Impact of Malnutrition on Long-Term Mortality in Elderly Patients with Acute Myocardial Infarction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Diagnosis of AMI
2.3. Mini Nutritional Assessment (MNA)
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Follow-Up Analysis
4. Discussion
Study Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kaiser, M.J.; Bauer, J.M.; Rämsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.S.; Charlton, K.E.; Maggio, M.; et al. Mini Nutritional Assessment International Group Frequency of malnutrition in older adults: A multinational perspective using the mini nutritional assessment. J. Am. Geriatr. Soc. 2010, 58, 1734–1738. [Google Scholar] [CrossRef]
- Rojer, A.G.; Kruizenga, H.M.; Trappenburg, M.C.; Reijnierse, E.M.; Sipila, S.; Narici, M.V. The prevalence of malnutrition according to the new ESPEN definition in four diverse populations. Clin. Nutr. 2016, 35, 758–762. [Google Scholar] [CrossRef]
- Bonetti, L.; Terzoni, S.; Lusignani, M.; Negri, M.; Froldi, M.; Destrebecq, A. Prevalence of malnutrition among older people in medical and surgical wards in hospital and quality of nutritional care: A multicenter, cross-sectional study. J. Clin. Nurs. 2017, 26, 5082–5092. [Google Scholar] [CrossRef]
- Wolters, M.; Volkert, D.; Streicher, M.; Kiesswetter, E.; Torbahn, G.; O’Connor, E.M.; O’Keeffe, M.; Kelly, M.; O’Herlihy, E.; O’Toole, P.W.; et al. Prevalence of malnutrition using harmonized definitions in older adults from different settings—A MaNuEL study. Clin. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Doehner, W.; Anker, S.D. Nutrition, metabolism, and the complex pathophysiology of cachexia in chronic heart failure. Cardiovasc Res. 2007, 73, 298–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salva, A.; Coll-Planas, L.; Bruce, S.; De Groot, L.; Andrieu, S.; Abellan, G.; Vellas, B. Task Force on Nutrition and Ageing of the IAGG and the IANA. Nutritional assessment of residents in long-term care facilities (LTCFs): Recommendations of the task force on nutrition and ageing of the IAGG European region and the IANA. J. Nutr. Health Aging 2009, 13, 475–483. [Google Scholar] [PubMed]
- Komici, K.; Vitale, D.F.; Leosco, D.; Mancini, A.; Corbi, G.; Bencivenga, L.; Mezzani, A.; Trimarco, B.; Morisco, C.; Ferrara, N.; et al. Pressure injuries in elderly with acute myocardial infarction. Clin. Interv. Aging 2017, 12, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.J.; Buitrago, G.; Rodríguez, N.; Gómez, G.; Sulo, S.; Gómez, C.; Partridge, J.; Misas, J.; Dennis, R.; Alba, M.J.; et al. Clinical and economic outcomes associated with malnutrition in hospitalized patients. Clin. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Corbi, G.; Conti, V.; Davinelli, S.; Scapagnini, G.; Filippelli, A.; Ferrara, N. Dietary Phytochemicals in Neuroimmunoaging: A New Therapeutic Possibility for Humans? Front. Pharmacol. 2016, 7, 364. [Google Scholar] [CrossRef] [PubMed]
- Lundin, H.; Sääf, M.; Strender, L.E.; Mollasaraie, H.A.; Salminen, H. Mini nutritional assessment and 10-year mortality in free-living elderly women: A prospective cohort study with 10-year follow-up. Eur. J. Clin. Nutr. 2012, 66, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Carro, A.; Kaski, J.C. Myocardial Infarction in the Elderly. Aging Dis. 2011, 2, 116–137. [Google Scholar] [PubMed]
- De Lucia, C.; Femminella, G.D.; Rengo, G.; Ruffo, A.; Parisi, V.; Pagano, G.; Liccardo, D.; Cannavo, A.; Iacotucci, P.; Komici, K.; et al. Risk of acute myocardial infarction after transurethral resection of prostate in elderly. BMC Surg. 2013, 13, S35. [Google Scholar] [CrossRef] [PubMed]
- Eagle, K.A.; Lim, M.J.; Dabbous, O.H.; Pieper, K.S.; Goldberg, R.J.; Van de Werf, F.; Goodman, S.G.; Granger, C.B.; Steg, P.G.; Gore, J.M.; et al. A validated prediction model for all forms of acute coronary syndrome: Estimating the risk of 6-month postdischarge death in an international registry. JAMA 2004, 291, 2727–2733. [Google Scholar] [CrossRef] [PubMed]
- Bucholz, E.M.; Krumholz, H.A.; Krumholz, H.M. Underweight, Markers of Cachexia, and Mortality in Acute Myocardial Infarction: A Prospective Cohort Study of Elderly Medicare Beneficiaries. PLoS Med. 2016, 13, e1001998. [Google Scholar] [CrossRef] [PubMed]
- Sujino, Y.; Tanno, J.; Nakano, S.; Funada, S.; Hosoi, Y.; Senbonmatsu, T.; Nishimura, S. Impact of hypoalbuminemia, frailty, and body mass index on early prognosis in older patients (>= 85 years) with ST-elevation myocardial infarction. J. Cardiol. 2015, 66, 263–268. [Google Scholar] [CrossRef]
- Yoo, S.H.; Kook, H.Y.; Hong, Y.J.; Kim, J.H.; Ahn, Y.; Jeong, M.H. Influence of undernutrition at admission on clinical outcomes in patients with acute myocardial infarction. J. Cardiol. 2017, 69, 555–560. [Google Scholar] [CrossRef]
- Killip, T.; Kimball, J.T. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am. J. Cardiol. 1967, 20, 457–464. [Google Scholar] [CrossRef]
- Aragam, K.G.; Tamhane, U.U.; Kline-Rogers, E.; Li, J.; Fox, K.A.; Goodman, S.G.; Eagle, K.A.; Gurm, H.S. Does simplicity compromise accuracy in ACS risk prediction? A retrospective analysis of the TIMI and GRACE risk scores. PLoS ONE 2009, 4, e7947. [Google Scholar] [CrossRef]
- Mendis, S.; Thygesen, K.; Kuulasmaa, K.; Giampaoli, S.; Mahonen, M.; Ngu Blackett, K.; Lisheng, L. Writing group on behalf of the participating experts of the WHO consultation for revision of WHO definition of myocardial infarction. World Health Organization definition of myocardial infarction: 2008–09 revision. Int. J. Epidemiol. 2011, 40, 139–146. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Third universal definition of myocardial infarction. Eur. Heart J. 2012, 33, 2551–2567. [Google Scholar] [CrossRef] [Green Version]
- Guigoz, Y.V.B.; Garry, P.J. Mini nutritonal assessment: A practical assessment tool for grading the nutritional state of elderly patients. Facts Res. Gerontol. 1994, 4, 15–59. [Google Scholar]
- Van Calster, B.; Vickers, A.J. Calibration of Risk Prediction Models:Impact on Decision-Analytic Performance. Med. Decis. Mak. 2015, 35, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J. Decision analysis for the evaluation of diagnostic tests, prediction models and molecular markers. Am. Stat. 2008, 62, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Cronin, A.M.; Elkin, E.B.; Gonen, M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med. Inform. Decis. Mak. 2008, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.J.; Coresh, J. Adjusting survival curves for confounders: A review and a new method. Am. J. Epidemiol. 1996, 143, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Van Wissen, J.; van Stijn, M.F.; Doodeman, H.J.; Houdijk, A.P. Mini Nutritional Assessment and Mortality after Hip Fracture Surgery in the Elderly. J. Nutr. Health Aging 2016, 20, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Malafarina, V.; Reginster, J.Y.; Cabrerizo, S.; Bruyère, O.; Kanis, J.A.; Martinez, J.A.; Zulet, M.A. Nutritional Status and Nutritional Treatment Are Related to Outcomes and Mortality in Older Adults with Hip Fracture. Nutrients 2018, 10, E555. [Google Scholar] [CrossRef] [PubMed]
- Basta, G.; Chatzianagnostou, K.; Paradossi, U.; Botto, N.; Del Turco, S.; Taddei, A.; Berti, S.; Mazzone, A. The prognostic impact of objective nutritional indices in elderly patients with ST-elevation myocardial infarction undergoing primary coronary intervention. Int. J. Cardiol. 2016, 221, 987–992. [Google Scholar] [CrossRef]
- Wada, H.; Dohi, T.; Miyauchi, K.; Doi, S.; Konishi, H.; Naito, R.; Tsuboi, S.; Ogita, M.; Kasai, T.; Okazaki, S.; et al. Prognostic impact of nutritional status assessed by the Controlling Nutritional Status score in patients with stable coronary artery disease undergoing percutaneous coronary intervention. Clin. Res. Cardiol. 2017, 106, 875–883. [Google Scholar] [CrossRef]
- Wada, H.; Dohi, T.; Miyauchi, K.; Shitara, J.; Endo, H.; Doi, S.; Konishi, H.; Naito, R.; Tsuboi, S.; Ogita, M.; et al. Pre-procedural neutrophil-to-lymphocyte ratio and long-term cardiac outcomes after percutaneous coronary intervention for stable coronary artery disease. Atherosclerosis 2017, 265, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.W.; Wong, C.K.; Herbison, P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am. Heart J. 2007, 153, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Rengo, G.; Pagano, G.; Filardi, P.P.; Femminella, G.D.; Parisi, V.; Cannavo, A.; Liccardo, D.; Komici, K.; Gambino, G.; D’Amico, M.L.; et al. Prognostic Value of Lymphocyte G Protein-Coupled Receptor Kinase-2 Protein Levels in Patients With Heart Failure. Circ. Res. 2016, 118, 1116–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breathett, K.; Allen, L.A.; Udelson, J.; Davis, G.; Bristow, M. Changes in Left Ventricular Ejection Fraction Predict Survival and Hospitalization in Heart Failure With Reduced Ejection Fraction. Circ. Heart Fail. 2016, 9, e002962. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, A.; Kohashi, K.; Morisawa, T.; Kosugi, M.; Endoh, I.; Kusama, Y.; Atarashi, H.; Shimizu, W. Nutritional status is associated with inflammation and predicts a poor outcome in patients with chronic heart Failure. J. Atheroscler. Thromb. 2016, 23, 713–717. [Google Scholar] [CrossRef]
- Baldacci, S.; Maio, S.; Simoni, M.; Cerrai, S.; Sarno, G.; Silvi, P.; Di Pede, F.; Borbotti, M.; Pala, A.P.; Bresciani, M.; et al. ARGA study group. The ARGA study with general practitioners: Impact of medical education on asthma/rhinitis management. Respir. Med. 2012, 106, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Izawa, K.P.; Watanabe, S.; Hirano, Y.; Yamamoto, S.; Oka, K.; Suzuki, N.; Kida, K.; Suzuki, K.; Osada, N.; Omiya, K.; et al. The relation between Geriatric Nutritional Risk Index and muscle mass, muscle strength, and exercise capacity in chronic heart failure patients. Int. J. Cardiol. 2014, 177, 1140–1141. [Google Scholar] [CrossRef]
- Rengo, G.; Galasso, G.; Piscione, F.; Golino, L.; Fortunato, F.; Zincarelli, C.; Cassese, S.; Abete, P.; Chiariello, M.; Rengo, F.; et al. An active lifestyle improves outcome of primary angioplasty in elderly patients with acute myocardial infarction. Am. Heart J. 2007, 154, 352–360. [Google Scholar] [CrossRef]
- Drewnowski, A.; Ewans, W.J. Nutrition, Physical Activity, and Quality of Life in Older Adults: Summary. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Allard, J.P.; Keller, H.; Jeejeebhoy, K.N.; Laporte, M.; Duerksen, D.R.; Gramlich, L.; Payette, H.; Bernier, P.; Vesnaver, E.; Davidson, B.; et al. Malnutrition at Hospital Admission-Contributors and Effect on Length of Stay: A Prospective Cohort Study From the Canadian Malnutrition Task Force. JPEN J. Parenter. Enteral. Nutr. 2016, 40, 487–497. [Google Scholar] [CrossRef]
- Heidegger, C.P.; Berger, M.M.; Graf, S.; Zingg, W.; Darmon, P.; Costanza, M.C.; Thibault, R.; Pichard, C. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: A randomised controlled clinical trial. Lancet 2013, 381, 385–393. [Google Scholar] [CrossRef]
- Kutsogiannis, J.; Alberda, C.; Gramlich, L.; Cahill, N.E.; Wang, M.; Day, A.G.; Dhaliwal, R.; Heyland, D.K. Early use of supplemental parenteral nutrition in critically ill patients: Results of an international multicenter observational study. Crit. Care Med. 2011, 39, 2691–2699. [Google Scholar] [CrossRef] [PubMed]

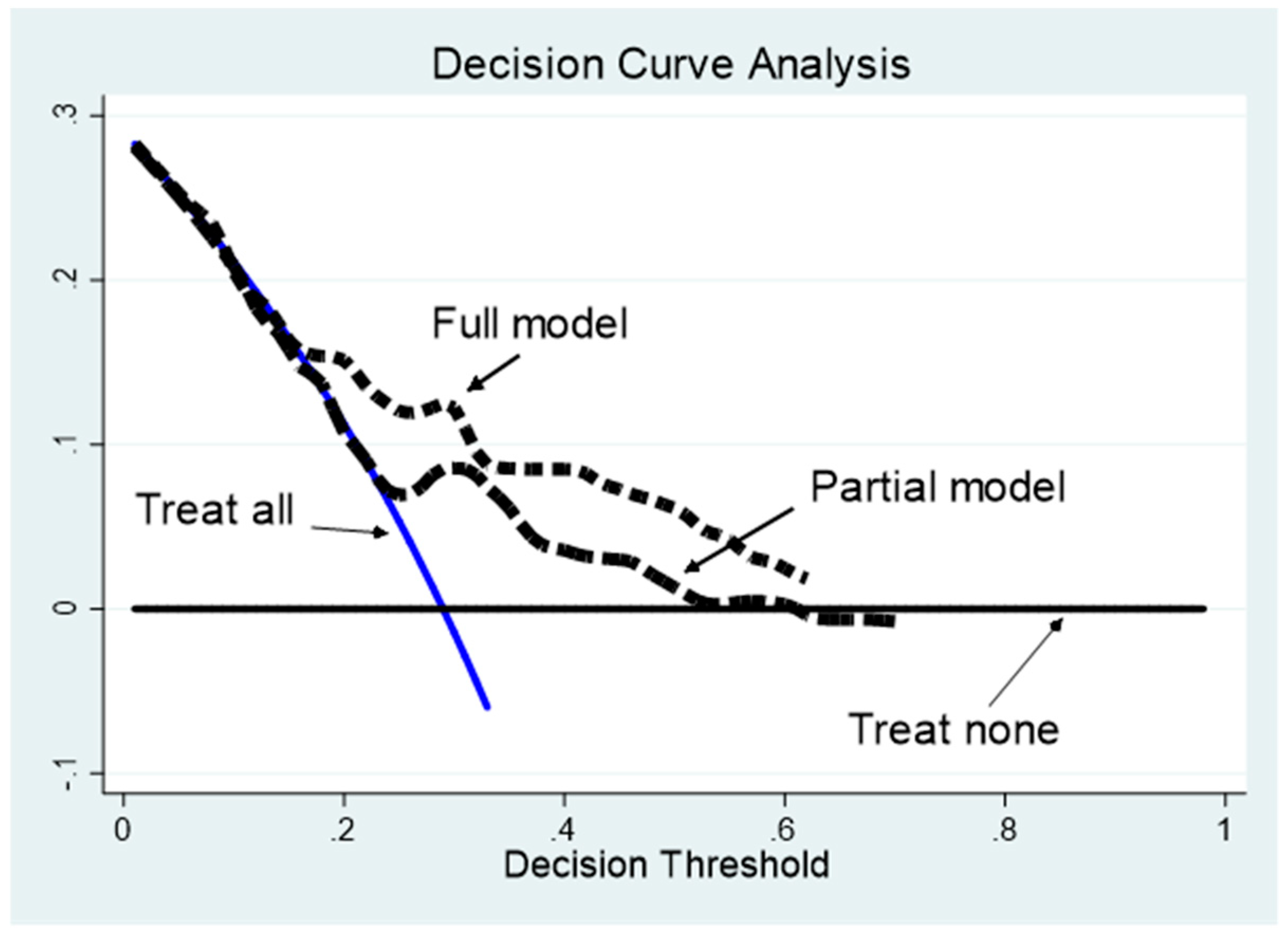
| Population Characteristics | All (174) | Survivors (N = 131) | Non-survivors (N = 43) | p-Value |
|---|---|---|---|---|
| Age, years ± SD | 74.26 ± 7.08 | 73.73 ± 7.16 | 75.86 ± 6.65 | 0.078 |
| Gender, male (n, %) | 114 (65) | 86 (65.6) | 28 (65.1) | 0.544 |
| BMI, kg/m2 ± SD | 27.56 ± 5.58 | 27.39 ± 5.03 | 28.03 ± 7.02 | 0.582 |
| LVEF, % ± SD | 39.89 ± 8.49 | 40.96 ± 7.59 | 36.67 ± 10.17 | 0.014 |
| Heart Rate bpm, ± SD | 78.56 ± 16.77 | 77.98 ± 15.94 | 80.33 ± 19.18 | 0.473 |
| SBP, mmHg ± SD | 128.70 ± 23.12 | 131.26 ± 22.52 | 120.88 ± 23.42 | 0.013 |
| STEMI, (n, %) | 92 (52.9) | 69 (52.6) | 23 (53.4) | 0.532 |
| Killip Class (III, IV n, %) | 41 (23.5) | 15 (11.4) | 26 (60.4) | <0.0001 |
| GRACE Score, ± SD | 150.01 ± 24.38 | 146.34 ± 22.48 | 161.45 ± 26.75 | <0.01 |
| MNA, ± SD | 22.15 ± 4.67 | 22.81 ± 4.45 | 20.13 ± 4.83 | <0.01 |
| DM, (n, %) | 62 (35.6) | 45 (34.3) | 17 (39.5) | 0.354 |
| Hypertension, (n, %) | 127 (73) | 99 (75.5) | 28 (65.1) | 0.127 |
| Smokers, (n, %) | 78 (44.8) | 59 (45.0) | 19(44.1) | 0.352 |
| COPD, (n, %) | 38 (21.8) | 27 (20.6) | 11 (25.5) | 0.537 |
| Hemoglobin, mg/dl ± SD | 13.05 ± 1.89 | 13.12 ± 1.85 | 12.84 ± 1.99 | 0.42 |
| WBC × 1000 /µl, ± SD | 10.37 ± 3.25 | 10.14 ± 3.82 | 11.08 ± 3.87 | 0.111 |
| Glycemia, mg/dl, ± SD | 135.7 ± 53.57 | 133.0 ± 43.48 | 143.93 ± 66.82 | 0.326 |
| GFR ml/kg/m2, ± SD | 72.38 ± 28.38 | 74.71 ± 27.18 | 65.12 ± 31.42 | 0.080 |
| Albumin mg/dl, ± SD | 3.72 ± 0.62 | 3.78 ± 0.64 | 3.54 ± 0.52 | 0.013 |
| Troponin I ng/ml, ± SD | 20.54 ± 23.62 | 14.08 ± 12.11 | 40.27 ± 36.28 | <0.0001 |
| Statins, (n, %) | 168(97.1) | 128 (97.7) | 40 (95.2) | 0.569 |
| ASA, (n, %) | 170 (97.7) | 130 (98.6) | 40 (95.2) | 0.248 |
| Beta-blockers, (n, %) | 137 (78.7) | 106 (81) | 31 (72.5) | 0.315 |
| ACEi/ARBs, (n, %) | 112 (64.3) | 85 (64.9) | 27 (61.9) | 0.716 |
| Independent Variables | HR (95% CI) | p-Value | Global R2 = 34.50% Fraction R2 |
|---|---|---|---|
| Age | 1.02 (0.98–1.07) | 0.265 | NA |
| Gender | 1.15 (0.52–2.55) | 0.723 | NA |
| BMI | 1.01 (0.96–1.06) | 0.536 | NA |
| LVEF | 0.96 (0.93–1.01) | 0.089 | NA |
| DM | 1.50 (0.78–2.90) | 0.221 | NA |
| MNA + 1 SD | 0.56 (0.42–0.73) | < 0.0001 | 16.70% |
| Albumin | 0.68 (0.39–3.31) | 0.221 | NA |
| GRACE Score +1 SD | 1.76 (1.34–2.32) | < 0.0001 | 17.80% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komici, K.; Vitale, D.F.; Mancini, A.; Bencivenga, L.; Conte, M.; Provenzano, S.; Grieco, F.V.; Visaggi, L.; Ronga, I.; Cittadini, A.; et al. Impact of Malnutrition on Long-Term Mortality in Elderly Patients with Acute Myocardial Infarction. Nutrients 2019, 11, 224. https://doi.org/10.3390/nu11020224
Komici K, Vitale DF, Mancini A, Bencivenga L, Conte M, Provenzano S, Grieco FV, Visaggi L, Ronga I, Cittadini A, et al. Impact of Malnutrition on Long-Term Mortality in Elderly Patients with Acute Myocardial Infarction. Nutrients. 2019; 11(2):224. https://doi.org/10.3390/nu11020224
Chicago/Turabian StyleKomici, Klara, Dino Franco Vitale, Angela Mancini, Leonardo Bencivenga, Maddalena Conte, Sandra Provenzano, Fabrizio Vincenzo Grieco, Lucia Visaggi, Ilaria Ronga, Antonio Cittadini, and et al. 2019. "Impact of Malnutrition on Long-Term Mortality in Elderly Patients with Acute Myocardial Infarction" Nutrients 11, no. 2: 224. https://doi.org/10.3390/nu11020224








