Fish Oil Supplementation Reduces Inflammation but Does Not Restore Renal Function and Klotho Expression in an Adenine-Induced CKD Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Real-Time PCR
2.3. Histological Evaluation
2.4. Western Blotting
2.5. Statistical Analyses
3. Results
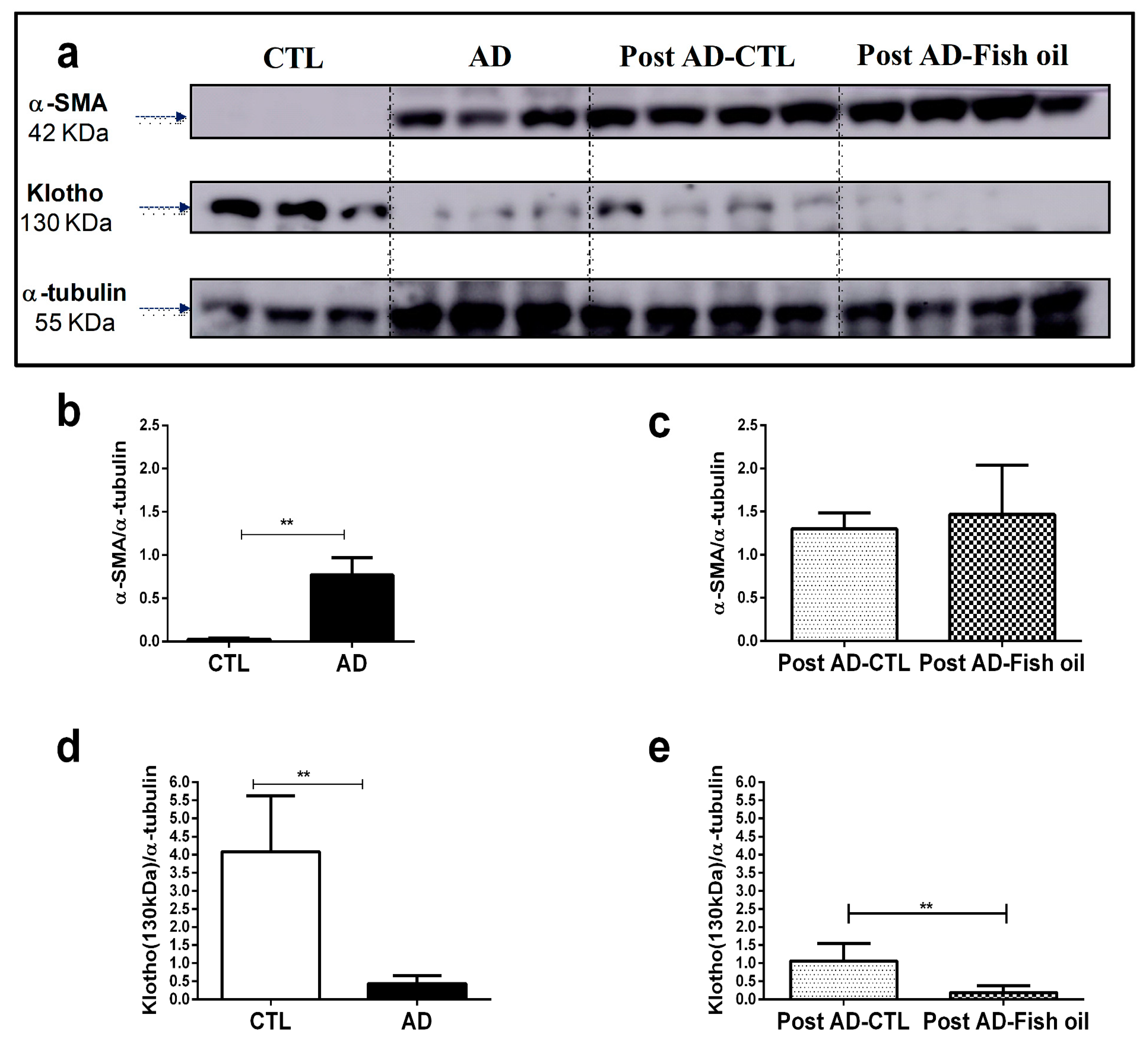
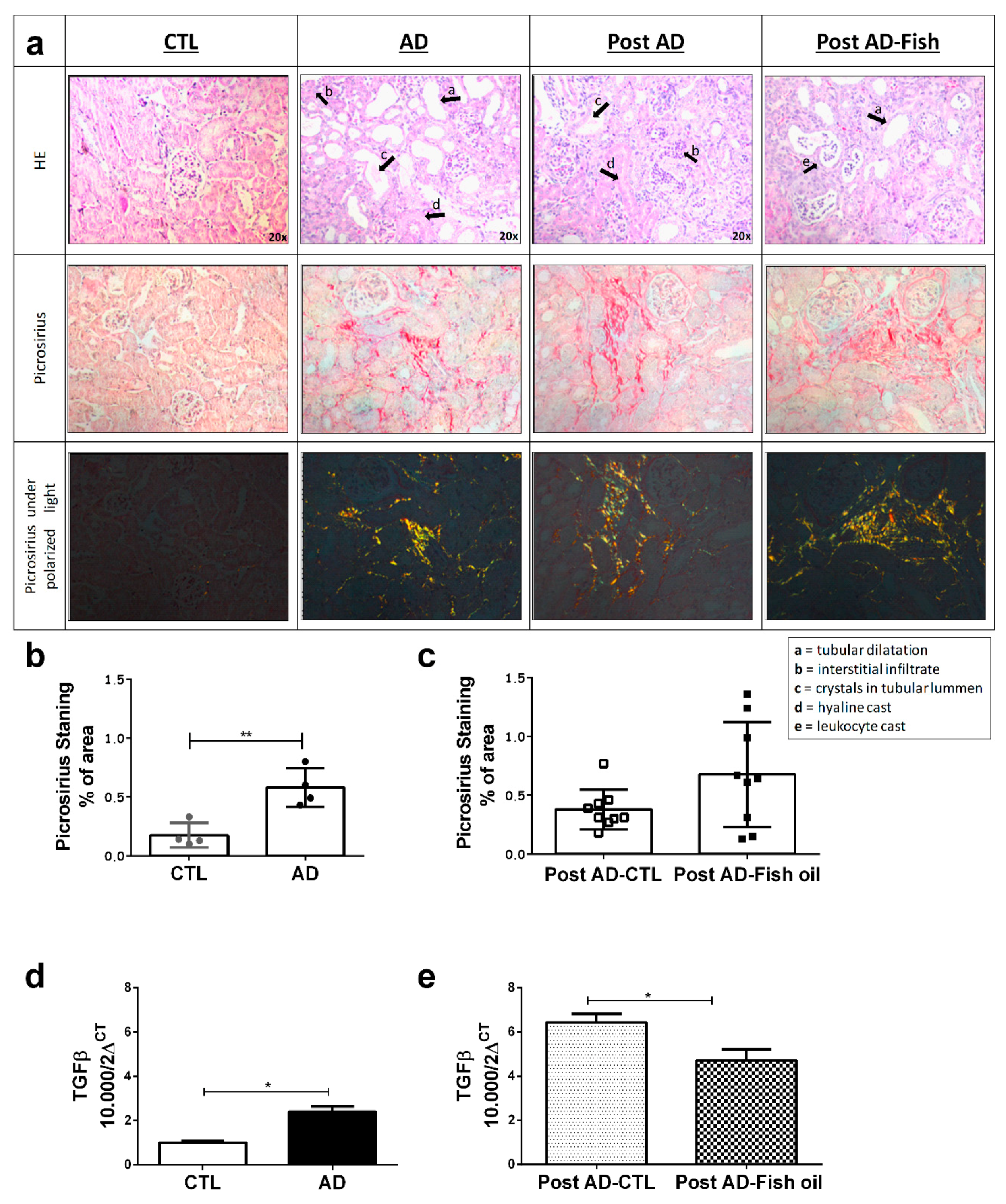
3.1. Adenine Supplementation Induces Inflammation, Loss of Renal Function, and Klotho Reduction
3.2. Fish Oil Supplementation Reduces Pro-Inflammatory Markers but Does Not Improve Renal Function
3.3. Fish Oil Supplementation Does Not Revert Progressive Renal Fibrosis
3.4. Fish Oil Does Not Restore Renal Klotho
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Braga, T.T.; Agudelo, J.S.; Camara, N.O. Macrophages during the fibrotic process: M2 as friend and foe. Front. Immunol. 2015, 6, 602. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shi, M.; Zhang, J.; Pastor, J.; Nakatani, T.; Lanske, B.; Razzaque, M.S.; Rosenblatt, K.P.; Baum, M.G.; Kuro-o, M.; et al. Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010, 24, 3438–3450. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Kuro-o, M.; Moe, O.W. Klotho and chronic kidney disease. Contrib. Nephrol. 2013, 180, 47–63. [Google Scholar] [PubMed]
- De Borst, M.H.; Vervloet, M.G.; ter Wee, P.M.; Navis, G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF23-klotho in chronic kidney disease. J. Am. Soc. Nephrol. 2011, 22, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Izquierdo, M.C.; Sanchez-Nino, M.D.; Suarez-Alvarez, B.; Lopez-Larrea, C.; Jakubowski, A.; Blanco, J.; Ramirez, R.; Selgas, R.; Ruiz-Ortega, M.; et al. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFkB. J. Am. Soc. Nephrol. 2011, 22, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Gobe, G.C.; Peake, J.M.; Coombes, J.S. Omega-3 polyunsaturated fatty acids in the treatment of kidney disease. Am. J. Kidney Dis. 2010, 56, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, E.K.; Geleijnse, J.M.; Kromhout, D.; Stijnen, T.; Gemen, E.F.; Kusters, R.; Giltay, E.J. Effect of omega-3 fatty acids on kidney function after myocardial infarction: The alpha omega trial. Clin. J. Am. Soc. Nephrol. 2014, 9, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- An, W.S.; Kim, H.J.; Cho, K.H.; Vaziri, N.D. Omega-3 fatty acid supplementation attenuates oxidative stress, inflammation, and tubulointerstitial fibrosis in the remnant kidney. Am. J. Physiol. Ren. Physiol. 2009, 297, F895–F903. [Google Scholar] [CrossRef] [PubMed]
- Baia, L.C.; Van den Berg, E.; Vervloet, M.G.; Heilberg, I.P.; Navis, G.; Bakker, S.J.; Geleijnse, J.M.; Kromhout, D.; Soedamah-Muthu, S.S.; De Borst, M.H.; et al. Fish and omega-3 fatty acid intake in relation to circulating fibroblast growth factor 23 levels in renal transplant recipients. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Montes, G.S.; Junqueira, L.C. The use of the picrosirius-polarization method for the study of the biopathology of collagen. Memórias do Instituto Oswaldo Cruz 1991, 86 (Suppl. 3), 1–11. [Google Scholar] [CrossRef] [PubMed]
- Correa-Costa, M.; Braga, T.T.; Semedo, P.; Hayashida, C.Y.; Bechara, L.R.; Elias, R.M.; Barreto, C.R.; Silva-Cunha, C.; Hyane, M.I.; Goncalves, G.M.; et al. Pivotal role of toll-like receptors 2 and 4, its adaptor molecule Myd88, and inflammasome complex in experimental tubule-interstitial nephritis. PLoS ONE 2011, 6, e29004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitani, H.; Ishizaka, N.; Aizawa, T.; Ohno, M.; Usui, S.; Suzuki, T.; Amaki, T.; Mori, I.; Nakamura, Y.; Sato, M.; et al. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 2002, 39, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Izquierdo, M.C.; Sanchez-Nino, M.D.; Ucero, A.C.; Egido, J.; Ruiz-Ortega, M.; Ramos, A.M.; Putterman, C.; Ortiz, A. TWEAK and the progression of renal disease: Clinical translation. Nephrol. Dial Transplant. 2014, 29 (Suppl. 1), i54–i62. [Google Scholar] [CrossRef]
- Ohyama, Y.; Kurabayashi, M.; Masuda, H.; Nakamura, T.; Aihara, Y.; Kaname, T.; Suga, T.; Arai, M.; Aizawa, H.; Matsumura, Y.; et al. Molecular cloning of rat klotho cdna: Markedly decreased expression of klotho by acute inflammatory stress. Biochem. Biophys. Res. Commun. 1998, 251, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Feger, M.; Mia, S.; Pakladok, T.; Nicolay, J.P.; Alesutan, I.; Schneider, S.W.; Voelkl, J.; Lang, F. Down-regulation of renal klotho expression by shiga toxin 2. Kidney Blood Press. Res. 2014, 39, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Banerjee, S.; Dey, N.; LeJeune, W.S.; Sarkar, P.S.; Brobey, R.; Rosenblatt, K.P.; Tilton, R.G.; Choudhary, S. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via rela (serine)536 phosphorylation. Diabetes 2011, 60, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, Y.; Ishikawa, K.; Yasuda, O.; Oguro, R.; Hanasaki, H.; Kida, I.; Takemura, Y.; Ohishi, M.; Katsuya, T.; Rakugi, H. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates nf-kappab activation. Endocrine 2009, 35, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ruan, X.Z.; Powis, S.H.; Fernando, R.; Mon, W.Y.; Wheeler, D.C.; Moorhead, J.F.; Varghese, Z. Epa and dha reduce LPS-induced inflammation responses in hk-2 cells: Evidence for a ppar-gamma-dependent mechanism. Kidney Int. 2005, 67, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Yang, H.T.; Hou, Y.C.; Chiu, Y.S.; Chiu, W.C. Dietary fish oil reduces systemic inflammation and ameliorates sepsis-induced liver injury by up-regulating the peroxisome proliferator-activated receptor gamma-mediated pathway in septic mice. J. Nutr. Biochem. 2014, 25, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Olauson, H.; Lindberg, K.; Amin, R.; Edvardsson, K.; Lindholm, B.; Andersson, G.; Wernerson, A.; Sabbagh, Y.; Schiavi, S.; et al. A novel model of adenine-induced tubulointerstitial nephropathy in mice. BMC Nephrol. 2013, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Aizawa, R.; Hori, M.; Ozaki, H. Progressive renal dysfunction and macrophage infiltration in interstitial fibrosis in an adenine-induced tubulointerstitial nephritis mouse model. Histochem. Cell Biol. 2009, 131, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Edvardsson, V.; Palsson, R.; Olafsson, I.; Hjaltadottir, G.; Laxdal, T. Clinical features and genotype of adenine phosphoribosyltransferase deficiency in iceland. Am. J. Kidney Dis. 2001, 38, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Engle, S.J.; Stockelman, M.G.; Chen, J.; Boivin, G.; Yum, M.N.; Davies, P.M.; Ying, M.Y.; Sahota, A.; Simmonds, H.A.; Stambrook, P.J.; et al. Adenine phosphoribosyltransferase-deficient mice develop 2,8-dihydroxyadenine nephrolithiasis. Proc. Natl. Acad. Sci. USA 1996, 93, 5307–5312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, L.; Lin, W.; Yin, S.; Duan, A.; Liu, Z.; Cao, W. Rhein reverses klotho repression via promoter demethylation and protects against kidney and bone injuries in mice with chronic kidney disease. Kidney Int. 2017, 91, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Koh, N.; Fujimori, T.; Nishiguchi, S.; Tamori, A.; Shiomi, S.; Nakatani, T.; Sugimura, K.; Kishimoto, T.; Kinoshita, S.; Kuroki, T.; et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem. Biophys. Res. Commun. 2001, 280, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shi, M.; Zhang, J.; Addo, T.; Cho, H.J.; Barker, S.L.; Ravikumar, P.; Gillings, N.; Bian, A.; Sidhu, S.S.; et al. Renal production, uptake, and handling of circulating αklotho. J. Am. Soc. Nephrol. 2016, 27, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Li, Y.; Chen, F.; Yin, S.; Liu, Z.; Cao, W. Klotho preservation via histone deacetylase inhibition attenuates chronic kidney disease-associated bone injury in mice. Sci. Rep. 2017, 7, 46195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.C.; Shi, M.; Zhang, J.; Quinones, H.; Griffith, C.; Kuro-o, M.; Moe, O.W. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2011, 22, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, E.K.; Geleijnse, J.M.; Kromhout, D.; Giltay, E.J. No effect of n-3 fatty acids on high-sensitivity c-reactive protein after myocardial infarction: The alpha omega trial. Eur. J. Prev. Cardiol. 2014, 21, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Geelen, A.; Brouwer, I.A.; Schouten, E.G.; Kluft, C.; Katan, M.B.; Zock, P.L. Intake of n-3 fatty acids from fish does not lower serum concentrations of c-reactive protein in healthy subjects. Eur. J. Clin. Nutr. 2004, 58, 1440–1442. [Google Scholar] [CrossRef] [PubMed]
- Madsen, T.; Schmidt, E.B.; Christensen, J.H. The effect of n-3 fatty acids on c-reactive protein levels in patients with chronic renal failure. J. Ren. Nutr. 2007, 17, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.B.; Caldas, H.C.; Toloni, L.D.; Baptista, M.A.; Fernandes, I.M.; Abbud-Filho, M. Supplementation with omega-3 polyunsaturated fatty acids and experimental tacrolimus-induced nephrotoxicity. Exp. Clin. Transplant. 2014, 12, 522–527. [Google Scholar] [PubMed]
- Devassy, J.G.; Yamaguchi, T.; Monirujjaman, M.; Gabbs, M.; Ravandi, A.; Zhou, J.; Aukema, H.M. Distinct effects of dietary flax compared to fish oil, soy protein compared to casein, and sex on the renal oxylipin profile in models of polycystic kidney disease. Prostaglandins Leukot Essent Fatty Acids 2017, 123, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Nagasu, H.; Morita, Y.; Yamaguchi, T.P.; Kanwar, Y.S.; Kashihara, N. Klotho protects against mouse renal fibrosis by inhibiting Wnt signaling. Am. J. Physiol. Ren. Physiol. 2012, 303, F1641–F1651. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, H.; Yoshida, T.; Shiohira, S.; Kohei, J.; Mitobe, M.; Kurosu, H.; Kuro-o, M.; Nitta, K.; Tsuchiya, K. Reduced klotho expression level in kidney aggravates renal interstitial fibrosis. Am. J. Physiol. Ren. Physiol. 2012, 302, F1252–F1264. [Google Scholar] [CrossRef] [PubMed]
- Adema, A.Y.; van Ittersum, F.J.; Hoenderop, J.G.; de Borst, M.H.; Nanayakkara, P.W.; Ter Wee, P.M.; Heijboer, A.C.; Vervloet, M.G.; NIGRAM consortium. Reduction of oxidative stress in chronic kidney disease does not increase circulating α-klotho concentrations. PLoS ONE 2016, 11, e0144121. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, C.; Nie, L.; Zhao, X.; Gu, J.; Guan, X.; Wang, S.; Xiao, T.; Xu, X.; He, T.; et al. Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J. Am. Soc. Nephrol. 2015, 26, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; David, V.; Martin, A.; Huang, J.; Li, H.; Jiao, Y.; Gu, W.; Quarles, L.D. A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse ckd model. PLoS ONE 2012, 7, e44161. [Google Scholar] [CrossRef] [PubMed]
- Neyra, J.A.; Hu, M.C. Potential application of klotho in human chronic kidney disease. Bone 2017, 100, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Asai, O.; Nakatani, K.; Tanaka, T.; Sakan, H.; Imura, A.; Yoshimoto, S.; Samejima, K.; Yamaguchi, Y.; Matsui, M.; Akai, Y.; et al. Decreased renal α-klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int. 2012, 81, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Zhang, H.; Lin, J.; Zhang, C.; Wu, Q.; Ding, X. Elevated klotho promoter methylation is associated with severity of chronic kidney disease. PLoS ONE 2013, 8, e79856. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Chang, S.C.; Wu, M.S. Suppression of klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int. 2012, 81, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, H.; Vieth, E.; Lee, J.; Segar, M.; Liu, Y.; Nephew, K.P.; Matei, D. TGF-β induces global changes in DNA methylation during the epithelial-to-mesenchymal transition in ovarian cancer cells. Epigenetics 2014, 9, 1461–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Andres, O.; Sanchez-Nino, M.D.; Moreno, J.A.; Ruiz-Ortega, M.; Ramos, A.M.; Sanz, A.B.; Ortiz, A. Downregulation of kidney protective factors by inflammation: Role of transcription factors and epigenetic mechanisms. Am. J. Physiol. Ren. Physiol. 2016, 311, F1329–F1340. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Kaneko, Y.; Yawata, T.; Uyama, H.; Ozono, S.; Motomiya, Y.; Hirao, Y. Reversibility of adenine-induced renal failure in rats. Clin. Exp. Nephrol. 1999, 3, 82–88. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henao Agudelo, J.S.; Baia, L.C.; Ormanji, M.S.; Santos, A.R.P.; Machado, J.R.; Saraiva Câmara, N.O.; Navis, G.J.; De Borst, M.H.; Heilberg, I.P. Fish Oil Supplementation Reduces Inflammation but Does Not Restore Renal Function and Klotho Expression in an Adenine-Induced CKD Model. Nutrients 2018, 10, 1283. https://doi.org/10.3390/nu10091283
Henao Agudelo JS, Baia LC, Ormanji MS, Santos ARP, Machado JR, Saraiva Câmara NO, Navis GJ, De Borst MH, Heilberg IP. Fish Oil Supplementation Reduces Inflammation but Does Not Restore Renal Function and Klotho Expression in an Adenine-Induced CKD Model. Nutrients. 2018; 10(9):1283. https://doi.org/10.3390/nu10091283
Chicago/Turabian StyleHenao Agudelo, Juan S., Leandro C. Baia, Milene S. Ormanji, Amandda R. P. Santos, Juliana R. Machado, Niels O. Saraiva Câmara, Gerjan J. Navis, Martin H. De Borst, and Ita P. Heilberg. 2018. "Fish Oil Supplementation Reduces Inflammation but Does Not Restore Renal Function and Klotho Expression in an Adenine-Induced CKD Model" Nutrients 10, no. 9: 1283. https://doi.org/10.3390/nu10091283
APA StyleHenao Agudelo, J. S., Baia, L. C., Ormanji, M. S., Santos, A. R. P., Machado, J. R., Saraiva Câmara, N. O., Navis, G. J., De Borst, M. H., & Heilberg, I. P. (2018). Fish Oil Supplementation Reduces Inflammation but Does Not Restore Renal Function and Klotho Expression in an Adenine-Induced CKD Model. Nutrients, 10(9), 1283. https://doi.org/10.3390/nu10091283




