Whey Protein Concentrate WPC-80 Intensifies Glycoconjugate Catabolism and Induces Oxidative Stress in the Liver of Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. WPC-80 Composition
2.2. Animals
- C7—control group receiving 0.9% NaCl for 7 days
- C14—control group receiving 0.9% NaCl for 14 days
- C21—control group receiving 0.9% NaCl for 21 days
- 0.3 WPC 7—a group receiving WPC-80 at a dose of 0.3 g/kg body weight for 7 days
- 0.3 WPC 14—a group receiving WPC-80 at a dose of 0.3 g/kg body weight for 14 days
- 0.3 WPC 21—a group receiving WPC-80 at a dose of 0.3 g/kg body weight for 21 days
- 0.5 WPC 7—a group receiving WPC-80 at a dose of 0.5 g/kg body weight for 7 days
- 0.5 WPC 14—a group receiving WPC-80 at a dose of 0.5 g/kg body weight for 14 days
- 0.5 WPC 21—a group receiving WPC-80 at a dose of 0.5 g/kg body weight for 21 days
2.3. Preparation of Homogenates
2.4. Evaluation of the Activity of Lysosomal Exoglycosidases
2.4.1. Evaluation of HEX Activity
2.4.2. Evaluation of GLU Activity
2.4.3. Evaluation of GAL Activity
2.4.4. Evaluation of MAN Activity
2.4.5. Evaluation of FUC Activity
2.5. Evaluation of Oxidative Stress Markers
2.5.1. Evaluation of GSH Concentration
2.5.2. Evaluation of MDA Concentration
2.6. Assessment of Pro-Inflammatory Cytokines
2.7. Assessment of Total Protein Concentration
2.8. Histological Examination
2.9. Statistical Analysis
3. Results
3.1. WPC-80 Composition
3.2. Characteristics of the Animals
3.3. Activity of Lysosomal Exoglycosidases
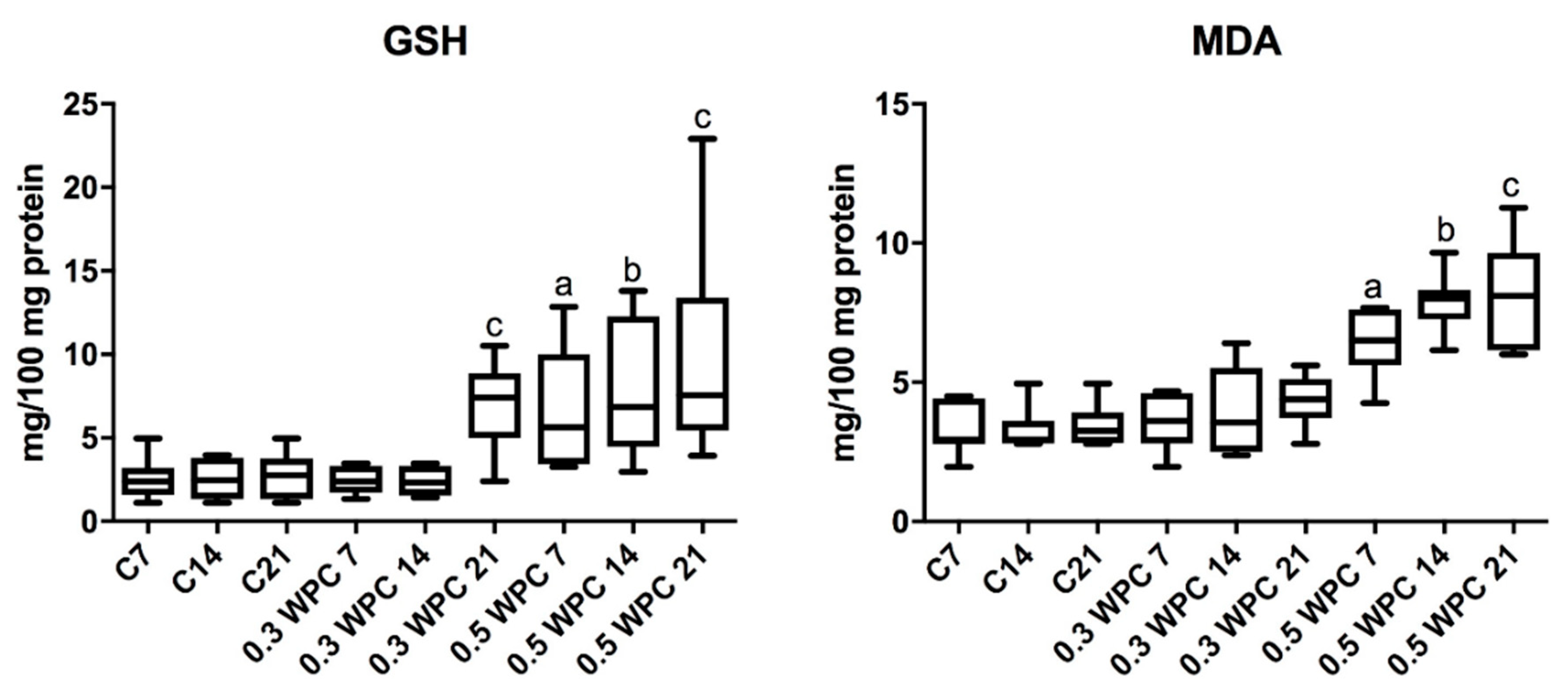
3.4. Oxidative stress Markers
3.5. Pro-inflammatory Cytokines
3.6. Correlations
3.7. Histological Examination
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of interest
References
- Kita, M.; Obara, K.; Kondo, S.; Umeda, S.; Ano, Y. Effect of supplementation of a whey peptide rich in tryptophan-tyrosine-related peptides on cognitive performance in healthy adults: A randomized, double-blind, placebo-controlled study. Nutrients 2018, 10, 887. [Google Scholar] [CrossRef] [PubMed]
- Teba, C.D.; da Silva, E.M.M.; Chávez, D.W.H.; de Carvalho, C.W.P; Ascheri, J.L.R. Effects of whey protein concentrate, feed moisture and temperature on the physicochemical characteristics of a rice-based extruded flour. Food Chem. 2017, 228, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, M.; Maciejczyk, M.; Koprowicz, T.; Mikołuć, B.; Milewska, A.; Zalewska, A.; Car, H. Whey protein concentrate wpc-80 improves antioxidant defense systems in the salivary glands of 14-month wistar rats. Nutrients 2018, 10, 782. [Google Scholar] [CrossRef] [PubMed]
- Nabuco, H.C.G.; Tomeleri, C.M.; Sugihara Junior, P.; Fernandes, R.R.; Cavalcante, E.F.; Antunes, M.; Ribeiro, A.S.; Teixeira, D.C.; Silva, A.M.; Sardinha, L.B.; et al. Effects of whey protein supplementation pre- or post-resistance training on muscle mass, muscular strength and functional capacity in pre-conditioned older women: A randomized clinical trial. Nutrients 2018, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Ignowski, E.; Winter, A.N.; Duval, N.; Fleming, H.; Wallace, T.; Manning, E.; Koza, L.; Huber, K.; Serkova, N.J.; Linseman, D.A. The cysteine-rich whey protein supplement, Immunocal®, preserves brain glutathione and improves cognitive, motor and histopathological indices of traumatic brain injury in a mouse model of controlled cortical impact. Free Radic. Biol. Med. 2018, 124, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rúa, R.; Keijer, J.; Palou, A.; van Schothorst, E.M.; Oliver, P. Long-term intake of a high-protein diet increases liver triacylglycerol deposition pathways and hepatic signs of injury in rats. J. Nutr. Biochem. 2017, 46, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Jean, C.; Rome, S.; Mathé, V.; Huneau, J.F.; Aattouri, N.; Fromentin, G.; Achagiotis, C.L.; Tomé, D. Metabolic evidence for adaptation to a high protein diet in rats. J. Nutr. 2001, 131, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Diez-Fernandez, C.; Häberle, J. Targeting CPS1 in the treatment of Carbamoyl phosphate synthetase 1 (CPS1) deficiency, a urea cycle disorder. Expert Opin. Ther. Targets 2017, 21, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Chojnowska, S.; Kępka, A.; Szajda, S.D.; Waszkiewicz, N.; Bierć, M.; Zwierz, K. Exoglycosidase markers of diseases. Biochem. Soc. Trans. 2011, 39, 406–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witek, B.; Rochon-Szmejchel, D.; Stanisławska, I.; Łyp, M.; Wróbel, K.; Zapała, A.; Kamińska, A.; Kołataj, A. Activities of lysosomal enzymes in alloxan-induced diabetes in the mouse. Adv. Exp. Med. Biol. 2018, 1040, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Lysek-Gladysinska, M.; Wieczorek, A.; Walaszczyk, A.; Jelonek, K.; Jozwik, A.; Pietrowska, M.; Dörr, W.; Gabrys, D.; Widlak, P. Long-term effects of low-dose mouse liver irradiation involve ultrastructural and biochemical changes in hepatocytes that depend on lipid metabolism. Radiat. Environ. Biophys. 2018, 57, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Kossakowska, A.; Szulimowska, J.; Klimiuk, A.; Knaś, M.; Car, H.; Niklińska, W.; Ładny, J.R.; Chabowski, A.; Zalewska, A. lysosomal exoglycosidase profile and secretory function in the salivary glands of rats with streptozotocin-induced diabetes. J. Diabetes Res. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Bee Ling, T.; Mohd Esa, N.; Winnie-Pui-Pui, L. Nutrients and oxidative stress: Friend or foe? Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Żukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch. Oral Biol. 2018, 92, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Pietrucha, B.; Heropolitanska-Pliszka, E.; Maciejczyk, M.; Car, H.; Sawicka-Powierza, J.; Motkowski, R.; Karpinska, J.; Hryniewicka, M.; Zalewska, A.; Pac, M.; et al. Comparison of selected parameters of redox homeostasis in patients with ataxia-telangiectasia and nijmegen breakage syndrome. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Żukowski, P.; Maciejczyk, M.; Matczuk, J.; Kurek, K.; Waszkiel, D.; Zendzian-Piotrowska, M.; Zalewska, A. Effect of N-Acetylcysteine on antioxidant defense, oxidative modification and salivary gland function in a rat model of insulin resistance. Oxid. Med. Cell. Longev. 2018, 2018, 6581970. [Google Scholar] [CrossRef] [PubMed]
- Fejfer, K.; Buczko, P.; Niczyporuk, M.; Ładny, J.R.; Hady, R.H.; Knaś, M.; Waszkiel, D.; Klimiuk, A.; Zalewska, A.; Maciejczyk, M. Oxidative modification of biomolecules in the nonstimulated and stimulated saliva of patients with morbid obesity treated with bariatric surgery. Biomed. Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Camiletti-Moirón, D.; Aparicio, V.A.; Nebot, E.; Medina, G.; Martínez, R.; Kapravelou, G.; Andrade, A.; Porres, J.M.; López-Jurado, M.; Aranda, P. High-protein diet induces oxidative stress in rat brain: Protective action of high-intensity exercise against lipid peroxidation. Nutr. Hosp. 2015, 31, 866–874. [Google Scholar] [CrossRef]
- Gu, C.; Xu, H. Effect of oxidative damage due to excessive protein ingestion on pancreas function in mice. Int. J. Mol. Sci. 2010, 11, 4591–4600. [Google Scholar] [CrossRef] [PubMed]
- Kołodziej, U.; Maciejczyk, M.; Niklińska, W.; Waszkiel, D.; Żendzian-Piotrowska, M.; Żukowski, P.; Zalewska, A. Chronic high-protein diet induces oxidative stress and alters the salivary gland function in rats. Arch. Oral Biol. 2017, 84, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kong, B.; Han, J.; Sun, C.; Li, P. Structure and antioxidant activity of whey protein isolate conjugated with glucose via the Maillard reaction under dry-heating conditions. Food Struct. 2014, 1, 145–154. [Google Scholar] [CrossRef]
- Hassan, A.M.; Abdel-Aziem, S.H.; Abdel-Wahhab, M.A. Modulation of DNA damage and alteration of gene expression during aflatoxicosis via dietary supplementation of Spirulina (Arthrospira) and whey protein concentrate. Ecotoxicol. Environ. Saf. 2012, 79, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Tokajuk, A.; Karpińska, O.; Zakrzeska, A.; Bienias, K.; Prokopiuk, S.; Kozłowska, H.; Kasacka, I.; Chabielska, E.; Car, H. Dysfunction of aorta is prevented by whey protein concentrate-80 in venous thrombosis-induced rats. J. Funct. Foods 2016, 27, 365–375. [Google Scholar] [CrossRef]
- Zalewska, A.; Knaś, M.; Maciejczyk, M.; Waszkiewicz, N.; Klimiuk, A.; Choromańska, M.; Matczuk, J.; Waszkiel, D.; Car, H. Antioxidant profile, carbonyl and lipid oxidation markers in the parotid and submandibular glands of rats in different periods of streptozotocin induced diabetes. Arch. Oral Biol. 2015, 60, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, U.; Maciejczyk, M.; Miasko, A.; Matczuk, J.; Knas, M.; Zukowski, P.; Zendzian-Piotrowska, M.; Borys, J.; Zalewska, A. Oxidative modification in the salivary glands of high fat-diet induced insulin resistant rats. Front. Physiol. 2017, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Krętowski, A.; Waszkiel, D.; Bortnik, P.; Czarniecka-Bargłowska, K.; Kocisz, M.; Szulimowska, J.; Czajkowski, M.; et al. Exposure to Ti4Al4V titanium alloy leads to redox abnormalities, oxidative stress and oxidative damage in patients treated for mandible fractures. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, J.; Zalewska, A.; Popko, J.; Zwierz, K. Optimization of an enzymatic method for the determination of lysosomal N-acetyl-β-d-hexosaminidase and β-glucuronidase in synovial fluid. Clin. Chem. Lab. Med. 2006, 44, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Chojnowska, S.; Minarowska, A.; Waszkiewicz, N.; Kępka, A.; Zalewska-Szajda, B.; Gościk, E.; Kowal, K.; Olszewska, E.; Konarzewska-Duchnowska, E.; Minarowski, Ł.; et al. The activity of N-acetyl-β-D-hexosaminidase A and B and β-glucuronidase in nasal polyps and hypertrophic nasal concha. Otolaryngol. Pol. 2014, 68, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Moron, M.; Depierre, J.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta-Gen. Subj. 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Sims-Robinson, C.; Bakeman, A.; Rosko, A.; Glasser, R.; Feldman, E.L. the role of oxidized cholesterol in diabetes-induced lysosomal dysfunction in the brain. Mol. Neurobiol. 2016, 53, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Pásztói, M.; Nagy, G.; Géher, P.; Lakatos, T.; Tóth, K.; Wellinger, K.; Pócza, P.; György, B.; Holub, M.C.; Kittel, Á.; et al. Gene expression and activity of cartilage degrading glycosidases in human rheumatoid arthritis and osteoarthritis synovial fibroblasts. Arthritis Res. Ther. 2009, 11, R68. [Google Scholar] [CrossRef] [PubMed]
- Ramessur, K.T.; Greenwell, P.; Nash, R.; Dwek, M. V Breast cancer invasion is mediated by beta-N-acetylglucosaminidase (beta-NAG) and associated with a dysregulation in the secretory pathway of cancer cells. Br. J. Biomed. Sci. 2010, 67, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Hultberg, B.; Pålsson, B.; Isaksson, A.; Masson, P. Beta-hexosaminidase in bile and plasma from patients with cholestasis. Liver 1995, 15, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Humaloja, K.; Salaspuro, M.; Roine, R.P. Biliary excretion of dolichols and β-hexosaminidase—Effect of ethanol and glucagon. Lipids 1997, 32, 1169–1172. [Google Scholar] [CrossRef] [PubMed]
- Maenhout, T.M.; Poll, A.; Wuyts, B.; Lecocq, E.; Van Vlierberghe, H.; De Buyzere, M.L.; Delanghe, J.R. Microheterogeneity of Serum β-Hexosaminidase in Chronic Alcohol Abusers in a Driver’s License Regranting Program. Alcohol. Clin. Exp. Res. 2013, 37, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Pesta, D.H.; Samuel, V.T. A high-protein diet for reducing body fat: Mechanisms and possible caveats. Nutr. Metab. 2014, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Fawzy Montaser, M.; Amin Sakr, M.; Omar Khalifa, M. Alpha-l-fucosidase as a tumour marker of hepatocellular carcinoma. Arab. J. Gastroenterol. 2012, 13, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, M.R.; Campbell, J.L.; Sprague, G.; Slater, K.; Noffsinger, J.K.; Peterson, C.M. Comparative evaluation of the clinical utility of three markers of ethanol intake: The effect of gender. Alcohol. Clin. Exp. Res. 1993, 17, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Moreno-Càceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; ten Dijke, P. TGF-β signalling and liver disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
- Shimoi, K.; Nakayama, T. Glucuronidase deconjugation in inflammation. Methods Enzymol. 2005, 400, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulou, E.C.; Fouzas, S.; Douros, K.; Triantaphyllidou, I.E.; Malavaki, C.; Priftis, K.N.; Karamanos, N.K.; Anthracopoulos, M.B. Increased β-glucuronidase activity in bronchoalveolar lavage fluid of children with bacterial lung infection: A case-control study. Respirology 2015, 20, 1248–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Zhang, Y.; Xiong, X.; Harville, E.W.; Karmin, O.; Qian, X. Salivary and serum inflammatory mediators among pre-conception women with periodontal disease. BMC Oral Health 2016, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Choromańska, M.; Klimiuk, A.; Kostecka-Sochoń, P.; Wilczyńska, K.; Kwiatkowski, M.; Okuniewska, N.; Waszkiewicz, N.; Zalewska, A.; Maciejczyk, M. Antioxidant defence, oxidative stress and oxidative damage in saliva, plasma and erythrocytes of dementia patients. Can salivary AGE be a marker of dementia? Int. J. Mol. Sci. 2017, 18, e2205. [Google Scholar] [CrossRef] [PubMed]
- Jordá, A.; Zaragozá, R.; Portolés, M.; Báguena-Cervellera, R.; Renau-Piqueras, J. Long-term high-protein diet induces biochemical and ultrastructural changes in rat liver mitochondria. Arch. Biochem. Biophys. 1988, 265, 241–248. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Szulimowska, J.; Skutnik, A.; Taranta-Janusz, K.; Wasilewska, A.; Wiśniewska, N.; Zalewska, A. Salivary Biomarkers of Oxidative Stress in Children with Chronic Kidney Disease. J. Clin. Med. 2018, 7, e209. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, S.; Kim, B.; Kwon, J. Toll-like receptor 7 affects the pathogenesis of non-alcoholic fatty liver disease. Sci. Rep. 2016, 6, 27849.02. [Google Scholar] [CrossRef] [PubMed]
- Elias-Miró, M.; Jiménez-Castro, M.B.; Rodés, J.; Peralta, C. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radic. Res. 2013, 47, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Witek, B.; Ochwanowska, E.; Stanisławska, I.; Wróbel, A.; Mierzwa, W.; Kołątaj, A.; Bagnicka, E. Effect of testosterone on the activity of lysosomal enzymes in the mouse liver and kidney maintained on the different protein level in diet. Vet. Med.-Sci. Pract. 2007, 63, 1084–1089. [Google Scholar]
- Colombo, J.-P.; Cervantes, H.; Kokorovic, M.; Pfister, U.; Perritaz, R. Effect of different protein diets on the distribution of amino acids in plasma, liver and brain in the rat. Ann. Nutr. Metab. 1992, 36, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.; Keshavarzian, A.; Mutlu, G. Hyperalbuminemia and elevated transaminases associated with high-protein diet. Scand. J. Gastroenterol. 2006, 41, 759–760. [Google Scholar] [CrossRef] [PubMed]
- French, W.W.; Dridi, S.; Shouse, S.A.; Wu, H.; Hawley, A.; Lee, S.O.; Gu, X.; Baum, J.I. A high-protein diet reduces weight gain, decreases food intake, decreases liver fat deposition and improves markers of muscle metabolism in obese Zucker rats. Nutrients 2017, 9, e587. [Google Scholar] [CrossRef] [PubMed]






| General Composition | % |
|---|---|
| Protein | 78.2 |
| Fat | 6.72 |
| Carbohydrates | 7.9 |
| Ash | 2.7 |
| Dietary fibre | <0.5 |
| Humidity | 4.5 |
| The calorific value | 1712 kJ/100 g |
| WBC (× 1012/L) | RBC (M/μL) | HGB (g/dL) | HTC (%) | MCV (fL) | MCH (pg) | MCHC (g/dL) | PLT (× 109/L) | |
|---|---|---|---|---|---|---|---|---|
| C7 | 3.3 (2.8–3.7) | 7.1 (6.6–9.9) | 13.8 (13.6–13.9) | 40.0 (38.9–43.0) | 58.5 (58.0–59.0) | 20.5 (18.3–21.1) | 35.2 (34.8–35.6) | 781.5 (635.0–928.0) |
| C14 | 2.9 (2.5–3.8) | 7.0 (6.7–7.4) | 14.0 (12.8–15.0) | 41.6 (39.4–45.2) | 59.0 (57.0–62.00) | 19.9 (19.0–21.2) | 33.2 (31.1–34.5) | 825.0 (747.0–881.0) |
| C21 | 3.5 (2.5–4.8) | 7.4 (6.8–8.9) | 15.3 (12.3–17.4) | 49.2 (36.2–52.2) | 58.5 (56.0–59.0) | 19.5 (18.2–20.5) | 33.2 (31.1–33.3) | 713.5 (406.0–821.0) |
| 0.3 WPC 7 | 3.6 (2.8–4.9) | 7.3 (6.3–7.9) | 14.9 (12.9–15.8) | 43.0 (26.5–46.0) | 58.5 (57.0–59.0) | 20.2 (19.8–20.8) | 34.7 (34.4–35.4) | 751.5 (627.0–914.0) |
| 0.3 WPC 14 | 3.3 (2.4–4.0) | 7.1 (6.4–8.1) | 14.1 (12.7–15.3) | 42.1 (37.2–44.8) | 57.5 (54.0–62.0) | 19.6 (18.0–20.5) | 34.2 (31.8–34.7) | 743.5 (462.0–920.0) |
| 0.3 WPC 21 | 3.2 (2.5–4.8) | 7.4 (6.5–8.7) | 14.8 (13.2–16.4) | 42.6 (36.7–49.5) | 58.0 (55.0–60.0) | 20.0 (18.0–21.0) | 35.1 (33.0–36.4) | 751.0 (667.0–855.0) |
| 0.5 WPC 7 | 3.9 (3.2–5.0) | 6.9 (6.4–8.9) | 14.2 (13.0–17.8) | 40.9 (37.3–50.8) | 58.0 (57.0–61.0) | 20.4 (20.0–20.6) | 35.0 (33.8–35.7) | 760.0 (589.0–930.0) |
| 0.5 WPC 14 | 3.2 (2.6–3.9) | 7.5 (6.6–8.0) | 13.9 (13.3–16.0) | 44.0 (39.8–54.0) | 59.0 (42.9–60.0) | 19.8 (14.2–20.0) | 33.5 (18.0–35.6) | 720.0 (331.0–778.0) |
| 0.5 WPC 21 | 3.7 (2.8–5.4) | 7.8 (6.6–8.9) | 15.1 (13.7–16.6) | 45.0 (38.7–49.7) | 57.5 (50.0–61.0) | 19.8 (17.9–20.9) | 33.7 (32.0–35.3) | 709.5 (599.0–880.0) |
| ALT (U/L) | AST (U/L) | Bilirubin (mg/dL) | Albumin (μmol/L) | Cr (mg/dL) | UA (μmol/L) | Urea (mmol/L) | TC (mmol/L) | Glucose (mg/dL) | |
|---|---|---|---|---|---|---|---|---|---|
| C7 | 52.6 (34.1–64.7) | 100.3 (65.9–133.1) | 1.6 (1.5–1.7) | 419.9 (416.5–432.8) | 0.5 (0.4–0.6) | 27.0 (26.0–36.0) | 6.4 (4.3–6.7) | 80.7 (61.2–98.7) | 94.2 (68.0–102.5) |
| C14 | 52.0 (42.8–66.6) | 88.0 (76.0–117.7) | 1.7 (1.0–2.0) | 420.4 (337.0–435.8) | 0.4 (0.4–0.5) | 27.0 (24.0–34.0) | 6.3 (5.6–8.6) | 83.3 (51.3–97.0) | 89.0 (83.0–92.0) |
| C21 | 47.2 (38.1–73.8) | 91.5 (75.9–116.8) | 2.0 (1.8–2.3) | 417.2 (385.3–435.4) | 0.5 (0.4–0.7) | 26.0 (16.0–34.0) | 8.2 (6.5–8.5) | 84.0 (78.6–95.6) | 89.0 (71.3–100.0) |
| 0.3 WPC 7 | 59.0 (35.5–88.4) | 122.2 (93.6–132.4) | 1.2 (1.0–1.3) | 418.7 (368.2–447.8) | 0.4 (0.3–0.5) | 24.0 (17.0–43.0) | 5.8 (4.6–6.1) | 70.4 (40.9–98.5) | 89.0 (78.0–104.0) |
| 0.3 WPC 14 | 55.2 (50.8–61.6) | 115.1 (106.0–135.9) | 1.1 (0.9–1.9) | 461.3 (432.3–500.8) | 0.5 (0.4–0.6) | 29.0 (24.0–45.0) | 7.8 (5.8–8.6) | 88.7 (62.7–101.4) | 85.0 (72.0–105.5) |
| 0.3 WPC 21 | 57.0 (49.1–76.8) | 103.3 (80.0–141.0) | 1.2 (0.9–2.0) | 419.7 (384.0–471.6) | 0.5 (0.5–0.6) | 36.0 (27.0–39.0) | 8.1 (7.2–9.2) | 90.1 (69.1–95.0) | 82.5 (68.0–96.00) |
| 0.5 WPC 7 | 57.5 (42.7–81.2) | 86.0 (73.0–158.7) | 2.0 (1.6–2.4) | 460.4 (445.3–486.4) | 0.6 (0.5–0.8) | 29.0 (21.0–45.0) | 6.0 (5.3–7.2) | 79.7 (63.7–82.1) | 90.5 (83.0–97.0) |
| 0.5 WPC 14 | 57.9 (49.6–99.8) | 109.4 (107.6–148.4) | 1.7 (1.4–2.1) | 430.0 (401.7–442.7) | 0.5 (0.5–0.7) | 33.0 (24.0–45.0) | 6.9 (5.7–7.8) | 88.8 (70.3–89.7) | 78.0 (74.0–98.00) |
| 0.5 WPC 21 | 101.1 (77.5–126.0)c | 173.0 (164.6–216.2)c | 1.3 (0.8–2.4) | 464.7 (388.6–469.7) | 0.7 (06–0.9)c | 33.0 (23.0–36.0) | 9.7 (8.9–10.4)c | 78.0 (58.4–95.2) | 85.5 (73.0–101.0) |
| Pair of Variable | Group | r | p |
|---|---|---|---|
| MAN & MDA | 0.3 WPC 14 | 0.886 | 0.003 |
| GLU & MDA | 0.5 WPC 7 | 0.812 | 0.005 |
| HEX & MDA | 0.5 WPC 7 | 0.607 | 0.05 |
| HEX & Cr | 0.5 WPC 7 | 0.650 | 0.05 |
| GLU & GSH | 0.5 WPC 14 | 0.771 | 0.04 |
| GLU & IL-1β | 0.5 WPC 14 | 0.650 | 0.05 |
| GLU & IL-1β | 0.5 WPC 21 | 0.810 | 0.005 |
| HEX & ALT | 0.5 WPC 21 | 0.741 | 0.001 |
| HEX & AST | 0.5 WPC 21 | 0.690 | 0.04 |
| GLU & ALT | 0.5 WPC 21 | 0.920 | <0.0001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żebrowska-Gamdzyk, M.; Maciejczyk, M.; Zalewska, A.; Guzińska-Ustymowicz, K.; Tokajuk, A.; Car, H. Whey Protein Concentrate WPC-80 Intensifies Glycoconjugate Catabolism and Induces Oxidative Stress in the Liver of Rats. Nutrients 2018, 10, 1178. https://doi.org/10.3390/nu10091178
Żebrowska-Gamdzyk M, Maciejczyk M, Zalewska A, Guzińska-Ustymowicz K, Tokajuk A, Car H. Whey Protein Concentrate WPC-80 Intensifies Glycoconjugate Catabolism and Induces Oxidative Stress in the Liver of Rats. Nutrients. 2018; 10(9):1178. https://doi.org/10.3390/nu10091178
Chicago/Turabian StyleŻebrowska-Gamdzyk, Marta, Mateusz Maciejczyk, Anna Zalewska, Katarzyna Guzińska-Ustymowicz, Anna Tokajuk, and Halina Car. 2018. "Whey Protein Concentrate WPC-80 Intensifies Glycoconjugate Catabolism and Induces Oxidative Stress in the Liver of Rats" Nutrients 10, no. 9: 1178. https://doi.org/10.3390/nu10091178





