Post-Prandial Changes in Salivary Glucocorticoids: Effects of Dietary Cholesterol and Associations with Bile Acid Excretion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population/Recruitment
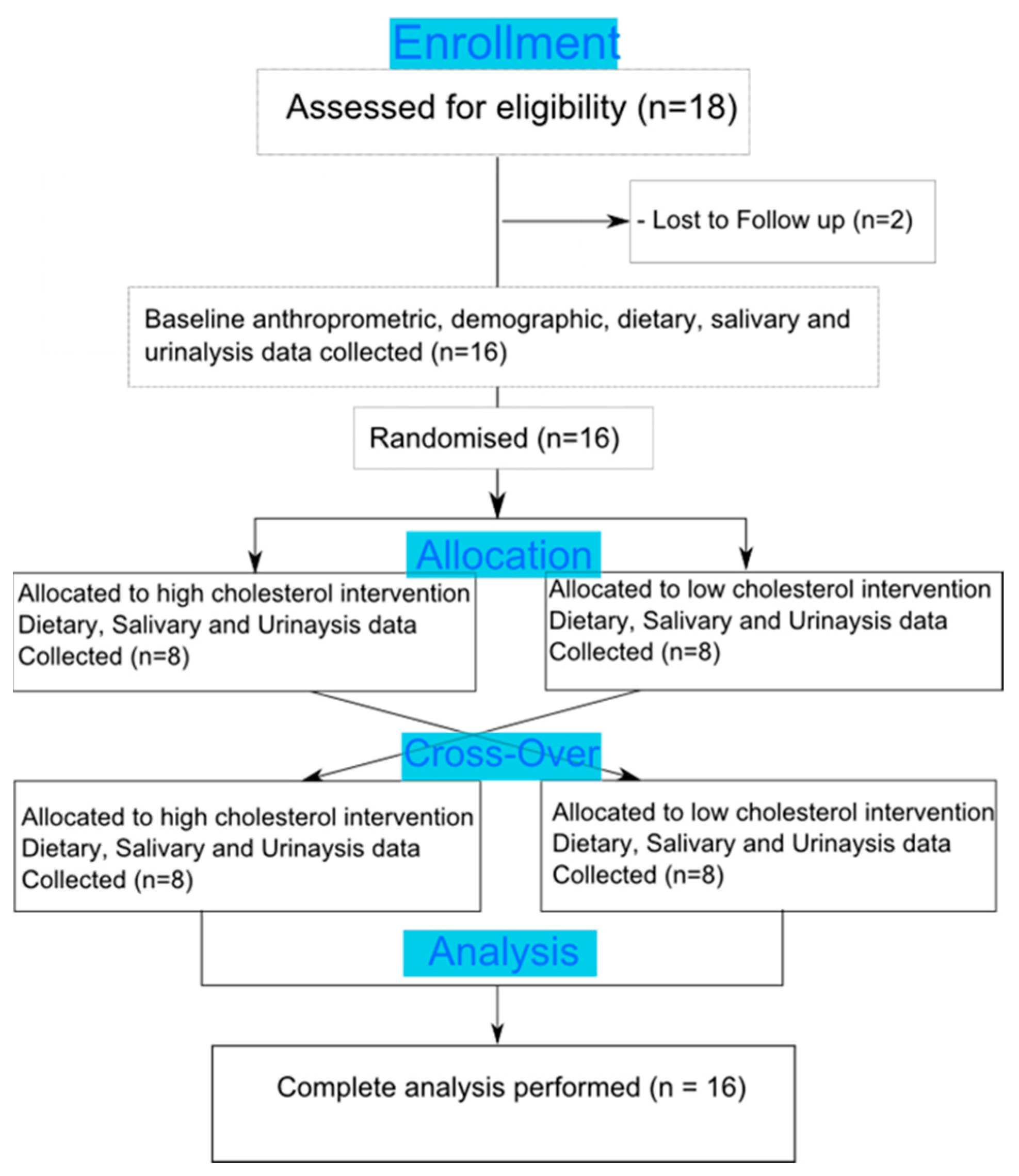
2.2. Study Design
2.3. Saliva and Urine Sampling
2.4. Cortisol/Cortisone Extraction & Analysis
2.5. Urine Analysis—FRAP, TBARS, Total Phenolics, and Bile Acids
2.6. Statistical Analysis
3. Results
3.1. Postprandial Effects on Salivary Glucocorticoids
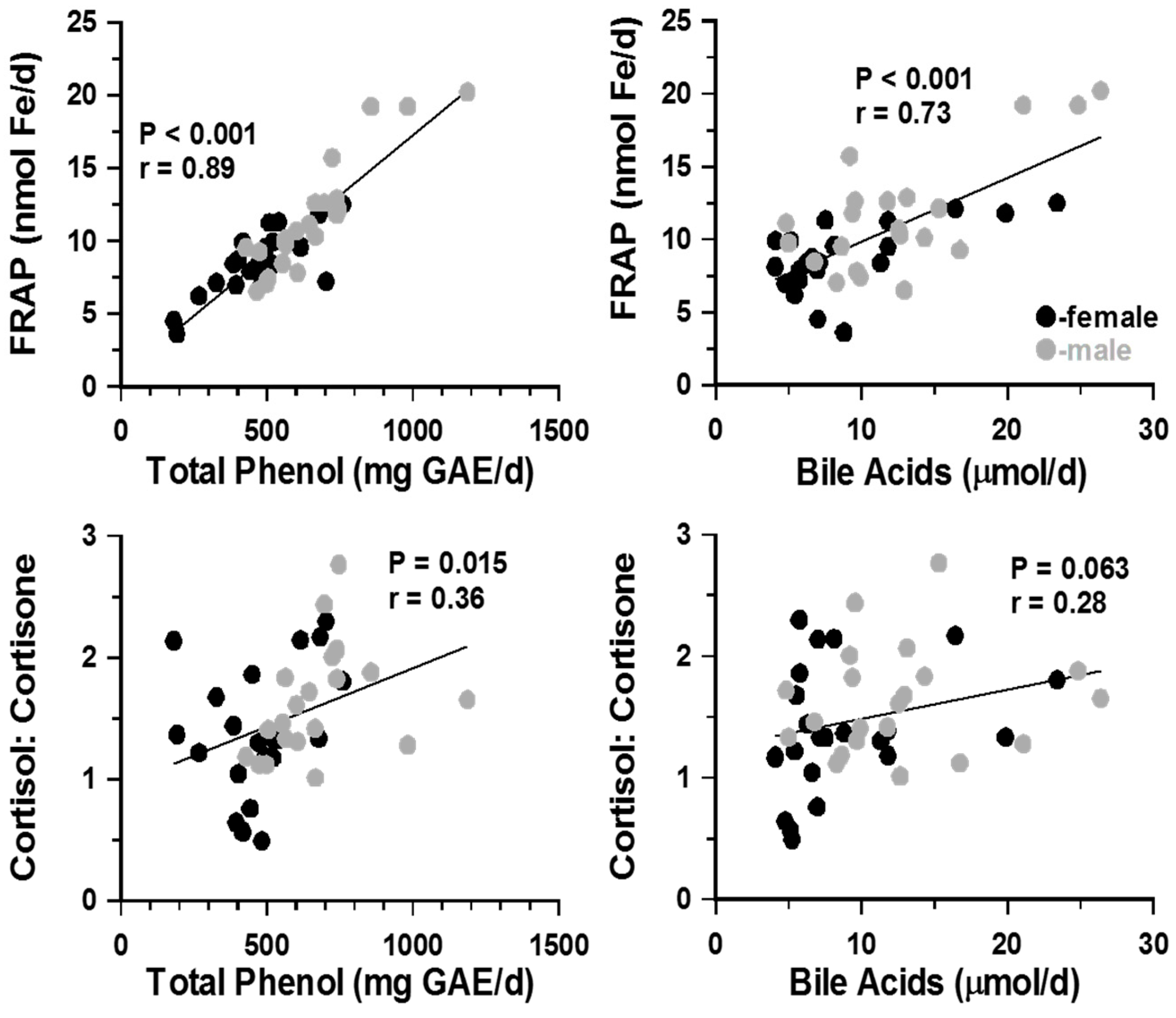
3.2. Urinary Steroids and Markers of Lipid Peroxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| 11β-HSD1 | 11β-Hydroxysteroid dehydrogenase type 1 |
| ELISA | Enzyme linked immunosorbant assy |
| FRAP | Ferric reducing antioxidant power |
| GAE | Gallic acid equivalent |
| GC | Glucocorticoids |
| TBARS | Thiobarbituric acid reactive substances |
References
- Quigley, M.E.; Yen, S.S. A mid-day surge in cortisol levels. J. Clin. Endocrinol. Metab. 1979, 49, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Fehm, H.L.; Holl, R.; Klein, E.; Voigt, K.H. The meal-related peak in plasma cortisol is not mediated by radioimmunoassayable ACTH. Clin. Physiol. Biochem. 1983, 1, 329–333. [Google Scholar] [PubMed]
- Iranmanesh, A.; Lawson, D.; Dunn, B.; Veldhuis, J.D. Glucose Ingestion Selectively Amplifies ACTH and Cortisol Secretory-Burst Mass and Enhances Their Joint Synchrony in Healthy Men. J. Clin. Endocrinol. Metab. 2011, 96, 2882–2888. [Google Scholar] [CrossRef]
- Pivonello, R.; Isidori, A.M.; De Martino, M.C.; Newell-Price, J.; Biller, B.M.K.; Colao, A. Complications of Cushing’s syndrome: State of the art. Lancet Diabetes Endocrinol. 2016, 4, 611–629. [Google Scholar] [CrossRef]
- Rose, A.J.; Herzig, S. Metabolic control through glucocorticoid hormones: An update. Mol. Cell. Endocrinol. 2013, 380, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Marciani, L.; Cox, E.F.; Hoad, C.L.; Totman, J.J.; Costigan, C.; Singh, G.; Shepherd, V.; Chalkley, L.; Robinson, M.; Ison, R.; et al. Effects of various food ingredients on gall bladder emptying. Eur. J. Clin. Nutr. 2013, 67, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.J.; Rutters, F.; Lemmens, S.G.; Born, J.M.; Westerterp-Plantenga, M.S. Effects of single macronutrients on serum cortisol concentrations in normal weight men. Physiol. Behav. 2010, 101, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Stimson, R.H.; Mohd-Shukri, N.A.; Bolton, J.L.; Andrew, R.; Reynolds, R.M.; Walker, B.R. The Postprandial Rise in Plasma Cortisol in Men Is Mediated by Macronutrient-Specific Stimulation of Adrenal and Extra-Adrenal Cortisol Production. J. Clin. Endocrinol. Metab. 2014, 99, 160–168. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. (2) Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [PubMed]
- Benedict, C.; Hallschmid, M.; Scheibner, J.; Niemeyer, D.; Schultes, B.; Merl, V.; Fehm, H.L.; Born, J.; Kern, W. Gut Protein Uptake and Mechanisms of Meal-Induced Cortisol Release. J. Clin. Endocrinol. Metab. 2005, 90, 1692–1696. [Google Scholar] [CrossRef]
- Lemmens, S.G.; Born, J.M.; Martens, M.J.; Westerterp-Plantenga, M.S.; A Martens, E. Lack of effect of high-protein vs. high-carbohydrate meal intake on stress-related mood and eating behavior. Nutr. J. 2011, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Slag, M.F.; Ahmed, M.; Gannon, M.C.; Nuttall, F.Q. Meal stimulation of cortisol secretion: A protein induced effect. Metabolism 1981, 30, 1104–1108. [Google Scholar] [CrossRef]
- Vicennati, V.; Ceroni, L.; Gagliardi, L.; Gambineri, A.; Pasquali, R. Comment: Response of the hypothalamic-pituitary-adrenocortical axis to high-protein/fat and high-carbohydrate meals in women with different obesity phenotypes. J. Clin. Endocrinol. Metab. 2002, 87, 3984–3988. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, J.D.; Iranmanesh, A.; Roelfsema, F.; Aoun, P.; Takahashi, P.; Miles, J.M.; Keenan, D.M. Tripartite Control of Dynamic ACTH-Cortisol Dose Responsiveness by Age, Body Mass Index, and Gender in 111 Healthy Adults. J. Clin. Endocrinol. Metab. 2011, 96, 2874–2881. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, A.D.; Macfarlane, D.P.; O’Flaherty, E.; Livingstone, D.E.; Mitic, T.; McConnell, K.M.; McKenzie, S.M.; Davies, E.; Reynolds, R.M.; Thiesson, H.C.; et al. Bile acids modulate glucocorticoid metabolism and the hypothalamic–pituitary–adrenal axis in obstructive jaundice. J. Hepatol. 2010, 52, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.A.; Hartman, L.R.; Souness, G.W.; Morris, D.J. Possible endogenous regulators of steroid inactivating enzymes and glucocorticoid-induced Na+ retention. Steroids 1994, 59, 352–356. [Google Scholar] [CrossRef]
- Dubois, C.; Armand, M.; Mekki, N.; Portugal, H.; Pauli, A.M.; Bernard, P.M.; Lafont, H.; Lairon, D. Effects of increasing amounts of dietary cholesterol on postprandial lipemia and lipoproteins in human subjects. J. Lipid Res. 1994, 35, 1993–2007. [Google Scholar] [PubMed]
- Beaumier-Gallon, G.; Dubois, C.; Portugal, H.; Lairon, D. Postprandial studies on dietary cholesterol in human subjects using stable isotopes and gas chromatography–mass spectrometry analysis. Atherosclerosis 1998, 141, 141. [Google Scholar] [CrossRef]
- Alphonse, P.A.S.; Jones, P.J.H. Revisiting Human Cholesterol Synthesis and Absorption: The Reciprocity Paradigm and its Key Regulators. Lipids 2015, 51, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Hornsby, P.J.; Crivello, J.F. The role of lipid peroxidation and biological antioxidants in the function of the adrenal cortex. Part 2. Mol. Cell Endocrinol. 1983, 30, 123–147. [Google Scholar] [CrossRef]
- Szelényi, P.; Révész, K.; Konta, L.; Tüttő, A.; Mandl, J.; Kereszturi, E.; Csala, M. Inhibition of microsomal cortisol production by (-)-epigallocatechin-3-gallate through a redox shift in the endoplasmic reticulum-A potential new target for treating obesity-related diseases. BioFactors 2013, 39, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Madhu, S.V.; Shukla, R.; Prabhu, K.M.; Gambhir, J.K. Postprandial hypertriglyceridemia and oxidative stress in patients of type 2 diabetes mellitus with macrovascular complications. Clin. Chim. Acta 2005, 359, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Berryman, C.E.; Grieger, J.A.; West, S.G.; Chen, C.-Y.O.; Blumberg, J.B.; Rothblat, G.H.; Sankaranarayanan, S.; Kris-Etherton, P.M. Acute Consumption of Walnuts and Walnut Components Differentially Affect Postprandial Lipemia, Endothelial Function, Oxidative Stress, and Cholesterol Efflux in Humans with Mild Hypercholesterolemia. J. Nutr. 2013, 143, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Al-Dujaili, E.; Kenyon, C.; Nicol, M.; Mason, J. Liquorice and glycyrrhetinic acid increase DHEA and deoxycorticosterone levels in vivo and in vitro by inhibiting adrenal SULT2A1 activity. Mol. Cell Endocrinol. 2011, 336, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.; Smail, N.F.; Almoosawi, S.; Davidson, I.; Al-Dujaili, E.A.S. Intake of polyphenol-rich pomegranate pure juice influences urinary glucocorticoids, blood pressure and homeostasis model assessment of insulin resistance in human volunteers. J. Nutr. Sci. 2012, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Kovar, J.; Poledne, R. Sex differences in the response of postprandial lipemia to a change from a low-fat low-cholesterol diet to a high-fat high-cholesterol diet. Physiol. Res. 2000, 49, 233–239. [Google Scholar]
- Al-Dujaili, E.A.; Baghdadi, H.H.; Howie, F.; Mason, J.I. Validation and application of a highly specific and sensitive ELISA for the estimation of cortisone in saliva, urine and in vitro cell-culture media by using a novel antibody. Steroids 2012, 77, 703–709. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolyndic-phosphotungstic reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Yagi, K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol. Biol. 1998, 108, 101–106. [Google Scholar]
- Fausa, O. Quantitative determination of serum bile acids using a purified 3 alpha-hydroxysteroid dehydrogenase. Scand. J. Gastroenterol. 1975, 10, 747–752. [Google Scholar]
- Basu, R.; Singh, R.; Basu, A.; Johnson, C.; Rizza, R.A. Effect of Nutrient Ingestion on Total-Body and Splanchnic Cortisol Production in Humans. Diabetes 2006, 55, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Bühler, H.; Perschel, F.H.; Fitzner, R.; Hierholzer, K. Endogenous inhibitors of 11 beta-OHSD: existence and possible significance. Steroids 1994, 59, 131–135. [Google Scholar] [CrossRef]
- Martens, E.A.; Lemmens, S.G.; Adam, T.C.; Westerterp-Plantenga, M.S. Sex differences in HPA axis activity in response to a meal. Physiol. Behav. 2012, 106, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Raven, P.W.; Taylor, N.F. Sex differences in the human metabolism of cortisol. Endocr. Res. 1996, 22, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Toogood, A.A.; Taylor, N.F.; Shalet, S.M.; Monson, J.P. Sexual dimorphism of cortisol metabolism is maintained in elderly subjects and is not oestrogen dependent. Clin. Endocrinol. 2000, 52, 61–66. [Google Scholar] [CrossRef]
- Taylor, N.F.; Monson, J.P.; Wood, P.J.; Kelly, W.F.; Weaver, J.U. Sexual dimorphism in 11 beta hydroxysteroid dehydrogenase activity and its relation to fat distribution and insulin sensitivity; a study in hypopituitary subjects. Clin. Endocrinol. 1998, 49, 13–20. [Google Scholar]
- Bochem, A.E.; Holleboom, A.G.; Romijn, J.A.; Hoekstra, M.; Dallinga-Thie, G.M.; Motazacker, M.M.; Hovingh, G.K.; Kuivenhoven, J.A.; Stroes, E.S.G.; et al. High density lipoprotein as a source of cholesterol for adrenal steroidogenesis: a study in individuals with low plasma HDL-C. J. Lipid Res. 2013, 54, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Neumann, H.; Karrasch, T.; Liebisch, G.; Schäffler, A. Bile Acid Metabolome after an Oral Lipid Tolerance Test by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). PLoS ONE 2016, 11, e0148869. [Google Scholar] [CrossRef] [PubMed]

| Gender (n) | Female (8) Mean (SD) | Male (8) Mean (SD) |
|---|---|---|
| Age (years) | 22 ± 0.6 | 23 ± 0.5 |
| Weight (kg) | 62.9 ± 3.8 | 70.7 ± 4.7 |
| Height (m) | 1.66 ± 0.02 | 1.75 ± 0.01 |
| BMI (kg/m2) | 22.8 ± 1.5 | 22.7± 1.3 |
| Systolic BP (mmHg) | 121 ± 1.9 | 125 ± 3.5 |
| Diastolic BP (mmHg) | 70 ± 2.7 | 71 ± 3.5 |
| Basal Caloric intake (kcal/day) | 2432 ± 201 | 2649 ± 394 |
| % Carbohydrate intake | 64.1 ± 2.5 | 65.5 ± 2.8 |
| % Fat intake | 17.7 ± 1.5 | 18.4 ± 1.9 |
| % Protein intake | 18.2 ± 1.4 | 16.1 ± 1.5 |
| Basal Cholesterol (mg/day) | 261 ± 48 | 269 ± 42 |
| Female Cortisol:Cortisone | |||||||
| Collection Time | AM | PRE | 15 min | 30 min | 90 min | PM | |
| Basal | Mean | 1.57 | 1.99 | 1.90 | 1.97 | 1.70 | 1.48 |
| ± SD | 0.30 | 0.31 | 0.32 | 0.31 | 0.23 | 0.29 | |
| High Cholesterol | Mean | 1.68 | 1.91 | 2.16 | 2.87 | 1.90 | 2.63 |
| ± SD | 0.17 | 0.26 | 0.37 | 0.81 | 0.27 | 0.33 | |
| Low Cholesterol | Mean | 1.43 | 1.56 | 2.26 | 1.66 | 1.69 | 1.42 |
| ± SD | 0.14 | 0.30 | 0.88 | 0.39 | 0.19 | 0.22 | |
| Male Cortisol:Cortisone | |||||||
| Collection Time | AM | PRE | 15 min | 30 min | 90 min | PM | |
| Basal | Mean | 1.39 | 1.09 | 0.99 | 1.69 | 1.86 | 1.96 |
| ± SD | 0.31 | 0.21 | 0.15 | 0.17 | 0.33 | 0.54 | |
| High Cholesterol | Mean | 1.28 | 1.12 | 1.53 | 1.47 | 1.86 | 1.76 |
| ± SD | 0.14 | 0.14 | 0.10 | 0.17 | 0.22 | 0.16 | |
| Low Cholesterol | Mean | 0.98 | 1.00 | 0.93 | 1.37 | 1.31 | 1.00 |
| ± SD | 0.14 | 0.14 | 0.10 | 0.17 | 0.22 | 0.16 | |
| Female | Male | |||||
|---|---|---|---|---|---|---|
| Basal | Low Cholesterol | High Cholesterol | Basal | Low Cholesterol | High Cholesterol | |
| Bile Acids (µmol/day) | 8.22 ± 1.37 | 8.22 ± 1.87 | 9.64 ± 2.47 | 13.91 ± 2.18 | 11.88 ± 2.61 | 12.46 ± 1.49 |
| Total Phenol (mg GAE/day) | 469.8 ± 61.9 | 478.2 ± 41.9 | 479.8 ± 67.2 | 697.7 ± 67.4 | 655.5 ± 97.2 | 634.8 ± 36.5 |
| FRAP (mmole Fe2/day) | 8.36 ± 0.85 | 8.83 ± 0.74 | 8.68 ± 1.01 | 13.11 ± 1.91 | 11.33 ± 1.71 | 10.35 ± 0.57 |
| TBARS (µmol/day) | 3.65 ± 0.47 | 2.98 ± 0.5 | 1.96 ± 0.17 | 3.22 ± 0.27 | 3.43 ± 0.34 | 2.48 ± 0.21 |
| Cortisol (nmol/day) | 57.7 ± 6.56 | 60.4 ± 8.91 | 68.6 ± 12.0 | 78.9 ± 11.6 | 89.0 ± 12.2 | 69.1 ± 10.5 |
| Cortisone (nmol/day) | 32.2 ± 2.9 | 61.4 ± 7.34 | 52.7 ± 6.21 | 45 ± 6.34 | 64.7 ± 12.0 | 45.3 ± 8.51 |
| Cortisol:Cortisone | 1.79 ± 0.14 | 1.02 ± 0.12 | 1.34 ± 0.19 | 1.76 ± 0.15 | 1.45 ± 0.12 | 1.65 ± 0.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, G.W.; Kenyon, C.J.; Al-Dujaili, E.A.S. Post-Prandial Changes in Salivary Glucocorticoids: Effects of Dietary Cholesterol and Associations with Bile Acid Excretion. Nutrients 2019, 11, 360. https://doi.org/10.3390/nu11020360
Anderson GW, Kenyon CJ, Al-Dujaili EAS. Post-Prandial Changes in Salivary Glucocorticoids: Effects of Dietary Cholesterol and Associations with Bile Acid Excretion. Nutrients. 2019; 11(2):360. https://doi.org/10.3390/nu11020360
Chicago/Turabian StyleAnderson, Graham W., Christopher J. Kenyon, and Emad A.S. Al-Dujaili. 2019. "Post-Prandial Changes in Salivary Glucocorticoids: Effects of Dietary Cholesterol and Associations with Bile Acid Excretion" Nutrients 11, no. 2: 360. https://doi.org/10.3390/nu11020360





