Choline Supplementation Prevents a Hallmark Disturbance of Kwashiorkor in Weanling Mice Fed a Maize Vegetable Diet: Hepatic Steatosis of Undernutrition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Murine Diet
2.2. Animals
2.3. Animal Protocol
2.4. Food Intake
2.5. Body Composition
2.6. Metabolite Concentrations
2.7. mRNA Quantification by Quantitative Reverse-Transcriptase (RT) PCR
2.8. Statistical Analyses
3. Results
3.1. Comparison of Study Diets
3.2. Weight Gain and Feed Intake
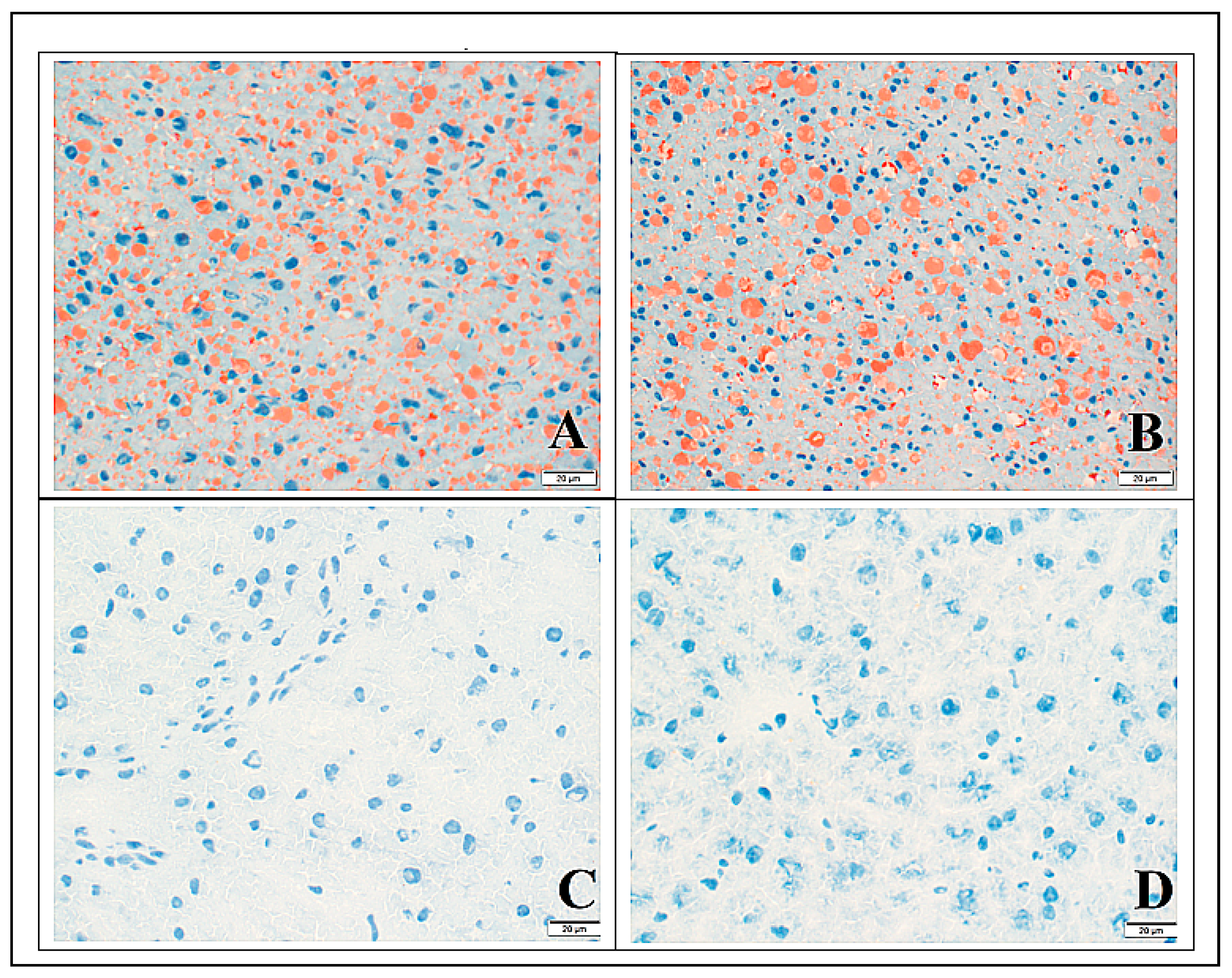
3.3. Histology
3.4. Liver Metabolites
3.5. Transcriptional Targets
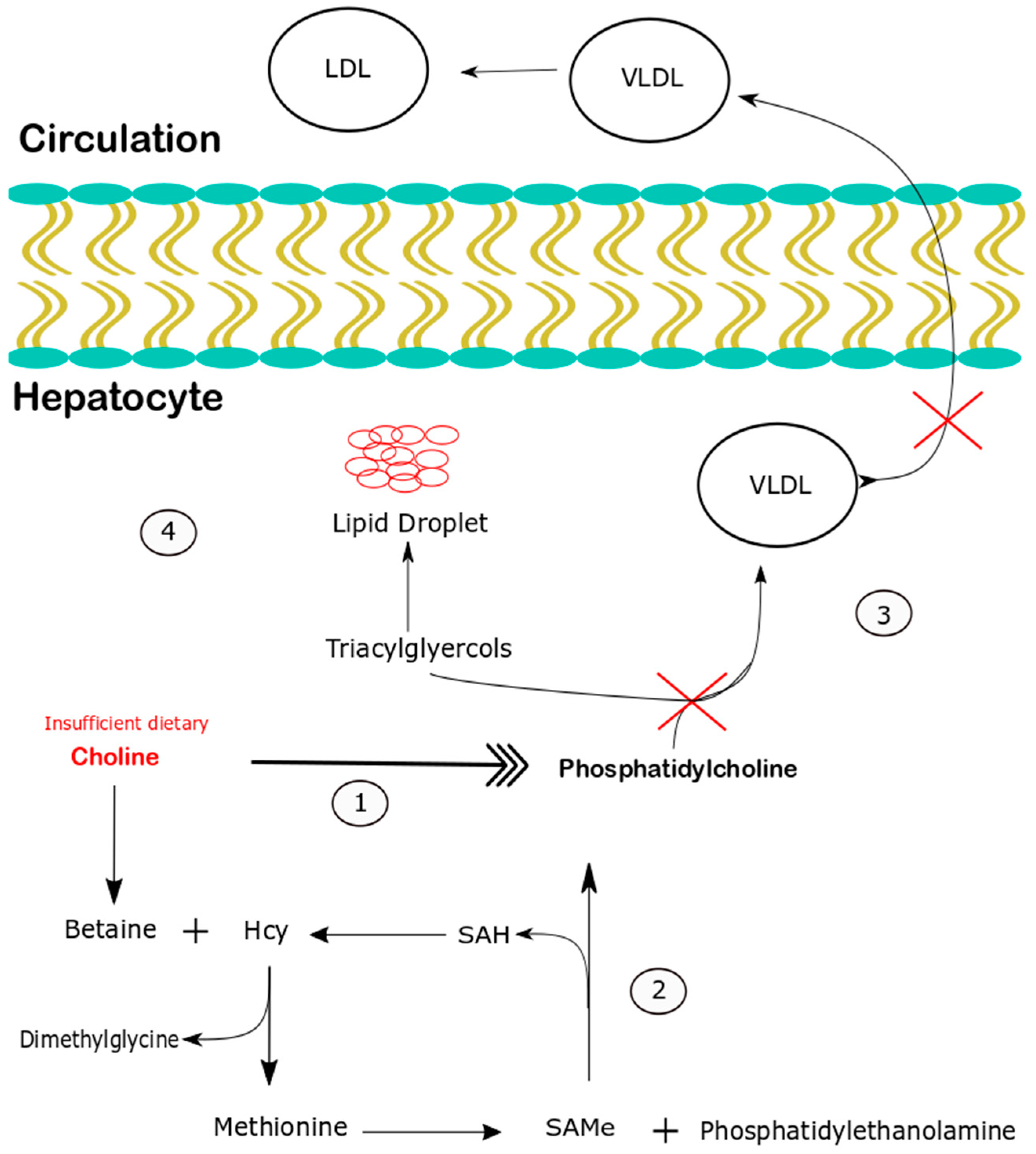
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Munthali, T.; Jacobs, C.; Sitali, L.; Dambe, R.; Michelo, C. Mortality and morbidity patterns in under-five children with severe acute malnutrition (SAM) in Zambia: A five-year retrospective review of hospital-based records (2009–2013). Arch. Public Health 2015, 73, 23. [Google Scholar] [CrossRef] [PubMed]
- Briend, A. Kwashiorkor: Still an Enigma–The Search Must Go on. In CMAM Forum Technical Brief; CMAM Forum: Copenhagen, Denmark, 2014. [Google Scholar]
- Davies, J.N.P. The essential pathology of kwashiorkor. Lancet 1948, 1, 317–320. [Google Scholar] [CrossRef]
- Davies, J.N. The pathology of dietary liver disease in tropical Africa. Ann. N. Y. Acad. Sci. 1954, 57, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Waterlow, J.C. Fatty liver disease in infants in the British West Indies. Med. Res. Counc. Spec. Rep. Ser. (Lond.) 1948, 263, 346–354. [Google Scholar]
- Truswell, A.S.; Hansen, J.D.L.; Watson, C.E.; Wannenburg, P. Relation of Serum Lipids and Lipoproteins to Fatty Liver in Kwashiorkor. Am. J. Clin. Nutr. 1969, 22, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Badaloo, A.; Reid, M.; Soares, D.; Forrester, T.; Jahoor, F. Relation between liver fat content and the rate of VLDL apolipoprotein B-100 synthesis in children with protein-energy malnutrition. Am. J. Clin. Nutr. 2005, 81, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Manary, M.J.; Heikens, G.T.; Golden, M. Kwashiorkor: More hypothesis testing is needed to understand the aetiology of oedema. Malawi Med. J. 2009, 21, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Keusch, G.T.; Denno, D.M.; Black, R.E.; Duggan, C.; Guerrant, R.L.; Lavery, J.V.; Nataro, J.P.; Rosenberg, I.H.; Ryan, E.T.; Tarr, P.I.; et al. Environmental Enteric Dysfunction: Pathogenesis, Diagnosis, and Clinical Consequences. Clin. Infect. Dis. 2014, 59, S207–S212. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Mar, M.-H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.M.; Kies, C.V. Influence of sulfur-amino acid content variation in plant vs. animal protein on serum and tissue lipids in rats. Plant Foods Hum. Nutr. Dordr. Neth. 1990, 40, 297–308. [Google Scholar] [CrossRef]
- Sullivan, J.; Ndekha, M.; Maker, D.; Hotz, C.; Manary, M.J. The quality of the diet in Malawian children with kwashiorkor and marasmus. Matern. Child. Nutr. 2006, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Veteläinen, R.; Van Vliet, A.; Van Gulik, T.M. Essential pathogenic and metabolic differences in steatosis induced by choline or methione-choline deficient diets in a rat model. J. Gastroenterol. Hepatol. 2007, 22, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.L.; Dubin, M.D.; Moukarzel, A.A.; Jenden, D.J.; Roch, M.; Rice, K.M.; Gornbein, J.; Ament, M.E. Choline deficiency: A cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology 1995, 22, 1399–1403. [Google Scholar] [PubMed]
- Yao, Z.M.; Vance, D.E. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J. Biol. Chem. 1988, 263, 2998–3004. [Google Scholar] [PubMed]
- Kennedy, E.P.; Weiss, S.B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J. Biol. Chem. 1956, 222, 193–214. [Google Scholar] [PubMed]
- Chew, T.W.; Jiang, X.; Yan, J.; Wang, W.; Lusa, A.L.; Carrier, B.J.; West, A.A.; Malysheva, O.V.; Brenna, J.T.; Gregory, J.F., 3rd. Folate intake, MTHFR genotype, and sex modulate choline metabolism in mice. J. Nutr. 2011, 141, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Vance, D.E.; Ridgway, N.D. The methylation of phosphatidylethanolamine. Prog. Lipid Res. 1988, 27, 61–79. [Google Scholar] [CrossRef]
- Vermeulen, P.S.; Lingrell, S.; Yao, Z.; Vance, D.E. Phosphatidylcholine biosynthesis is required for secretion of truncated apolipoprotein Bs from McArdle RH7777 cells only when a neutral lipid core is formed. J. Lipid Res. 1997, 38, 447–458. [Google Scholar] [PubMed]
- Melse-Boonstra, A.; Rozendaal, M.; Rexwinkel, H.; Gerichhausen, M.J.; van den Briel, T.; Bulux, J.; Solomons, N.W.; West, C.E. Determination of discretionary salt intake in rural Guatemala and Benin to determine the iodine fortification of salt required to control iodine deficiency disorders: Studies using lithium-labeled salt. Am. J. Clin. Nutr. 1998, 68, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Badoe, E.O.; Appeadu-Mensah, W.; Hesse, A.; Maddy, S.O. The daily water, sodium and potassium excretion in urine of Ghanaian children aged 5 to 12 years. West Afr. J. Med. 2005, 24, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Fiorotto, M.L.; Davis, T.A.; Sosa, H.A.; Villegas-Montoya, C.; Estrada, I.; Fleischmann, R. Ribosome abundance regulates the recovery of skeletal muscle protein mass upon recuperation from postnatal undernutrition in mice. J. Physiol. 2014, 592, 5269–5286. [Google Scholar] [CrossRef] [PubMed]
- Best, C.H.; Channon, H.J. The action of choline and other substances in the prevention and cure of fatty livers. Biochem. J. 1935, 29, 2651–2658. [Google Scholar] [CrossRef] [PubMed]
- Engel, R.W. Anemia and edema of chronic choline deficiency in the rat. J. Nutr. 1948, 36, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.A. EDF Statistics for Goodness of Fit and Some Comparisons. J. Am. Stat. Assoc. 1974, 69, 730–737. [Google Scholar] [CrossRef]
- Brown, M.B.; Forsythe, A.B. Robust Tests for the Equality of Variances. J. Am. Stat. Assoc. 1974, 69, 364–367. [Google Scholar] [CrossRef]
- Welch, B.L. On the Comparison of Several Mean Values: An Alternative Approach. Biometrika 1951, 38, 330–336. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Salvi, F.; Gadda, G. Human choline dehydrogenase: Medical promises and biochemical challenges. Arch. Biochem. Biophys. 2013, 537, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lin, L.; Qin, G.; Lu, X.; Fiorotto, M.; Dixit, V.D.; Sun, Y. Ablations of ghrelin and ghrelin receptor exhibit differential metabolic phenotypes and thermogenic capacity during aging. PLoS ONE 2011, 6, e16391. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.I.; Ueland, P.M.; Kvalheim, G.; Lien, E.A. Determination of Choline, Betaine, and Dimethylglycine in Plasma by a High-Throughput Method Based on Normal-Phase Chromatography–Tandem Mass Spectrometry. Clin. Chem. 2003, 49, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Oyebode, O.; Oti, S.; Chen, Y.-F.; Lilford, R.J. Salt intakes in sub-Saharan Africa: A systematic review and meta-regression. Popul. Health Metr. 2016, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Golden, M.H. The consequences of protein deficiency in man and its relationship to the features of kwashiorkor. Nutr. Adapt. Man. 1985, 169–185. [Google Scholar] [CrossRef]
- Lobe, S.L.; Bernstein, M.C.; German, R.Z. Life-long protein malnutrition in the rat (Rattus norvegicus) results in altered patterns of craniofacial growth and smaller individuals. J. Anat. 2006, 208, 795–812. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.P.; Kelly, K.B.; Lewis, E.D.; Leonard, K.-A.; Goruk, S.; Curtis, J.M.; Vine, D.F.; Proctor, S.D.; Field, C.J.; Jacobs, R.L. Choline deficiency impairs intestinal lipid metabolism in the lactating rat. J. Nutr. Biochem. 2015, 26, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.M.; Zhu, X.; Zeisel, S.H. Phosphatidylethanolamine-N-methyltransferase activity and dietary choline regulate liver-plasma lipid flux and essential fatty acid metabolism in mice. J. Nutr. 2003, 133, 3386–3391. [Google Scholar] [CrossRef] [PubMed]
- Al Rajabi, A.; Castro, G.S.F.; da Silva, R.P.; Nelson, R.C.; Thiesen, A.; Vannucchi, H.; Vine, D.F.; Proctor, S.D.; Field, C.J.; Curtis, J.M.; et al. Choline Supplementation Protects against Liver Damage by Normalizing Cholesterol Metabolism in Pemt/Ldlr Knockout Mice Fed a High-Fat Diet. J. Nutr. 2014, 144, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Hongu, N.; Mynatt, R.L.; Sachan, D.S. Choline supplementation increases tissue concentrations of carnitine and lowers body fat in guinea pigs. J. Nutr. Biochem. 1998, 9, 464–470. [Google Scholar] [CrossRef]
- Dahlhoff, C.; Worsch, S.; Sailer, M.; Hummel, B.A.; Fiamoncini, J.; Uebel, K.; Obeid, R.; Scherling, C.; Geisel, J.; Bader, B.L.; et al. Methyl-donor supplementation in obese mice prevents the progression of NAFLD, activates AMPK and decreases acyl-carnitine levels. Mol. Metab. 2014, 3, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Lykke, M.; Hother, A.-L.; Hansen, C.F.; Friis, H.; Mølgaard, C.; Michaelsen, K.F.; Briend, A.; Larsen, T.; Sangild, P.T.; Thymann, T. Malnutrition induces gut atrophy and increases hepatic fat infiltration: Studies in a pig model of childhood malnutrition. Am. J. Transl. Res. 2013, 5, 543–554. [Google Scholar] [PubMed]
- Noga, A.A.; Zhao, Y.; Vance, D.E. An Unexpected Requirement for PhosphatidylethanolamineN-Methyltransferase in the Secretion of Very Low Density Lipoproteins. J. Biol. Chem. 2002, 277, 42358–42365. [Google Scholar] [CrossRef] [PubMed]
- Noga, A.A.; Vance, D.E. Insights into the requirement of phosphatidylcholine synthesis for liver function in mice. J. Lipid Res. 2003, 44, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Miller, J.W.; da Costa, K.A.; Nadeau, M.; Smith, D.; Selhub, J.; Zeisel, S.H.; Mason, J.B. Severe folate deficiency causes secondary depletion of choline and phosphocholine in rat liver. J. Nutr. 1994, 124, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Caballero, F.; Fernández, A.; Matías, N.; Martínez, L.; Fucho, R.; Elena, M.; Caballeria, J.; Morales, A.; Fernández-Checa, J.C.; García-Ruiz, C. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis: Impact on mitochondrial S-adenosyl-l-methionine and glutathione. J. Biol. Chem. 2010, 285, 18528–18536. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Suzuki, S.; Sato, Y.; Itoh, T.; Umegaki, K. Evaluation of Methionine Content in a High-Fat and Choline-Deficient Diet on Body Weight Gain and the Development of Non-Alcoholic Steatohepatitis in Mice. PLoS ONE 2016, 11, e0164191. [Google Scholar] [CrossRef] [PubMed]
- Wilson, F.A.; Holtrop, G.; Calder, A.G.; Anderson, S.E.; Lobley, G.E.; Rees, W.D. Effects of methyl-deficient diets on methionine and homocysteine metabolism in the pregnant rat. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1531–E1540. [Google Scholar] [CrossRef] [PubMed]



| Food Ingredient | Content (g/kg) |
|---|---|
| Maize meal | 300 |
| Peeled onions | 105 |
| Mustard greens | 135 |
| Turnip greens | 135 |
| Mangos | 90 |
| Bananas | 65 |
| Non-iodized salt | 15 |
| Raw cane sugar | 155 |
| Maize Vegetable Diet | Control Chow | MVD% of Control Chow | |
|---|---|---|---|
| Metabolizable Energy, kcal/kg | 3693 | 3683 | 100.3 |
| Protein, g/kg | 64 | 183 | 35.0 |
| Carbohydrate, g/kg | 784 | 581 | 134.9 |
| Fat, g/kg | 34 | 71 | 47.9 |
| Fiber, g/kg | 15 | 28 | 53.6 |
| Ash, g/kg | 40 | 41 | 97.6 |
| Moisture, g/kg | 64 | 97 | 66.0 |
| One Carbon Micronutrients and Sulfur Amino Acids | |||
| Choline, mg/kg | 489 | 1620 | 30.2 |
| Betaine, mg/kg | 240 | 900 | 26.7 |
| Folic Acid, mg/kg | 0.15 | 2.46 | 6.1 |
| Pyridoxine, mg/kg | 2.46 | 11.9 | 20.7 |
| Cysteine, mg/kg | 1150 | 3700 | 31.1 |
| Methionine, mg/kg | 1020 | 4600 | 22.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
May, T.; Klatt, K.C.; Smith, J.; Castro, E.; Manary, M.; Caudill, M.A.; Jahoor, F.; Fiorotto, M.L. Choline Supplementation Prevents a Hallmark Disturbance of Kwashiorkor in Weanling Mice Fed a Maize Vegetable Diet: Hepatic Steatosis of Undernutrition. Nutrients 2018, 10, 653. https://doi.org/10.3390/nu10050653
May T, Klatt KC, Smith J, Castro E, Manary M, Caudill MA, Jahoor F, Fiorotto ML. Choline Supplementation Prevents a Hallmark Disturbance of Kwashiorkor in Weanling Mice Fed a Maize Vegetable Diet: Hepatic Steatosis of Undernutrition. Nutrients. 2018; 10(5):653. https://doi.org/10.3390/nu10050653
Chicago/Turabian StyleMay, Thaddaeus, Kevin C. Klatt, Jacob Smith, Eumenia Castro, Mark Manary, Marie A. Caudill, Farook Jahoor, and Marta L. Fiorotto. 2018. "Choline Supplementation Prevents a Hallmark Disturbance of Kwashiorkor in Weanling Mice Fed a Maize Vegetable Diet: Hepatic Steatosis of Undernutrition" Nutrients 10, no. 5: 653. https://doi.org/10.3390/nu10050653





