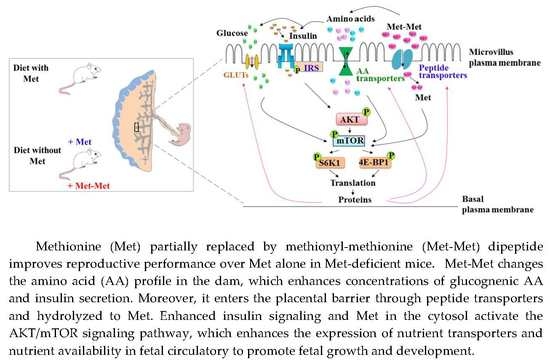
Methionine Partially Replaced by Methionyl-Methionine Dipeptide Improves Reproductive Performance over Methionine Alone in Methionine-Deficient Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptide Stability in Biological Fluids
2.2. Animals
2.3. In Vivo Optical Imaging
2.4. Maternal Studies
2.5. Amino Acid Analysis
2.6. Quantitative Reverse Transcription-PCR (qRT-PCR) and Western Blot Analysis
2.7. Statistics
3. Results
3.1. Stability of Met-Met in Biological Fluids and In Vivo Biodistribution
3.2. Reproductive Performance with Different Levels of Free Met
3.3. Reproductive Performance When Free Met Was Partially Substituted with Met-Met
3.4. Amino Acids in the Plasma of Dams and the Associated Fetal with Different Sources of Met
3.5. Plasma Glucose and Insulin in Dams With Different Sources of Met
3.6. Nutrient Transporters in the Placenta with Different Sources of Met
3.6.1. Amino Acid Transporters
3.6.2. Glucose Transporters
3.6.3. Peptide Transporters
3.7. Signaling Molecules in the Placenta with Different Sources of Met
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sutter, B.M.; Wu, X.; Laxman, S.; Tu, B.P. Methionine inhibits autophagy and promotes growth by inducing the SAM-responsive methylation of PP2A. Cell 2013, 154, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Janke, R.; Dodson, A.E.; Rine, J. Metabolism and epigenetics. Annu. Rev. Cell Dev. Biol. 2015, 31, 473–496. [Google Scholar] [CrossRef] [PubMed]
- Patrick, T.E.; Powers, R.W.; Daftary, A.R.; Ness, R.B.; Roberts, J.M. Homocysteine and folic acid are inversely related in black women with preeclampsia. Hypertension 2014, 43, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Kalhan, S.C. Metabolism of methionine in vivo: Impact of pregnancy, protein restriction, and fatty liver disease. Nestle Nutr. Workshop Ser. Pediatr. Program. 2009, 63, 121–131. [Google Scholar] [PubMed]
- Kalhan, S.C. Protein metabolism in pregnancy. Am. J. Clin. Nutr. 2000, 71, 1249S–1255S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalhan, S.C.; Marczewski, S.E. Methionine, homocysteine, one carbon metabolism and fetal growth. Rev. Endocr. Metab. Disord. 2012, 13, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.; Kottra, G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch. 2004, 447, 610–618. [Google Scholar] [PubMed]
- Smith, D.E.; Clémençon, B.; Hediger, M.A. Proton-coupled oligopeptide transporter family SLC15: Physiological, pharmacological and pathological implications. Mol. Aspects Med. 2013, 34, 323–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Webb, K.E., Jr.; Akers, M.R. Peptide-bound methionine can be a source of methionine for the synthesis of secreted proteins by mammary tissue explants from lactating mice. J. Nutr. 1996, 126, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.H.; Yang, J.Y.; Zhao, K.; Liu, H.Y.; Wu, Y.M.; Liu, J.X. Effects of methionine-containing dipeptides on as1 casein expression in bovine mammary epithelial cells. J. Anim. Feed Sci. 2007, 16, 325–329. [Google Scholar] [CrossRef]
- Zhou, M.M.; Wu, Y.M.; Liu, H.Y.; Liu, J.X. Effects of phenylalanine and threonine oligopeptides on milk protein synthesis in cultured bovine mammary epithelial cells. J. Anim. Physiol. Anim. Nutr. 2015, 99, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.X.; Wang, C.H.; Xu, Q.B.; Zhao, F.Q.; Liu, J.X.; Liu, H.Y. Methionyl-Methionine promotes α-s1 casein synthesis in bovine mammary gland explants by enhancing intracellular substrate availability and activating JAK2-STAT5 and mTOR-mediated signaling pathways. J. Nutr. 2015, 145, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Zhao, F.Q.; Liu, J.X.; Liu, H.Y. Dipeptide (Methionyl-Methionine) transport and its effect on β-Casein synthesis in bovine mammary epithelial cells. Cell. Physiol. Biochem. 2018, 47, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhang, L.; Wang, Y. The antimicrobial peptide cathelicidin-BF could be a potential therapeutic for Salmonella typhimurium infection. Microbiol. Res. 2015, 171, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997, 127, 838S. [Google Scholar] [CrossRef] [PubMed]
- Rogers, Q.R.; Harper, A.E. Amino acid diets and maximal growth in the rat. J. Nutr. 1965, 87, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Jung, Y.S.; Kim, S.J.; Kim, Y.S.; Choi, D.W.; Kim, Y.C. Alterations in sulfur amino acid metabolism in mice treated with silymarin: A novel mechanism of its action involved in enhancement of the antioxidant defense in liver. Planta Med. 2013, 79, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, M.; Rahman, S.M.; Hanafy, M.S.; Khalafalla, M.M.; EI-Shemy, H.A.; Nakamoto, Y.; Kita, Y.; Takanashi, K.; Matauda, F.; Murano, Y.; et al. Evaluation of amino acid content and nutritional quality of transgenic soybean seeds with high-level tryptophan accumulation. Mol. Breed. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Pu, Y.T.; Li, S.H.; Xiong, H.T.; Zhang, X.F.; Wang, Y.Z.; Du, H.H. Iron promotes intestinal development in neonatal piglets. Nutrients 2018, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Agnoux, A.M.; Antignac, J.P.; Boquien, C.Y.; David, A.; Desnots, E.; Ferchaud-Roucher, V.; Darmaun, D.; Parnet, P.; Alexandre-Gouabau, M.C. Perinatal protein restriction affects milk free amino acid and fatty acid profile in lactating rats: Potential role on pup growth and metabolic status. J. Nutr. Biochem. 2015, 26, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Jullian, M.; Hernandez, A.; Maurras, A.; Puget, K.; Amblard, M.; Martinez, J.; Subra, G. N-terminus FITC labeling of peptides on solid support: The truth behind the spacer. Tetrahedron Lett. 2009, 50, 260–263. [Google Scholar] [CrossRef]
- Wu, G.; Knabe, D.A.; Flynn, N.E. Amino acid metabolism in the small intestine: Biochemical bases and nutritional significance. In Biology of Metabolism of Growing Animals; Burrin, D.G., Mersmann, H.J., Eds.; Elsevier: New York, NY, USA, 2005; pp. 107–126. [Google Scholar]
- Rees, W.D.; Wilson, F.A.; Maloney, C.A. Sulfur amino acid metabolism in pregnancy: The impact of methionine in the maternal diet. J. Nutr. 2006, 136, 1701S–1705S. [Google Scholar] [CrossRef] [PubMed]
- Maze, I.; Noh, K.M.; Soshnev, A.A.; Alliset, C.D. Every amino acid matters: Essential contributions of histone variants to mammalian development and disease. Nat. Rev. Genet. 2014, 15, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Reimer, R.J.; Chaudhry, F.A.; Gray, A.T.; Edwards, R.H. Amino acid transport system A resembles system N in sequence but differs in mechanism. Proc. Natl. Acad. Sci. USA 2000, 97, 7715–7720. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, R.; Ohmori, T.; Hara, S.; Nakagomi, S.; Kanai-Azuma, M.; Kaneda-Nakashima, K.; Okuda, S.; Nagamori, S.; Kanai, Y. Essential roles of L-Type amino acid transporter 1 in syncytiotrophoblast development by presenting ausogenic 4F2hc. Mol. Cell. Biol. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.; Lee, V.W.; Mclean, M.; Athayde, N.; Lanzarone, V.; Khoshnow, Q.; Peek, M.J.; Cheung, N.W. The Association of falling insulin requirements with maternal biomarkers and placental dysfunction: A prospective study of women with preexisting diabetes in pregnancy. Diabetes Care 2017, 40, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, D.M.; Bain, J.R.; Reisetter, A.C.; Muehlbauer, M.J.; Nodzenski, M.; Stevens, R.D.; Ilkayeva, O.; Lowe, L.P.; Metzger, B.E.; Newgard, C.B.; et al. HAPO Study Cooperative Research Group. Metabolic networks and metabolites underlie associations between maternal glucose during pregnancy and newborn size at birth. Diabetes 2016, 65, 2039–2050. [Google Scholar] [CrossRef] [PubMed]
- Saenen, N.D.; Vrijens, K.; Janssen, B.G.; Roels, H.A.; Neven, K.Y.; Vanden, B.W.; Gyselaers, W.; Vanpoucke, C.; Lefebvre, W.; Boever, P.D.; et al. Lower placental leptin promoter methylation in association with fine particulate matter air pollution during pregnancy and placental nitrosative stress at birth in the ENVIRONAGE Cohort. Environ. Health Perspect. 2017, 125, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Gar, C.; Rottenkolber, M.; Prehn, C.; Adamski, J.; Seissler, J.; Lechner, A. Serum and plasma amino acids as markers of prediabetes, insulin resistance, and incident diabetes. Crit. Rev. Clin. Lab. Sci. 2018, 55, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Dimasuay, K.G.; Aitken, E.H.; Rosario, F.; Njie, M.; Glazier, J.; Rogerson, S.J.; Fowkes, F.J.; Beeson, J.G.; Powell, T.; Jansson, T.; et al. Inhibition of placental mTOR signaling provides a link between placental malaria and reduced birthweight. BMC Med. 2017, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Jewell, J.L.; Russell, R.C.; Guan, K.L. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 2013, 14, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicklin, P.; Bergman, P.; Zhang, B.; Triantafellow, E.; Wang, H.; Nyfeler, B.; Yang, H.; Hild, M.; Kung, C.; Wilson, C.; et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 2009, 136, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Abu-Remaileh, M.; Wyant, G.A.; Kim, C.; Laqtom, N.N.; Abbasi, M.; Chan, S.H.; Freinkman, E.; Sabatini, D.M. Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science 2017, 358, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, J.V.; Rosario, F.J.; Nijland, M.J.; McDonald, T.J.; Wu, G.; Kanai, Y.; Powell, T.L.; Nathanielsz, P.W.; Jansson, T. Down-regulation of placental mTOR, insulin/IGF-I signaling, and nutrient transporters in response to maternal nutrient restriction in the baboon. FASEB J. 2014, 28, 1294–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Dai, W.; Sun, Y.; Zhao, F.; Liu, J.; Liu, H. Methionine Partially Replaced by Methionyl-Methionine Dipeptide Improves Reproductive Performance over Methionine Alone in Methionine-Deficient Mice. Nutrients 2018, 10, 1190. https://doi.org/10.3390/nu10091190
Chen Q, Dai W, Sun Y, Zhao F, Liu J, Liu H. Methionine Partially Replaced by Methionyl-Methionine Dipeptide Improves Reproductive Performance over Methionine Alone in Methionine-Deficient Mice. Nutrients. 2018; 10(9):1190. https://doi.org/10.3390/nu10091190
Chicago/Turabian StyleChen, Qiong, Wenting Dai, Yalu Sun, Fengqi Zhao, Jianxin Liu, and Hongyun Liu. 2018. "Methionine Partially Replaced by Methionyl-Methionine Dipeptide Improves Reproductive Performance over Methionine Alone in Methionine-Deficient Mice" Nutrients 10, no. 9: 1190. https://doi.org/10.3390/nu10091190