Main Human Urinary Metabolites after Genipap (Genipa americana L.) Juice Intake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Subject Recruitment
2.2. Juice Preparation
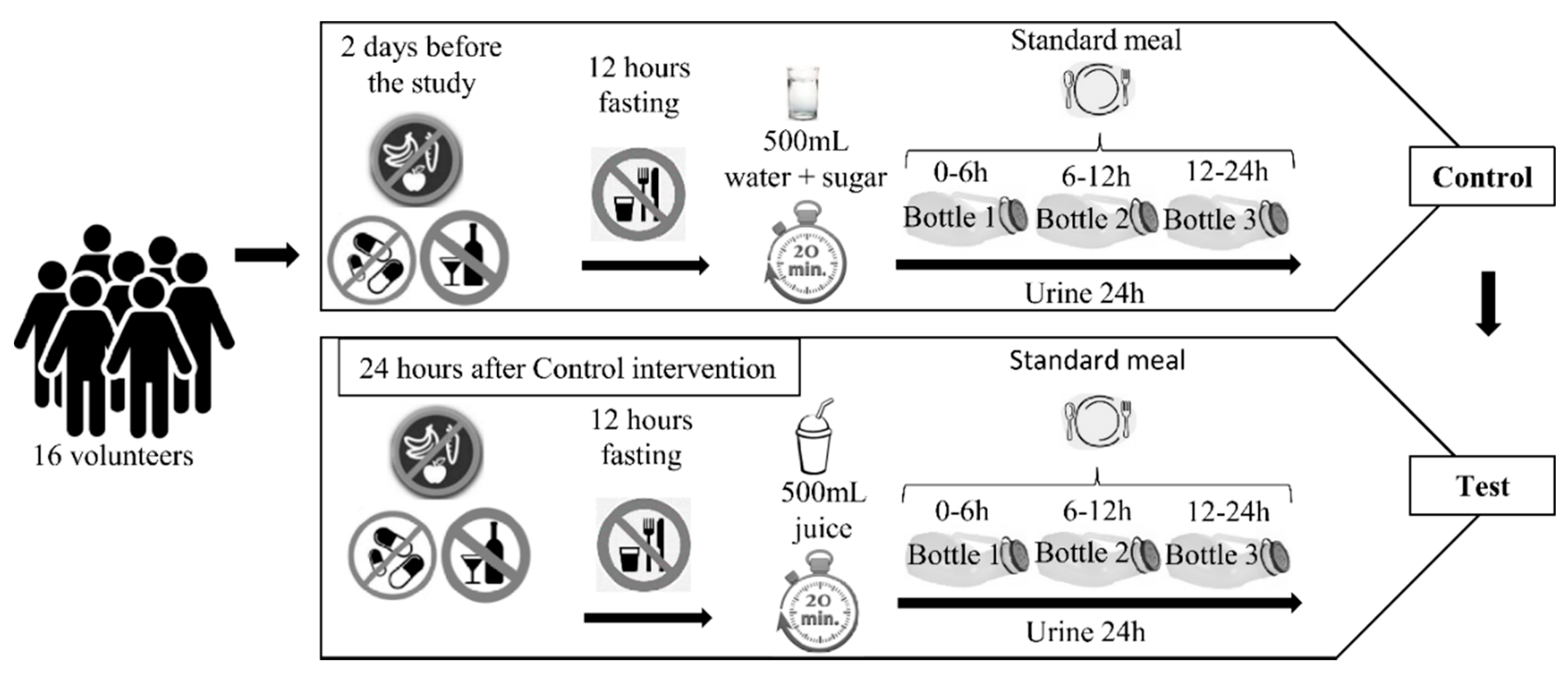
2.3. Study Design
2.4. Sample Preparation
2.5. UHPLC-HESI-Orbitrap-MS Analysis
2.6. Preprocessing and Pretreatment of Data
2.7. Data Analysis and Multi-Metabolite Biomarker Model
2.8. Annotation, Identification, and Interpretation
3. Results
3.1. Data Treatment and Analysis
3.2. Annotation and Tentative Identification
3.3. Calculation and Validation of a Multiplex Biomarker of Genipap Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Athersuch, T. Metabolome analyses in exposome studies: Profiling methods for a vast chemical space. Arch. Biochem. Biophys. 2016, 589, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P. The exposome: From concept to utility. Int. J. Epidemiol. 2012, 41, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Dragsted, L.O.; Gao, Q.; Praticò, G.; Manach, C.; Wishart, D.S.; Scalbert, A.; Feskens, E.J.M. Dietary and health biomarkers—Time for an update. Genes Nutr. 2017, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M. Biomarkers intersect with the exposome. Biomarkers 2012, 17, 483–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bando, K.; Kawahara, R.; Kunimatsu, T.; Sakai, J.; Kimura, J.; Funabashi, H.; Seki, T.; Bamba, T.; Fukusaki, E. Influences of biofluid sample collection and handling procedures on gc–ms based metabolomic studies. J. Biosci. Bioeng. 2010, 110, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Saude, E.J.; Sykes, B.D. Urine stability for metabolomic studies: Effects of preparation and storage. Metabolomics 2007, 3, 19–27. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics: Applications to food science and nutrition research. Trends Food Sci. Technol. 2008, 19, 482–493. [Google Scholar] [CrossRef]
- Martins, D.; Nunez, C. Secondary Metabolites from Rubiaceae Species. Molecules 2015, 20, 13422–13495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Figueiredo, R.W.; Maia, G.A.; de Holanda, L.F.F.; Monteiro, J.C.S. Características físicas e químicas do jenipapo. Pesquisa Agropecuária Brasileira 1986, 21, 421–428. [Google Scholar]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Anthony, S. Genipa Americana. In Agroforestree Database: A Tree Reference and Selection Guide Version 4.0; World Agroforestry Centre: Nairobi, Kenya, 2009. [Google Scholar]
- Zappi, D. GENIPA L. Flora Fanerogâmica do Estado de São Paulo; Instituto de Botânica: São Paulo, Brazil, 2007; Volume 5, pp. 344–345. [Google Scholar]
- Da Conceição, A.O.; Rossi, M.H.; de Oliveira, F.F.; Takser, L.; Lafond, J. Genipa americana (Rubiaceae) fruit extract affects mitogen-activated protein kinase cell pathways in human trophoblast–derived BeWo cells: Implications for placental development. J. Med. Food 2011, 14, 483–494. [Google Scholar]
- Morton, J. Fruits of Warm Climates; Creative Resource Systems: Miami, FL, USA, 1987. [Google Scholar]
- De Sousa Bentes, A.; Mercadante, A.Z. Influence of the Stage of Ripeness on the Composition of Iridoids and Phenolic Compounds in Genipap (Genipa americana L.). J. Agric. Food Chem. 2014, 62, 10800–10808. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Ming, L.C. Chemistry and pharmacology of some plants mentioned in the letter of Pero Vaz de Caminha. Etnobiol. Conserv. 2015, 4, 1–15. [Google Scholar] [CrossRef]
- Mitra, M. Gray Special Feature. Asia Pac. Biotech. News 2007, 11, 689–743. [Google Scholar] [CrossRef]
- Revilla, J. Apontamentos para a Cosmética Amazônica/Juan Revilla, 2nd ed.; INPA, Instituto Nacional de Pesquisas da Amazônia: Manaus, Brazil, 2002. [Google Scholar]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Statti, G.A.; Menichini, F. Biological and pharmacological activities of iridoids: Recent developments. Mini Rev. Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Ishimatsu, N.; Masuoka, C.; Yoshimitsu, H.; Tsuchihashi, R.; Okawa, M.; Kinjo, J.; Ikeda, T.; Nohara, T. Three new monoterpenoids from the fruit of Genipa americana. Chem. Pharm. Bull. 2007, 55, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Ueno, M.; Masuoka, C.; Ikeda, T.; Nohara, T. Iridoid glucosides from the fruit of Genipa americana. Chem. Pharm. Bull. 2005, 53, 1342–1344. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Shen, H.; Tang, F.R.; Arfuso, F.; Rajesh, M.; Wang, L.; Kumar, A.P.; Bian, J.; Goh, B.C.; Bishayee, A.; et al. Potential role of genipin in cancer therapy. Pharmacol. Res. 2018, 133, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, T.-B.; Jia, D.-H.; Sun, W.-Q.; Wang, C.-L.; Gu, A.-Z.; Yang, X.-M. Genipin inhibits the growth of human bladder cancer cells via inactivation of PI3K/Akt signaling. Oncol. Lett. 2018, 15, 2619–2624. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yin, F.; Guo, L.; Deng, X.; Hu, Y. Neuroprotection of geniposide against hydrogen peroxide induced PC12 cells injury: Involvement of PI3 kinase signal pathway. Acta Pharmacol. Sin. 2009, 30, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Lee, M.; Ryu, E.; Moon, A.; Jeong, C.-S.; Jung, Y.W.; Park, G.H.; Sung, G.-H.; Cho, H.; Kang, H. Genipin as a novel chemical activator of EBV lytic cycle. J. Microbiol. 2015, 53, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Hwa, J.S.; Mun, L.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Kwak, J.H.; Lee, D.-U.; Chang, K.C. Genipin selectively inhibits TNF-α-activated VCAM-1 but not ICAM-1 expression by upregulation of PPAR-γ in human endothelial cells. Korean J. Physiol. Pharmacol. 2011, 15, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.; Mncwangi, N.; Vermaak, I. Anti-Inflammatory Iridoids of Botanical Origin. Curr. Med. Chem. 2012, 19, 2104–2127. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.-J.; Song, Y.S.; Kim, H.-J.; Lee, Y.-H.; Hong, S.-M.; Kim, S.-J.; Kim, B.-C.; Jin, C.; Lim, C.-J.; Park, E.-H. Antiinflammatory effects of genipin, an active principle of gardenia. Eur. J. Pharmacol. 2004, 495, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Mikami Masaki; Takikawa Hajime Effect of genipin on the biliary excretion of cholephilic compounds in rats. Hepatol. Res. 2008, 38, 614–621. [CrossRef] [PubMed]
- Wu, S.; Wang, G.; Liu, Z.; Rao, J.; Lü, L.; Xu, W.; Wu, S.; Zhang, J. Effect of geniposide, a hypoglycemic glucoside, on hepatic regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Acta Pharmacol. Sin. 2009, 30, 202–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Kessner, D.; Chambers, M.; Burke, R.; Agus, D.; Mallick, P. ProteoWizard: Open source software for rapid proteomics tools development. Bioinformatics 2008, 24, 2534–2536. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Using metaboanalyst 3.0 for comprehensive metabolomics data analysis. In Current Protocols in Bioinformatics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Aidoud, N.; Delplanque, B.; Baudry, C.; Garcia, C.; Moyon, A.; Balasse, L.; Guillet, B.; Antona, C.; Darmaun, D.; Fraser, K.; et al. A combination of lipidomics, MS imaging, and PET scan imaging reveals differences in cerebral activity in rat pups according to the lipid quality of infant formulas. FASEB J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afgan, E.; Baker, D.; van den Beek, M.; Blankenberg, D.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Eberhard, C.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 2016, 44, W3–W10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN: A metabolite mass spectral database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Ruttkies, C.; Schymanski, E.L.; Wolf, S.; Hollender, J.; Neumann, S. MetFrag relaunched: Incorporating strategies beyond in silico fragmentation. J. Cheminformatics 2016, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Gerlich, M.; Neumann, S. MetFusion: Integration of compound identification strategies. J. Mass Spectrom 2013, 48. [Google Scholar] [CrossRef] [PubMed]
- Chagoyen, M.; Pazos, F. Tools for the functional interpretation of metabolomic experiments. Brief. Bioinform. 2013, 14, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.; Billington, R.; Ferrer, L.; Foerster, H.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016, 44, D471–D480. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Broadhurst, D.I.; Wilson, M.; Wishart, D.S. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics 2013, 9, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Akao, T.; Kobashi, K.; Aburada, M. Enzymic studies on the animal and intestinal bacterial metabolism of geniposide. Biol. Pharm. Bull. 1994, 17, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Feng, Q.; Xu, W.; Li, X.; Kang, Z.; Ren, Y.; Du, L. Genipin induced apoptosis associated with activation of the c-Jun NH2-terminal kinase and p53 protein in hela cells. Biol. Pharm. Bull. 2010, 33, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.N.; Choi, Y.-S.; Jung, H.-J.; Park, G.H.; Park, J.-M.; Moon, S.-K.; Cho, K.-H.; Kang, C.; Kang, I.; Oh, M.S.; et al. Genipin inhibits the inflammatory response of rat brain microglial cells. Int. Immunopharmacol. 2010, 10, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Miyagoshi, M.; Amagaya, S.; Ogihara, Y. Choleretic Actions of Iridoid Compounds. J. Pharmacobiodyn. 1988, 11, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Tallent, W.H. Two new antibiotic cyclopentanoid monoterpenes of plant origin. Tetrahedron 1964, 20, 1781–1787. [Google Scholar] [CrossRef]
- Ueda, S.; Iwahashi, Y.; Tokuda, H. Production of Anti-Tumor-Promoting Iridoid Glucosides in Genipa americana and Its Cell Cultures. J. Nat. Prod. 1991, 54, 1677–1680. [Google Scholar] [CrossRef] [PubMed]
- Abadio Finco, F.D.B.; Böser, S.; Graeve, L. Antiproliferative activity of bacaba (Oenocarpus bacaba) and jenipapo (Genipa americana L.) phenolic extracts: A comparison of assays. Nutr. Food Sci. 2013, 43, 98–106. [Google Scholar] [CrossRef]
- Baeza, G.; Sarriá, B.; Mateos, R.; Bravo, L. Dihydrocaffeic acid, a major microbial metabolite of chlorogenic acids, shows similar protective effect than a yerba mate phenolic extract against oxidative stress in HepG2 cells. Food Res. Int. 2016, 87, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.-H.; Terao, J. Antioxidant activity of caffeic acid and dihydrocaffeic acid in lard and human low-density lipoprotein. J. Agric. Food Chem. 1998, 46, 5062–5065. [Google Scholar] [CrossRef]
- Gröber, U.; Reichrath, J.; Holick, M.; Kisters, K. Vitamin K: An old vitamin in a new perspective. Dermatoendocrinol. 2014, 6, e968490. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sinclair, A.J.; Faiza, M.; Li, D.; Han, X.; Yin, H.; Wang, Y. Furan fatty acids—Beneficial or harmful to health? Prog. Lipid Res. 2017, 68, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Fang, Y.; Rong, A.; Wang, J. Flexible combination of multiple diagnostic biomarkers to improve diagnostic accuracy. BMC Med. Res. Methodol. 2015, 15, 94. [Google Scholar] [CrossRef] [PubMed]







| Meal | Food |
|---|---|
| Breakfast | Bread with butter, or bread with cheese, or bread with cheese and ham, or toast with butter, or cream crackers + yogurt * |
| Snack | Cream crackers + yogurt * |
| Lunch | Rice or pasta + beans + grilled (or roasted) chicken or meat + mashed potatoes Dessert: gelatin |
| Snack | Sandwich without salad + yogurt or other beverage * |
| Dinner | Pasta |
| Time Range | Metabolites | AUC | p-Value (t-Test) | Cut-Off Value |
|---|---|---|---|---|
| All times | 1R,6R-6-Hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate | 1 | 2.0454 × 10−21 | 5 |
| Hydroxyhydrocinnamic acid | 1 | 5.7497 × 10−28 | 2.64 | |
| multiplex pred | 1 | 6.8817 × 10−57 | 0.665 | |
| 3,4-dihydroxyphenylacetate | 0.99593 | 1.35 × 10−14 | 0.63 | |
| 3(7-dehydro)genipinic acid | 0.98008 | 7.1576 × 10−14 | 4.3 | |
| Nonate | 0.97737 | 3.9231 × 10−17 | 2.47 | |
| 12-demethylated-8-hydroxygenipinic acid | 0.95292 | 5.7703 × 10−7 | 0.00878 | |
| Dihydroxyhydrocinnamic acid | 0.93074 | 3.0562 × 10−18 | 37.8 | |
| Genipic acid | 0.85785 | 1.0072 × 10−9 | 0.322 | |
| Genipic acid glucuronide | 0.74423 | 0.55998 | 0.218 | |
| 0 to 6 h | Dihydroxyhydrocinnamic acid | 1 | 1.9091 × 10−14 | 48.9 |
| 1R,6R-6-Hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate | 1 | 9.9186 × 10−10 | 3.95 | |
| Hydroxyhydrocinnamic acid | 1 | 7.7957 × 10−12 | 3.56 | |
| Nonate | 1 | 8.2316 × 10−9 | 5.85 | |
| 3,4-dihydroxyphenylacetate | 1 | 1.6602 × 10−7 | 0.244 | |
| multiplex pred | 1 | 1.3766 × 10−22 | 0.633 | |
| Genipic acid | 0.98333 | 7.4173 × 10−8 | 0.342 | |
| 3(7-dehydro)genipinic acid | 0.95 | 5.951 × 10−7 | 4.14 | |
| 12-demethylated-8-hydroxygenipinic acid | 0.9375 | 2.574 × 10−4 | 0.0159 | |
| Genipic acid glucuronide | 0.93333 | 2.0461 × 10−4 | 0.17 | |
| 6 to 12 h | 1R,6R-6-Hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate | 1 | 6.1416 × 10−9 | 4.83 |
| Hydroxyhydrocinnamic acid | 1 | 1.7482 × 10−9 | 2.64 | |
| 12-Demethylated-8-hydroxygenipinic acid | 1 | 6.9629 × 10−5 | 0.00947 | |
| Nonate | 1 | 9.5177 × 10−6 | 3.09 | |
| 3,4-dihydroxyphenylacetate | 1 | 1.1069 × 10−4 | 0.892 | |
| multiplex pred | 1 | 2.2156 × 10−21 | 0.486 | |
| 3(7-dehydro)genipinic acid | 0.97917 | 1.3988 × 10−5 | 3.5 | |
| Dihydroxyhydrocinnamic acid | 0.93333 | 3.7546 × 10−6 | 36.8 | |
| Genipic acid | 0.77083 | 0.0073173 | 0.322 | |
| Genipic acid glucuronide | 0.7625 | 0.92915 | 0.374 | |
| 12 to 24 h | 1R,6R-6-Hydroxy-2-succinylcyclohexa-2,4-diene-1-carboxylate | 1 | 6.279 × 10−6 | 6.12 |
| Hydroxyhydrocinnamic acid | 1 | 2.1664 × 10−10 | 3.85 | |
| 3,4-dihydroxyphenylacetate | 1 | 9.8555 × 10−6 | 0.63 | |
| multiplex pred | 1 | 1.4956 × 10−16 | 0.665 | |
| 3(7-dehydro)genipinic acid | 0.99219 | 1.2466 × 10−5 | 1.74 | |
| 12-demethylated-8-hydroxygenipinic acid | 0.92929 | 0.002416 | 0.0106 | |
| Nonate | 0.92578 | 1.7992 × 10−5 | 7.13 | |
| Dihydroxyhydrocinnamic acid | 0.86528 | 1.8911 × 10−4 | 50 | |
| Genipic acid | 0.83984 | 8.338 × 10−4 | 0.318 | |
| Genipic acid glucuronide | 0.55469 | 0.22484 | 0.242 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dickson, L.; Tenon, M.; Svilar, L.; Fança-Berthon, P.; Lugan, R.; Martin, J.-C.; Vaillant, F.; Rogez, H. Main Human Urinary Metabolites after Genipap (Genipa americana L.) Juice Intake. Nutrients 2018, 10, 1155. https://doi.org/10.3390/nu10091155
Dickson L, Tenon M, Svilar L, Fança-Berthon P, Lugan R, Martin J-C, Vaillant F, Rogez H. Main Human Urinary Metabolites after Genipap (Genipa americana L.) Juice Intake. Nutrients. 2018; 10(9):1155. https://doi.org/10.3390/nu10091155
Chicago/Turabian StyleDickson, Livia, Mathieu Tenon, Ljubica Svilar, Pascale Fança-Berthon, Raphael Lugan, Jean-Charles Martin, Fabrice Vaillant, and Hervé Rogez. 2018. "Main Human Urinary Metabolites after Genipap (Genipa americana L.) Juice Intake" Nutrients 10, no. 9: 1155. https://doi.org/10.3390/nu10091155
APA StyleDickson, L., Tenon, M., Svilar, L., Fança-Berthon, P., Lugan, R., Martin, J.-C., Vaillant, F., & Rogez, H. (2018). Main Human Urinary Metabolites after Genipap (Genipa americana L.) Juice Intake. Nutrients, 10(9), 1155. https://doi.org/10.3390/nu10091155





