Fat Addiction: Psychological and Physiological Trajectory
Abstract
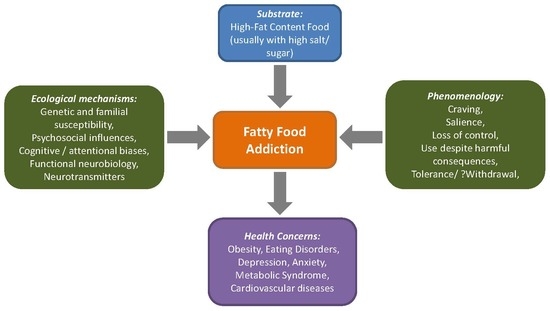
:1. Introduction
2. The Construct of Fat Rich Food Addiction
2.1. Defining Fatty Food Addiction in the Context of Nutrient Intake
2.2. Clinical and Epidemiological Implications of Addiction towards Fat
2.3. Measurement Approaches
3. Psychological Correlates of Addiction to Fat Rich Diets
3.1. Attentional Biases and Cognitive Functioning
3.2. Craving and Liking
4. Understanding the Physiological and Neurobiological Processes of Fat Food Addiction
4.1. Animal Models for Understanding the Addiction to Fat Rich Foods
4.2. Neurotransmitters Including Dopamine
4.3. Neuroimaging Correlates
4.4. Genetics Underpinnings
5. Conclusions, Limitations and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, K.P. Dietary spices in health and diseases: I. Ind. J. Physiol. Pharmacol. 2008, 52, 106–122. [Google Scholar]
- Kochhar, K.P. Dietary spices in health and diseases (II). Ind. J. Physiol. Pharmacol. 2008, 52, 327–354. [Google Scholar]
- Erlanson-Albertsson, C. Fat-rich food palatability and appetite regulation. In Fat Detection: Taste, Texture, and Post Ingestive Effects; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Drewnowski, A. Obesity, diets, and social inequalities. Nutr. Rev. 2009, 67 (Suppl. 1), S36–S39. [Google Scholar] [CrossRef]
- Popkin, B.M. Global nutrition dynamics: The world is shifting rapidly toward a diet linked with noncommunicable diseases. Am. J. Clin. Nutr. 2006, 84, 289–298. [Google Scholar] [CrossRef]
- Popkin, B.M.; Gordon-Larsen, P. The nutrition transition: Worldwide obesity dynamics and their determinants. Int. J. Obes. 2004, 28, S2–S9. [Google Scholar] [CrossRef]
- Hebebrand, J.; Albayrak, Ö.; Adan, R.; Antel, J.; Dieguez, C.; de Jong, J.; Leng, G.; Menzies, J.; Mercer, J.G.; Murphy, M.; et al. “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neurosci. Biobehav. Rev. 2014, 47, 295–306. [Google Scholar] [CrossRef]
- Gordon, E.L.; Ariel-Donges, A.H.; Bauman, V.; Merlo, L.J. What Is the Evidence for “Food Addiction?” A Systematic Review. Nutrients 2018, 10, 477. [Google Scholar] [CrossRef]
- Lerma-Cabrera, J.M.; Carvajal, F.; Lopez-Legarrea, P. Food addiction as a new piece of the obesity framework. Nutr. J. 2015, 15, 5. [Google Scholar] [CrossRef]
- Bassareo, V.; Gambarana, C. Food and Its Effect on the Brain: From Physiological to Compulsive Consumption. Front. Psychiatry 2019, 10, 209. [Google Scholar] [CrossRef]
- Fernandez-Aranda, F.; Karwautz, A.; Treasure, J. Food addiction: A transdiagnostic construct of increasing interest. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2018, 26, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Pelchat, M.L. Food addiction in humans. J. Nutr. 2009, 139, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Shriner, R.; Gold, M. Food addiction: An evolving nonlinear science. Nutrients 2014, 6, 5370–5391. [Google Scholar] [CrossRef] [PubMed]
- Golay, A.; Bobbioni, E. The role of dietary fat in obesity. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1997, 21 (Suppl. 3), S2–S11. [Google Scholar]
- Smilowitz, J.T.; German, J.B.; Zivkovic, A.M. Food Intake and Obesity: The Case of Fat. In Fat Detection: Taste, Texture, and Post Ingestive Effects; Frontiers in Neuroscience; Montmayeur, J.-P., le Coutre, J., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010; ISBN 978-1-4200-6775-0. [Google Scholar]
- Meule, A. A Critical Examination of the Practical Implications Derived from the Food Addiction Concept. Curr. Obes. Rep. 2019, 8, 11–17. [Google Scholar] [CrossRef]
- Cassin, S.E.; Buchman, D.Z.; Leung, S.E.; Kantarovich, K.; Hawa, A.; Carter, A.; Sockalingam, S. Ethical, Stigma, and Policy Implications of Food Addiction: A Scoping Review. Nutrients 2019, 11, 710. [Google Scholar] [CrossRef]
- Khan, N.A.; Besnard, P. Oro-sensory perception of dietary lipids: New insights into the fat taste transduction. Biochim. Biophys. Acta 2009, 1791, 149–155. [Google Scholar] [CrossRef]
- Mattes, R.D. Fat taste and lipid metabolism in humans. Physiol. Behav. 2005, 86, 691–697. [Google Scholar] [CrossRef]
- Drewnowski, A.; Mennella, J.A.; Johnson, S.L.; Bellisle, F. Sweetness and food preference. J. Nutr. 2012, 142, 1142S–1148S. [Google Scholar] [CrossRef]
- Sellayah, D.; Cagampang, F.R.; Cox, R.D. On the evolutionary origins of obesity: A new hypothesis. Endocrinology 2014, 155, 1573–1588. [Google Scholar] [CrossRef]
- Reddon, H.; Patel, Y.; Turcotte, M.; Pigeyre, M.; Meyre, D. Revisiting the evolutionary origins of obesity: Lazy versus peppy-thrifty genotype hypothesis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2018, 19, 1525–1543. [Google Scholar] [CrossRef] [PubMed]
- Genné-Bacon, E.A. Thinking evolutionarily about obesity. Yale J. Biol. Med. 2014, 87, 99–112. [Google Scholar] [PubMed]
- Clifton, P.M.; Keogh, J.B. A systematic review of the effect of dietary saturated and polyunsaturated fat on heart disease. Nutr. Metab. Cardiovasc. Dis. NMCD 2017, 27, 1060–1080. [Google Scholar] [CrossRef] [PubMed]
- Power, R.; Prado-Cabrero, A.; Mulcahy, R.; Howard, A.; Nolan, J.M. The Role of Nutrition for the Aging Population: Implications for Cognition and Alzheimer’s Disease. Annu. Rev. Food Sci. Technol. 2019, 10, 619–639. [Google Scholar] [CrossRef] [PubMed]
- Besnard, P.; Passilly-Degrace, P.; Khan, N.A. Taste of Fat: A Sixth Taste Modality? Physiol. Rev. 2016, 96, 151–176. [Google Scholar] [CrossRef]
- Val-Laillet, D.; Aarts, E.; Weber, B.; Ferrari, M.; Quaresima, V.; Stoeckel, L.E.; Alonso-Alonso, M.; Audette, M.; Malbert, C.H.; Stice, E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. NeuroImage Clin. 2015, 8, 1–31. [Google Scholar] [CrossRef]
- Schmidt, U.; Campbell, I.C. Treatment of eating disorders can not remain “brainless”: The case for brain-directed treatments. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2013, 21, 425–427. [Google Scholar] [CrossRef]
- Rogers, P.J. Food and drug addictions: Similarities and differences. Pharmacol. Biochem. Behav. 2017, 153, 182–190. [Google Scholar] [CrossRef]
- Hone-Blanchet, A.; Fecteau, S. Overlap of food addiction and substance use disorders definitions: Analysis of animal and human studies. Neuropharmacology 2014, 85, 81–90. [Google Scholar] [CrossRef]
- Meule, A.; Gearhardt, A.N. Food addiction in the light of DSM-5. Nutrients 2014, 6, 3653–3671. [Google Scholar] [CrossRef]
- Schreiber, L.R.N.; Odlaug, B.L.; Grant, J.E. The overlap between binge eating disorder and substance use disorders: Diagnosis and neurobiology. J. Behav. Addict. 2013, 2, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Citrome, L. A primer on binge eating disorder diagnosis and management. CNS Spectr. 2015, 20 (Suppl. 1), 44–50. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.C.; Van Wijk, M.; Rowsell, M. Symptoms of “food addiction” in binge eating disorder using the Yale Food Addiction Scale version 2.0. Appetite 2019, 133, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Linardon, J.; Messer, M. Assessment of food addiction using the Yale Food Addiction Scale 2.0 in individuals with binge-eating disorder symptomatology: Factor structure, psychometric properties, and clinical significance. Psychiatry Res. 2019, 279, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Burrows, T.; Skinner, J.; McKenna, R.; Rollo, M. Food Addiction, Binge Eating Disorder, and Obesity: Is There a Relationship? Behav. Sci. Basel Switz. 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pursey, K.M.; Stanwell, P.; Gearhardt, A.N.; Collins, C.E.; Burrows, T.L. The prevalence of food addiction as assessed by the Yale Food Addiction Scale: A systematic review. Nutrients 2014, 6, 4552–4590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penzenstadler, L.; Soares, C.; Karila, L.; Khazaal, Y. Systematic Review of Food Addiction as Measured With the Yale Food Addiction Scale: Implications for the Food Addiction Construct. Curr. Neuropharmacol. 2018, 16, 520. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, Y.; Han, Y.; Liu, Y.; Yang, K.; Zhen, S.; Wen, D. Psychosocial Correlates of Food Addiction and Its Association with Quality of Life in a Non-Clinical Adolescent Sample. Nutrients 2018, 10, 837. [Google Scholar] [CrossRef] [Green Version]
- Ayaz, A.; Nergiz-Unal, R.; Dedebayraktar, D.; Akyol, A.; Pekcan, A.G.; Besler, H.T.; Buyuktuncer, Z. How does food addiction influence dietary intake profile? PLoS ONE 2018, 13, e0195541. [Google Scholar] [CrossRef] [Green Version]
- Pursey, K.M.; Collins, C.E.; Stanwell, P.; Burrows, T.L. Foods and dietary profiles associated with “food addiction” in young adults. Addict. Behav. Rep. 2015, 2, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Ivezaj, V.; Wiedemann, A.A.; Grilo, C.M. Food addiction and bariatric surgery: A systematic review of the literature. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2017, 18, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Stein, R.I.; Eagon, J.C.; Klein, S. Bariatric surgery-induced weight loss causes remission of food addiction in extreme obesity. Obes. Silver Spring Md. 2014, 22, 1792–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevinçer, G.M.; Konuk, N.; Bozkurt, S.; Coşkun, H. Food addiction and the outcome of bariatric surgery at 1-year: Prospective observational study. Psychiatry Res. 2016, 244, 159–164. [Google Scholar] [CrossRef]
- Guerrero Pérez, F.; Sánchez-González, J.; Sánchez, I.; Jiménez-Murcia, S.; Granero, R.; Simó-Servat, A.; Ruiz, A.; Virgili, N.; López-Urdiales, R.; Montserrat-Gil de Bernabe, M.; et al. Food addiction and preoperative weight loss achievement in patients seeking bariatric surgery. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2018, 26, 645–656. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Preliminary validation of the Yale food addiction scale. Appetite 2009, 52, 430–436. [Google Scholar] [CrossRef] [Green Version]
- Gearhardt, A.N.; Roberto, C.A.; Seamans, M.J.; Corbin, W.R.; Brownell, K.D. Preliminary validation of the Yale Food Addiction Scale for children. Eat. Behav. 2013, 14, 508–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Tang, Z.; Guo, G.; Liu, X.; Xiao, S. The Chinese version of the Yale Food Addiction Scale: An examination of its validation in a sample of female adolescents. Eat. Behav. 2015, 18, 97–102. [Google Scholar] [CrossRef]
- Nantha, Y.S.; Patah, N.A.A.; Pillai, M.P. Preliminary validation of the Malay Yale Food Addiction Scale: Factor structure and item analysis in an obese population. Clin. Nutr. ESPEN 2016, 16, 42–47. [Google Scholar] [CrossRef]
- Brunault, P.; Ballon, N.; Gaillard, P.; Réveillère, C.; Courtois, R. Validation of the French version of the Yale Food Addiction Scale: An examination of its factor structure, reliability, and construct validity in a nonclinical sample. Can. J. Psychiatry 2014, 59, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Development of the Yale Food Addiction Scale Version 2.0. Psychol. Addict. Behav. J. Soc. Psychol. Addict. Behav. 2016, 30, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Wardle, J.; Guthrie, C.A.; Sanderson, S.; Rapoport, L. Development of the children’s eating behaviour questionnaire. J. Child Psychol. Psychiatry 2001, 42, 963–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cepeda-Benito, A.; Gleaves, D.H.; Williams, T.L.; Erath, S.A. The development and validation of the state and trait food-cravings questionnaires. Behav. Ther. 2000, 31, 151–173. [Google Scholar] [CrossRef]
- Schlundt, D.G.; Hargreaves, M.K.; Buchowski, M.S. The eating behavior patterns questionnaire predicts dietary fat intake in African American women. J. Am. Diet. Assoc. 2003, 103, 338–345. [Google Scholar] [PubMed]
- Cappelleri, J.C.; Bushmakin, A.G.; Gerber, R.A.; Leidy, N.K.; Sexton, C.C.; Karlsson, J.; Lowe, M.R. Evaluating the Power of Food Scale in obese subjects and a general sample of individuals: Development and measurement properties. Int. J. Obes. 2009, 33, 913–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batterink, L.; Yokum, S.; Stice, E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: An fMRI study. NeuroImage 2010, 52, 1696–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldman, R.L.; Canterberry, M.; Borckardt, J.J.; Madan, A.; Byrne, T.K.; George, M.S.; O’Neil, P.M.; Hanlon, C.A. Executive control circuitry differentiates degree of success in weight loss following gastric-bypass surgery. Obes. Silver Spring Md. 2013, 21, 2189–2196. [Google Scholar] [CrossRef] [Green Version]
- Rodrigue, C.; Ouellette, A.-S.; Lemieux, S.; Tchernof, A.; Biertho, L.; Bégin, C. Executive functioning and psychological symptoms in food addiction: A study among individuals with severe obesity. Eat. Weight Disord. EWD 2018, 23, 469–478. [Google Scholar] [CrossRef]
- Franken, I.H.A.; Nijs, I.M.T.; Toes, A.; van der Veen, F.M. Food addiction is associated with impaired performance monitoring. Biol. Psychol. 2018, 131, 49–53. [Google Scholar] [CrossRef]
- Steward, T.; Mestre-Bach, G.; Vintró-Alcaraz, C.; Lozano-Madrid, M.; Agüera, Z.; Fernández-Formoso, J.A.; Granero, R.; Jiménez-Murcia, S.; Vilarrasa, N.; García-Ruiz-de-Gordejuela, A.; et al. Food addiction and impaired executive functions in women with obesity. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2018, 26, 574–584. [Google Scholar] [CrossRef]
- Blume, M.; Schmidt, R.; Hilbert, A. Executive Functioning in Obesity, Food Addiction, and Binge-Eating Disorder. Nutrients 2018, 11, 54. [Google Scholar] [CrossRef] [Green Version]
- Ruddock, H.K.; Field, M.; Jones, A.; Hardman, C.A. State and trait influences on attentional bias to food-cues: The role of hunger, expectancy, and self-perceived food addiction. Appetite 2018, 131, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Frayn, M.; Sears, C.R.; von Ranson, K.M. A sad mood increases attention to unhealthy food images in women with food addiction. Appetite 2016, 100, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; Treat, T.A.; Hollingworth, A.; Corbin, W.R. The relationship between eating-related individual differences and visual attention to foods high in added fat and sugar. Eat. Behav. 2012, 13, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Havermans, R.C. “You Say it’s Liking, I Say it’s Wanting…”. On the difficulty of disentangling food reward in man. Appetite 2011, 57, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Mela, D.J. Why do we like what we like? J. Sci. Food Agric. 2001, 81, 10–16. [Google Scholar] [CrossRef]
- Pelchat, M.L. Of human bondage: Food craving, obsession, compulsion, and addiction. Physiol. Behav. 2002, 76, 347–352. [Google Scholar] [CrossRef]
- Méjean, C.; Deglaire, A.; Kesse-Guyot, E.; Hercberg, S.; Schlich, P.; Castetbon, K. Association between intake of nutrients and food groups and liking for fat (The Nutrinet-Santé Study). Appetite 2014, 78, 147–155. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Rizk, M.T.; Treat, T.A. The association of food characteristics and individual differences with ratings of craving and liking. Appetite 2014, 79, 166–173. [Google Scholar] [CrossRef]
- Volkow, N.; Wang, G.J.; Fowler, J.S.; Tomasi, D.; Baler, R. Food and drug reward: Overlapping circuits in human obesity and addiction. In Brain Imaging in Behavioral Neuroscience; Springer: Berlin, Germany, 2011; pp. 1–24. [Google Scholar]
- Fortuna, J.L. Sweet preference, sugar addiction and the familial history of alcohol dependence: Shared neural pathways and genes. J. Psychoact. Drugs 2010, 42, 147–151. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Lenoir, M.; Guillem, K. Neurobiology of addiction versus drug use driven by lack of choice. Curr. Opin. Neurobiol. 2013, 23, 581–587. [Google Scholar] [CrossRef]
- Ziauddeen, H.; Farooqi, I.S.; Fletcher, P.C. Obesity and the brain: How convincing is the addiction model? Nat. Rev. Neurosci. 2012, 13, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.; Sizemore, G.M. Animal models of addiction: Fat and sugar. Curr. Pharm. Des. 2011, 17, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Novelle, M.G.; Diéguez, C. Food Addiction and Binge Eating: Lessons Learned from Animal Models. Nutrients 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, J.W.; Vanderschuren, L.J.M.J.; Adan, R.A.H. Towards an animal model of food addiction. Obes. Facts 2012, 5, 180–195. [Google Scholar] [CrossRef]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D.; Faria, A.; Calhau, C. High-fat diet-induced obesity Rat model: A comparison between Wistar and Sprague-Dawley Rat. Adipocyte 2016, 5, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Avena, N.M. Examining the addictive-like properties of binge eating using an animal model of sugar dependence. Exp. Clin. Psychopharmacol. 2007, 15, 481–491. [Google Scholar] [CrossRef]
- Avena, N.M.; Rada, P.; Hoebel, B.G. Sugar and fat bingeing have notable differences in addictive-like behavior. J. Nutr. 2009, 139, 623–628. [Google Scholar] [CrossRef]
- Wong, K.J.; Wojnicki, F.H.W.; Corwin, R.L.W. Baclofen, raclopride, and naltrexone differentially affect intake of fat/sucrose mixtures under limited access conditions. Pharmacol. Biochem. Behav. 2009, 92, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Liang, N.-C.; Hajnal, A.; Norgren, R. Sham feeding corn oil increases accumbens dopamine in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1236–R1239. [Google Scholar] [CrossRef] [Green Version]
- Adachi, S.; Endo, Y.; Mizushige, T.; Tsuzuki, S.; Matsumura, S.; Inoue, K.; Fushiki, T. Increased levels of extracellular dopamine in the nucleus accumbens and amygdala of rats by ingesting a low concentration of a long-chain Fatty Acid. Biosci. Biotechnol. Biochem. 2013, 77, 2175–2180. [Google Scholar] [CrossRef]
- Dela Cruz, J.A.D.; Coke, T.; Bodnar, R.J. Simultaneous Detection of c-Fos Activation from Mesolimbic and Mesocortical Dopamine Reward Sites Following Naive Sugar and Fat Ingestion in Rats. J. Vis. Exp. JoVE 2016. [Google Scholar] [CrossRef]
- Dela Cruz, J.A.D.; Coke, T.; Karagiorgis, T.; Sampson, C.; Icaza-Cukali, D.; Kest, K.; Ranaldi, R.; Bodnar, R.J. c-Fos induction in mesotelencephalic dopamine pathway projection targets and dorsal striatum following oral intake of sugars and fats in rats. Brain Res. Bull. 2015, 111, 9–19. [Google Scholar] [CrossRef]
- Dela Cruz, J.A.D.; Icaza-Cukali, D.; Tayabali, H.; Sampson, C.; Galanopoulos, V.; Bamshad, D.; Touzani, K.; Sclafani, A.; Bodnar, R.J. Roles of dopamine D1 and D2 receptors in the acquisition and expression of fat-conditioned flavor preferences in rats. Neurobiol. Learn. Mem. 2012, 97, 332–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Gosnell, B.A.; Kelley, A.E. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J. Pharmacol. Exp. Ther. 1998, 285, 908–914. [Google Scholar] [PubMed]
- Ward, S.J.; Walker, E.A.; Dykstra, L.A. Effect of cannabinoid CB1 receptor antagonist SR141716A and CB1 receptor knockout on cue-induced reinstatement of Ensure and corn-oil seeking in mice. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2007, 32, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Brissard, L.; Leemput, J.; Hichami, A.; Passilly-Degrace, P.; Maquart, G.; Demizieux, L.; Degrace, P.; Khan, N.A. Orosensory Detection of Dietary Fatty Acids Is Altered in CB₁R−/− Mice. Nutrients 2018, 10, 1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterschmitt, Y.; Abdoul-Azize, S.; Murtaza, B.; Barbier, M.; Khan, A.S.; Millot, J.-L.; Khan, N.A. Fatty Acid Lingual Application Activates Gustatory and Reward Brain Circuits in the Mouse. Nutrients 2018, 10, 1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patriarca, L.; Magerowski, G.; Alonso-Alonso, M. Functional neuroimaging in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.J.; Cedernaes, J.; Schiöth, H.B. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: A meta-analysis of fMRI studies. PLoS ONE 2013, 8, e60393. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Yokum, S.; Orr, P.T.; Stice, E.; Corbin, W.R.; Brownell, K.D. The Neural Correlates of “Food Addiction”. Arch. Gen. Psychiatry 2011, 68, 808–816. [Google Scholar] [CrossRef]
- Hsu, J.-S.; Wang, P.-W.; Ko, C.-H.; Hsieh, T.-J.; Chen, C.-Y.; Yen, J.-Y. Altered brain correlates of response inhibition and error processing in females with obesity and sweet food addiction: A functional magnetic imaging study. Obes. Res. Clin. Pract. 2017, 11, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Rodriguez, O.; Burrows, T.; Pursey, K.M.; Stanwell, P.; Parkes, L.; Soriano-Mas, C.; Verdejo-Garcia, A. Food addiction linked to changes in ventral striatum functional connectivity between fasting and satiety. Appetite 2019, 133, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Loxton, N.J.; Levitan, R.D.; Kaplan, A.S.; Carter, J.C.; Kennedy, J.L. “Food addiction” and its association with a dopaminergic multilocus genetic profile. Physiol. Behav. 2013, 118, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Pedram, P.; Zhai, G.; Gulliver, W.; Zhang, H.; Sun, G. Two novel candidate genes identified in adults from the Newfoundland population with addictive tendencies towards food. Appetite 2017, 115, 71–79. [Google Scholar] [CrossRef]
- Yeh, J.; Trang, A.; Henning, S.M.; Wilhalme, H.; Carpenter, C.; Heber, D.; Li, Z. Food Cravings, Food Addiction, and a Dopamine-Resistant (DRD2 A1) Receptor Polymorphism in Asian American College Students. Asia Pac. J. Clin. Nutr. 2016, 25, 424–429. [Google Scholar]
- Cornelis, M.C.; Flint, A.; Field, A.E.; Kraft, P.; Han, J.; Rimm, E.B.; van Dam, R.M. A genome-wide investigation of food addiction. Obes. Silver Spring Md. 2016, 24, 1336–1341. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.S.; Murtaza, B.; Hichami, A.; Khan, N.A. A cross-talk between fat and bitter taste modalities. Biochimie 2019, 159, 3–8. [Google Scholar] [CrossRef]
- Love-Gregory, L.; Abumrad, N. CD36 genetics and the metabolic complications of obesity. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 527–534. [Google Scholar] [CrossRef]
- Mrizak, I.; Šerý, O.; Plesnik, J.; Arfa, A.; Fekih, M.; Bouslema, A.; Zaouali, M.; Tabka, Z.; Khan, N.A. The a allele of cluster of differentiation 36 (CD36) SNP 1761667 associates with decreased lipid taste perception in obese Tunisian women. Br. J. Nutr. 2015, 113, 1330–1337. [Google Scholar] [CrossRef] [Green Version]
- Melis, M.; Carta, G.; Pintus, S.; Pintus, P.; Piras, C.A.; Murru, E.; Manca, C.; Di Marzo, V.; Banni, S.; Tomassini Barbarossa, I. Polymorphism rs1761667 in the CD36 Gene Is Associated to Changes in Fatty Acid Metabolism and Circulating Endocannabinoid Levels Distinctively in Normal Weight and Obese Subjects. Front. Physiol. 2017, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Plesník, J.; Serý, O.; Khan, A.; Bielik, P.; Khan, N.A. The rs1527483, but not rs3212018, CD36 polymorphism associates with linoleic acid detection and obesity in Czech young adults. Br. J. Nutr. 2018, 119, 1–7. [Google Scholar] [CrossRef] [Green Version]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, S.; Kochhar, K.P.; Khan, N.A. Fat Addiction: Psychological and Physiological Trajectory. Nutrients 2019, 11, 2785. https://doi.org/10.3390/nu11112785
Sarkar S, Kochhar KP, Khan NA. Fat Addiction: Psychological and Physiological Trajectory. Nutrients. 2019; 11(11):2785. https://doi.org/10.3390/nu11112785
Chicago/Turabian StyleSarkar, Siddharth, Kanwal Preet Kochhar, and Naim Akhtar Khan. 2019. "Fat Addiction: Psychological and Physiological Trajectory" Nutrients 11, no. 11: 2785. https://doi.org/10.3390/nu11112785