TGGAGGTTCTGGATGAGAGC | AATGTCCAGGAAGTAGGTGAGG |
| PTGS2 | TCAAACAGTTTCTCTACAACAACTCC | ACATTTCTTCCCCCAGCAA |
| β-Actin | CTAAGGCCAACCGTGAAAAG | ACCAGAGGCATACAGGGACA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-J.; Lee, J.Y.; Kim, H.-J.; Kim, D.-H.; Lee, T.H.; Kang, M.S.; Park, W. Anti-Inflammatory Effects of Angelica sinensis (Oliv.) Diels Water Extract on RAW 264.7 Induced with Lipopolysaccharide. Nutrients 2018, 10, 647. https://doi.org/10.3390/nu10050647
Kim Y-J, Lee JY, Kim H-J, Kim D-H, Lee TH, Kang MS, Park W. Anti-Inflammatory Effects of Angelica sinensis (Oliv.) Diels Water Extract on RAW 264.7 Induced with Lipopolysaccharide. Nutrients. 2018; 10(5):647. https://doi.org/10.3390/nu10050647
Chicago/Turabian StyleKim, Young-Jin, Ji Young Lee, Hyun-Ju Kim, Do-Hoon Kim, Tae Hee Lee, Mi Suk Kang, and Wansu Park. 2018. "Anti-Inflammatory Effects of Angelica sinensis (Oliv.) Diels Water Extract on RAW 264.7 Induced with Lipopolysaccharide" Nutrients 10, no. 5: 647. https://doi.org/10.3390/nu10050647