Effect of Oral Nutritional Supplements with Sucromalt and Isomaltulose versus Standard Formula on Glycaemic Index, Entero-Insular Axis Peptides and Subjective Appetite in Patients with Type 2 Diabetes: A Randomised Cross-Over Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Design and Ethics Issues
2.1.2. Inclusion and Exclusion Criteria
2.1.3. Population, Sample Size, and Patient’s Selection
2.1.4. Anthropometric Assessment
2.2. Study Protocol
2.2.1. Oral Nutritional Supplements Composition
2.2.2. Experimental Protocol
Background Diet, Physical Activities and Other Measurements
Randomisation
2.3. Measurement of EIAP/GI and Subjective Appetite Evaluation
2.3.1. EIAP and Glycaemic Index
2.3.2. Subjective Appetite Assessment
2.3.3. Laboratory Determinations
2.4. Data Processing and Statistical Analyses
3. Results
3.1. Glycaemic Response and EIAP Concentrations
3.1.1. Insulin
3.1.2. GLP-1
3.1.3. GIP
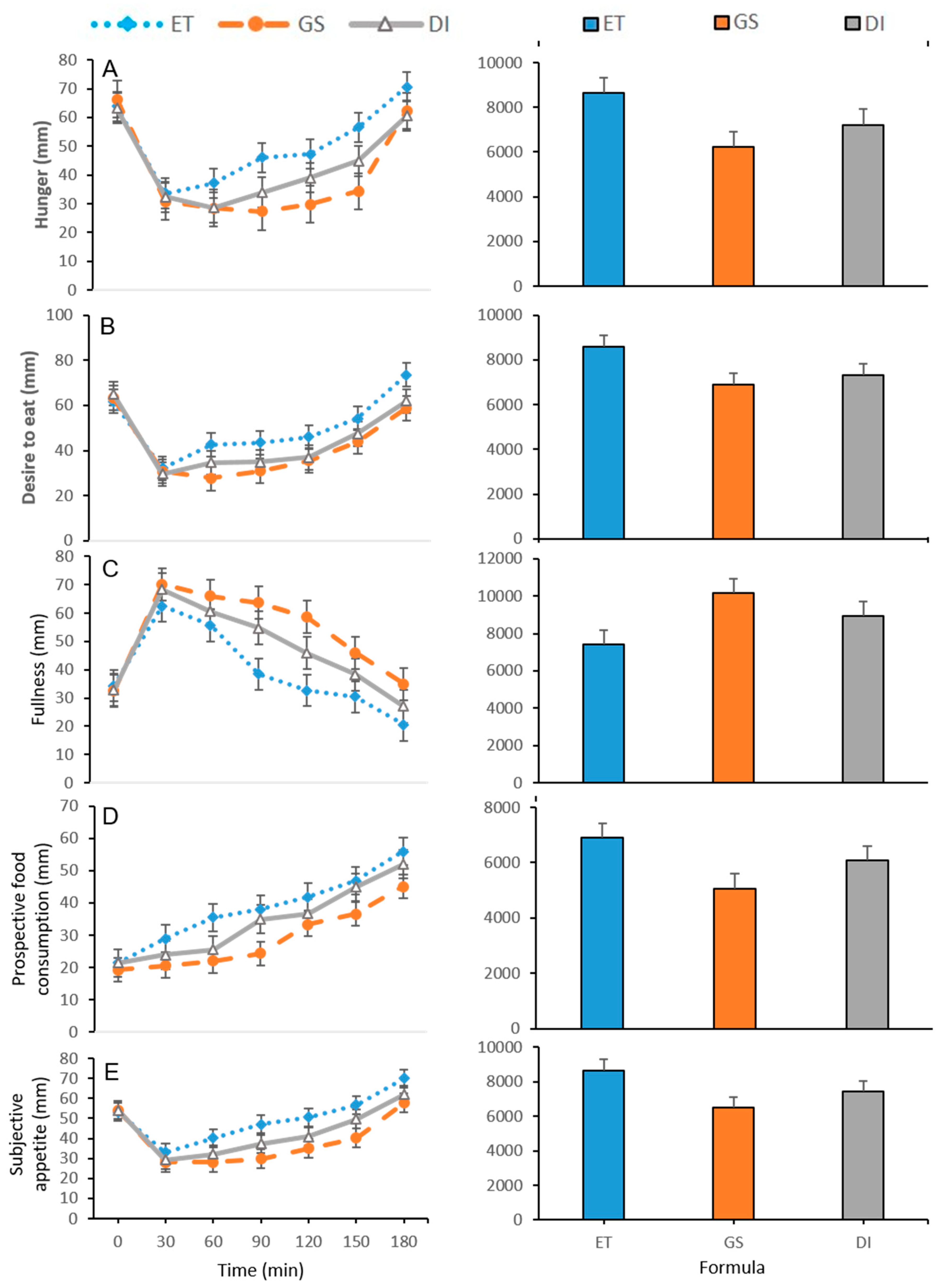
3.2. Subjective Appetite Measurements
3.3. Correlation Analysis Between EIAP, Serum Glucose and Subjective Appetite
3.4. Glycaemic Index and Glycaemic Load
3.5. EIAP and SA Relation with GI and GL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| BMI | Body mass index |
| DI | Isomaltulose and resistant starch supplement |
| DM2 | Diabetes mellitus type 2 |
| ET | Standard nutritional supplement not specific for diabetics |
| GB | Glucose solution or reference food |
| GI | Glycaemic index |
| GIP | Glucose-dependent insulinotropic polypeptide |
| GL | Glycaemic load |
| GLP-1 | Glucagon-like peptide 1 |
| GS | Resistant maltodextrin and sucromalt supplement |
| HbA1c | Glycated haemoglobin (HbA1c) |
| IDF | International Diabetes Federation |
| ONS-D | Oral nutritional supplements specific for diabetics |
| SEM | Standard error of the mean |
| VAS | Visual analogue scale |
| LGL | Low glycaemic load |
| AUCG | Area under the curve glucose |
References
- Rosen, E.D.; Kaestner, K.H.; Natarajan, R.; Patti, M.-E.; Sallari, R.; Sander, M.; Susztak, K. Epigenetics and Epigenomics: Implications for Diabetes and Obesity. Diabetes 2018, 67, 1923–1931. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. The Top 10 Causes of Death. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 31 March 2019).
- World Health Organization. Diabetes. 2018. Available online: https://www.who.int/en/news-room/fact-sheets/detail/diabetes (accessed on 31 March 2019).
- Unnikrishnan, R.; Pradeepa, R.; Joshi, S.R.; Mohan, V. Type 2 Diabetes: Demystifying the Global Epidemic. Diabetes 2017, 66, 1432–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Diabetes Federation (IDF). The 8th Edition of the Diabetes Atlas. 2017. Available online: http://diabetesatlas.org/resources/2017-atlas.html (accessed on 31 March 2019).
- International Diabetes Federation Guideline Development Group. Guideline for management of postmeal glucose in diabetes. Diabetes Res. Clin. Pract. 2014, 103, 256–268. [Google Scholar] [CrossRef]
- American Diabetes Association. 15. Diabetes Advocacy: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S152–S153. [Google Scholar] [CrossRef] [PubMed]
- Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes Recommendations for the nutritional management of patients with diabetes mellitus. Eur. J. Clin. Nutr. 2000, 54, 353–355. [CrossRef]
- Willett, W.; Manson, J.; Liu, S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am. J. Clin. Nutr. 2002, 76, 274S–280S. [Google Scholar] [CrossRef] [Green Version]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojo, O.; Ojo, O.O.; Adebowale, F.; Wang, X.-H. The Effect of Dietary Glycaemic Index on Glycaemia in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2018, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Niwano, Y.; Adachi, T.; Kashimura, J.; Sakata, T.; Sasaki, H.; Sekine, K.; Yamamoto, S.; Yonekubo, A.; Kimura, S. Is glycemic index of food a feasible predictor of appetite, hunger, and satiety? J. Nutr. Sci. Vitaminol. (Tokyo) 2009, 55, 201–207. [Google Scholar] [CrossRef]
- Sun, F.-H.; Li, C.; Zhang, Y.-J.; Wong, S.; Wang, L. Effect of Glycemic Index of Breakfast on Energy Intake at Subsequent Meal among Healthy People: A Meta-Analysis. Nutrients 2016, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Møller, B.K.; Raben, A.; Sloth, B.; Pedersen, D.; Tetens, I.; Holst, J.J.; Astrup, A. Glycemic and insulinemic responses as determinants of appetite in humans. Am. J. Clin. Nutr. 2006, 84, 1365–1373. [Google Scholar] [CrossRef]
- Elia, M.; Ceriello, A.; Laube, H.; Sinclair, A.J.; Engfer, M.; Stratton, R.J. Enteral nutritional support and use of diabetes-specific formulas for patients with diabetes: A systematic review and meta-analysis. Diabetes Care 2005, 28, 2267–2279. [Google Scholar] [CrossRef]
- Ojo, O.; Brooke, J. Evaluation of the Role of Enteral Nutrition in Managing Patients with Diabetes: A Systematic Review. Nutrients 2014, 6, 5142–5152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Luis DA, D.M. A randomized clinical trial with two enteral diabetes-specific supplements in patients with diabetes mellitus type 2: Metabolic effects. Eur. Rev. Med. Pharmacol. Sci. 2008, 12, 261–266. [Google Scholar]
- Voss, A.C.; Maki, K.C.; Garvey, W.T.; Hustead, D.S.; Alish, C.; Fix, B.; Mustad, V.A. Effect of two carbohydrate-modified tube-feeding formulas on metabolic responses in patients with type 2 diabetes. Nutrition 2008, 24, 990–997. [Google Scholar] [CrossRef]
- Sanz-Paris, A.; Boj-Carceller, D.; Lardies-Sanchez, B.; Perez-Fernandez, L.; Cruz-Jentoft, A. Health-Care Costs, Glycemic Control and Nutritional Status in Malnourished Older Diabetics Treated with a Hypercaloric Diabetes-Specific Enteral Nutritional Formula. Nutrients 2016, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Siow, P.C.; Peh, E.; Henry, C.J. Influence of rice, pea and oat proteins in attenuating glycemic response of sugar sweetened beverages. Eur. J. Nutr. 2018, 57, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Yabe, D.; Seino, Y.; Seino, Y. Incretin concept revised: The origin of the insulinotropic function of glucagon-like peptide-1 -the gut, the islets or both? J. Diabetes Investig. 2018, 9, 21–24. [Google Scholar] [CrossRef]
- Rojas, J.; Bermudez, V.; Palmar, J.; Martínez, M.S.; Olivar, L.C.; Nava, M.; Tomey, D.; Rojas, M.; Salazar, J.; Garicano, C.; et al. Pancreatic Beta Cell Death: Novel Potential Mechanisms in Diabetes Therapy. J. Diabetes Res. 2018, 2018, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Prinz, P. The role of dietary sugars in health: Molecular composition or just calories? Eur. J. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Giezenaar, C.; Trahair, L.G.; Luscombe-Marsh, N.D.; Hausken, T.; Standfield, S.; Jones, K.L.; Lange, K.; Horowitz, M.; Chapman, I.; Soenen, S. Effects of randomized whey-protein loads on energy intake, appetite, gastric emptying, and plasma gut-hormone concentrations in older men and women. Am. J. Clin. Nutr. 2017, 106, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef] [PubMed]
- Behall, K.M.; Scholfield, D.J.; Canary, J. Effect of starch structure on glucose and insulin responses in adults. Am. J. Clin. Nutr. 1988, 47, 428–432. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.M.; Nystrom, E.; Braunschweig, C.; Miles, J.; Compher, C.; the American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. Clinical Guidelines: Nutrition Support of Adult Patients with Hyperglycemia. J. Parenter. Enter. Nutr. 2013, 37, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Doola, R.; Todd, A.S.; Forbes, J.M.; Deane, A.M.; Presneill, J.J.; Sturgess, D.J. Diabetes-Specific Formulae Versus Standard Formulae as Enteral Nutrition to Treat Hyperglycemia in Critically Ill Patients: Protocol for a Randomized Controlled Feasibility Trial. JMIR Res. Protoc. 2018, 7, e90. [Google Scholar] [CrossRef]
- Devitt, A.A.; Williams, J.A.; Choe, Y.S.; Hustead, D.S.; Mustad, V.A. Glycemic responses to glycemia-targeted specialized-nutrition beverages with varying carbohydrates compared to a standard nutritional beverage in adults with type 2 diabetes. Adv. Biosci. Biotechnol. 2013, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Grysman, A.; Carlson, T.; Wolever, T.M.S. Effects of sucromalt on postprandial responses in human subjects. Eur. J. Clin. Nutr. 2008, 62, 1364–1371. [Google Scholar] [CrossRef]
- Maresch, C.C.; Petry, S.F.; Theis, S.; Bosy-Westphal, A.; Linn, T. Low Glycemic Index Prototype Isomaltulose-Update of Clinical Trials. Nutrients 2017, 9, 381. [Google Scholar] [CrossRef]
- Pfeiffer, A.F.H.; Keyhani-Nejad, F. High Glycemic Index Metabolic Damage—A Pivotal Role of GIP and GLP-1. Trends Endocrinol. Metab. 2018, 29, 289–299. [Google Scholar] [CrossRef]
- Holub, I.; Gostner, A.; Theis, S.; Nosek, L.; Kudlich, T.; Melcher, R.; Scheppach, W. Novel findings on the metabolic effects of the low glycaemic carbohydrate isomaltulose (Palatinose). Br. J. Nutr. 2010, 103, 1730–1737. [Google Scholar] [CrossRef]
- Kendall, F.E.; Marchand, O.; Haszard, J.J.; Venn, B.J. The Comparative Effect on Satiety and Subsequent Energy Intake of Ingesting Sucrose or Isomaltulose Sweetened Trifle: A Randomized Crossover Trial. Nutrients 2018, 10, 1504. [Google Scholar] [CrossRef] [PubMed]
- Hofman, Z.; De Van Drunen, J.; Kuipers, H. The Glycemic Index of standard and diabetes-specific enteral formulas. Asia Pac. J. Clin. Nutr. 2006, 15, 412–417. [Google Scholar] [PubMed]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Medical Association. Ethical Principles for Medical Research Involving Human Subjects. 64ª General Assembly. 2013. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 24 March 2019).
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, B.A.; Sturm, K.; Macintosh, C.G.; Feinle, C.; Horowitz, M.; Chapman, I.M. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur. J. Clin. Nutr. 2004, 58, 212–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation. 1981. Available online: https://apps.who.int/iris/handle/10665/39527 (accessed on 24 March 2019).
- Akilen, R.; Deljoomanesh, N.; Hunschede, S.; Smith, C.E.; Arshad, M.U.; Kubant, R.; Anderson, G.H. The effects of potatoes and other carbohydrate side dishes consumed with meat on food intake, glycemia and satiety response in children. Nutr. Diabetes 2016, 6, e195. [Google Scholar] [CrossRef]
- Mottalib, A.; Mohd-Yusof, B.-N.; Shehabeldin, M.; Pober, D.; Mitri, J.; Hamdy, O. Impact of Diabetes-Specific Nutritional Formulas versus Oatmeal on Postprandial Glucose, Insulin, GLP-1 and Postprandial Lipidemia. Nutrients 2016, 8, 443. [Google Scholar] [CrossRef]
- Alish, C.J.; Garvey, W.T.; Maki, K.C.; Sacks, G.S.; Hustead, D.S.; Hegazi, R.A.; Mustad, V.A. A Diabetes-Specific Enteral Formula Improves Glycemic Variability in Patients with Type 2 Diabetes. Diabetes Technol. Ther. 2010, 12, 419–425. [Google Scholar] [CrossRef]
- Ceriello, A.; Davidson, J.; Hanefeld, M.; Leiter, L.; Monnier, L.; Owens, D.; Tajima, N.; Tuomilehto, J. Postprandial hyperglycaemia and cardiovascular complications of diabetes: An update. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 453–456. [Google Scholar] [CrossRef]
- Yoshizane, C.; Mizote, A.; Yamada, M.; Arai, N.; Arai, S.; Maruta, K.; Mitsuzumi, H.; Ariyasu, T.; Ushio, S.; Fukuda, S. Glycemic, insulinemic and incretin responses after oral trehalose ingestion in healthy subjects. Nutr. J. 2017, 16. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Nakano, D.; Oriishi, T.; Torimura, T. Effects of isomaltulose on insulin resistance and metabolites in patients with non-alcoholic fatty liver disease: A metabolomic analysis. Mol. Med. Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016, 4, 525–536. [Google Scholar] [CrossRef]
- Holst, J.J. On the Physiology of GIP and GLP-1. Horm. Metab. Res. 2004, 36, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Nasteska, D.; Harada, N.; Suzuki, K.; Yamane, S.; Hamasaki, A.; Joo, E.; Iwasaki, K.; Shibue, K.; Harada, T.; Inagaki, N. Chronic Reduction of GIP Secretion Alleviates Obesity and Insulin Resistance Under High-Fat Diet Conditions. Diabetes 2014, 63, 2332. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Potential health risks from beverages containing fructose found in sugar or high-fructose corn syrup. Diabetes Care 2013, 36, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.A.; Samuel, V.T. The Sweet Path to Metabolic Demise: Fructose and Lipid Synthesis. Trends Endocrinol. Metab. 2016, 27, 719–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donner, T.W.; Wilber, J.F.; Ostrowski, D. D-tagatose, a novel hexose: Acute effects on carbohydrate tolerance in subjects with and without type 2 diabetes. Diabetes Obes. Metab. 1999, 1, 285–291. [Google Scholar] [CrossRef]
- Guerrero-Wyss, M.; Durán Agüero, S.; Angarita Dávila, L. D-Tagatose Is a Promising Sweetener to Control Glycaemia: A New Functional Food. BioMed Res. Int. 2018, 2018, 8718053. [Google Scholar] [CrossRef]
- Maeda, A.; Miyagawa, J.-I.; Miuchi, M.; Nagai, E.; Konishi, K.; Matsuo, T.; Tokuda, M.; Kusunoki, Y.; Ochi, H.; Murai, K.; et al. Effects of the naturally-occurring disaccharides, palatinose and sucrose, on incretin secretion in healthy non-obese subjects. J. Diabetes Investig. 2013, 4, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gil, A. Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical Trials. Adv. Nutr. (Bethesda Md.) 2019, 10, S31–S48. [Google Scholar] [CrossRef] [Green Version]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angarita, L.; Bermudez, V.; Reina, N.; Cisternas, S.; Díaz, W.; Escobar, M.C.; Carrasco, P.; Durán, S.; Buhring, K.; Buhring, R.; et al. New Insights into Alleviating Diabetes Mellitus: Role of Gut Microbiota and a Nutrigenomic Approach. In Diabetes Food Plan; Waisundara, V., Ed.; InTech: London, UK, 2018; ISBN 978-1-78923-274-5. [Google Scholar] [Green Version]
- Ang, M.; Linn, T. Comparison of the effects of slowly and rapidly absorbed carbohydrates on postprandial glucose metabolism in type 2 diabetes mellitus patients: A randomized trial. Am. J. Clin. Nutr. 2014, 100, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- König, D.; Theis, S.; Kozianowski, G.; Berg, A. Postprandial substrate use in overweight subjects with the metabolic syndrome after isomaltulose (Palatinose TM) ingestion. Nutrition 2012, 28, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Van Can, J.G.P.; van Loon, L.J.C.; Brouns, F.; Blaak, E.E. Reduced glycaemic and insulinaemic responses following trehalose and isomaltulose ingestion: Implications for postprandial substrate use in impaired glucose-tolerant subjects. Br. J. Nutr. 2012, 108, 1210–1217. [Google Scholar] [CrossRef]
- Sloth, B.; Due, A.; Larsen, T.M.; Holst, J.J.; Heding, A.; Astrup, A. The effect of a high-MUFA, low-glycaemic index diet and a low-fat diet on appetite and glucose metabolism during a 6-month weight maintenance period. Br. J. Nutr. 2008, 101, 1846–1858. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Rocca, A.S.; LaGreca, J.; Kalitsky, J.; Brubaker, P.L. Monounsaturated Fatty Acid Diets Improve Glycemic Tolerance through Increased Secretion of Glucagon-Like Peptide-1*. Endocrinology 2001, 142, 1148–1155. [Google Scholar] [CrossRef]
- Storm, H.; Holst, J.J.; Hermansen, K.; Thomsen, C. Differential effects of saturated and monounsaturated fats on postprandial lipemia and glucagon-like peptide 1 responses in patients with type 2 diabetes. Am. J. Clin. Nutr. 2003, 77, 605–611. [Google Scholar] [CrossRef]
- Printz, H.; Recke, B.; Fehmann, H.C.; Göke, B. No apparent benefit of liquid formula diet in NIDDM. Exp. Clin. Endocrinol. Diabetes 2009, 105, 134–139. [Google Scholar] [CrossRef]
- Noreberg, C.; Indar-Brown, K.; Madar, Z. Glycemic and insulinemic responses after ingestion of ethnic foods by NIDDM and healthy subjects. Am. J. Clin. Nutr. 1992, 55, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, P.C.; Galgani, F.J.; Díaz, B.E. Determinación del índice glicémico del alimento nutridiabetic® destinado a diabéticos tipo 2. Rev. Chil. Nutr. 2006, 33, 14–21. [Google Scholar] [CrossRef]
- Rizkalla, S.W.; Laromiguiere, M.; Champ, M.; Bruzzo, F.; Boillot, J.; Slama, G. Effect of baking process on postprandial metabolic consequences: Randomized trials in normal and type 2 diabetic subjects. Eur. J. Clin. Nutr. 2006, 61, 175. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Arumugam, V.; Haugabrooks, E.; Williamson, P.; Hendrich, S. Soluble dietary fibre (Fibersol-2) decreased hunger and increased satiety hormones in humans when ingested with a meal. Nutr. Res. 2015, 35, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Cani, P.D.; Daubioul, C.; Neyrinck, A.M. Impact of inulin and oligofructose on gastrointestinal peptides. Br. J. Nutr. 2005, 93, S157–S161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkmeyer, S.V.; Mattes, R.D. Effects of food attributes on hunger and food intake. Int. J. Obes. 2000, 24, 1167–1175. [Google Scholar] [CrossRef] [Green Version]
- Stafleu, A.; Hendriks, H.F.; Smeets, P.A.; Blom, W.A.; de Graaf, C. Biomarkers of satiation and satiety. Am. J. Clin. Nutr. 2004, 79, 946–961. [Google Scholar] [Green Version]
- Thomas, D.; Elliott, E.; Baur, L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst. Rev. 2007. [Google Scholar] [CrossRef]
- Bornet, F.R.J.; Jardy-Gennetier, A.-E.; Jacquet, N.; Stowell, J. Glycaemic response to foods: Impact on satiety and long-term weight regulation. Appetite 2007, 49, 535–553. [Google Scholar] [CrossRef]
- Van Dam, R.M.; Seidell, J.C. Carbohydrate intake and obesity. Eur. J. Clin. Nutr. 2007, 61, S75. [Google Scholar] [CrossRef]
- Flint, A.; Gregersen, N.T.; Gluud, L.L.; Møller, B.K.; Raben, A.; Tetens, I.; Verdich, C.; Astrup, A. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: A meta-analysis of test meal studies. Br. J. Nutr. 2007, 98, 17–25. [Google Scholar] [CrossRef]
- Giezenaar, C.; van der Burgh, Y.; Lange, K.; Hatzinikolas, S.; Hausken, T.; Jones, K.; Horowitz, M.; Chapman, I.; Soenen, S. Effects of Substitution, and Adding of Carbohydrate and Fat to Whey-Protein on Energy Intake, Appetite, Gastric Emptying, Glucose, Insulin, Ghrelin, CCK and GLP-1 in Healthy Older Men—A Randomized Controlled Trial. Nutrients 2018, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.P.F.; Ravestein, P.; van der Hijden, H.T.W.M.; Boers, H.M.; Mela, D.J. Effect of carbohydrate digestibility on appetite and its relationship to postprandial blood glucose and insulin levels. Eur. J. Clin. Nutr. 2010, 65, 47. [Google Scholar] [CrossRef] [PubMed]

| Composition | ET | DI | GS |
|---|---|---|---|
| Calories (kcal) | 105 | 104 | 93 |
| Protein (g) | 3.8 | 4.9 | 4.3 |
| Fat (g) | 2.5 | 3.8 | 3.5 |
| Saturates (g) | 0.4 | 0.5 | 0.3 |
| Monounsaturates (g) | 0.8 | 2.2 | 2.1 |
| Polyunsaturates (g) | 1.3 | 1.1 | 0.9 |
| Total carbohydrate (g) | 17.3 | 11.7 | 10.9 |
| Sugar (g) | 10.0 | 8.3 | 1.7 |
| Dietary Fibre (g) | 1.0 | 2.0 | 1.8 |
| Soluble (g) | 0.0 | 1.6 | 1.8 |
| Non-soluble (g) | 0.0 | 0.4 | 0.0 |
| Chromium (µg) | 12.7 | 12.0 | 5.0 |
| Portion size (mL) | 100 | 100 | 100 |
| Composition | ET | DI | GS |
|---|---|---|---|
| Calories (kcal) | 149 | 223 | 214 |
| Protein (g) | 5.3 | 10.5 | 9.9 |
| Fat (g) | 3.5 | 8.1 | 8.1 |
| Saturates (g) | 0.5 | 1.0 | 0.6 |
| Monounsaturated (g) | 1.1 | 4.7 | 4.9 |
| Polyunsaturated (g) | 1.8 | 2.3 | 2.0 |
| Total carbohydrate (g) | 25.0 | 25.0 | 25.0 |
| Sugar (g) | 14.1 | 8.3 | 0.0 |
| Dietary Fibre (g) | 0.9 | 4.3 | 4.1 |
| Soluble (g) | 0.0 | 3.4 | 4.1 |
| Non-soluble (g) | 0.0 | 0.8 | 0.0 |
| Chromium (µg) | 12.4 | 25.8 | 11.5 |
| Portion size (mL) | 141 | 214 | 230 |
| Sex | ||||||
|---|---|---|---|---|---|---|
| Female | Male | Total | ||||
| Mean | SEM * | Mean | SEM | Mean | SEM | |
| Age (years) | 54.75 | 1. 65 | 57.83 | 1.35 | 56.0 | 1.11 |
| Weight (cm) | 87.75 | 3.73 | 90.17 | 1.22 | 89.0 | 1.58 |
| Height (m) | 1.68 | 0.04 | 1.69 | 0.01 | 1.68 | 0.01 |
| BMC (kg/m2) | 30.90 | 0.44 | 31.04 | 0.36 | 30.8 | 0.26 |
| Waist circumference (cm) | 106.00 | 1.58 | 111.00 | 0.89 | 106 | 0.77 |
| Base glycaemia (mmol/L) | 7.51 | 0.40 | 6.75 | 0.30 | 7.05 | 0.26 |
| Total cholesterol (mg/dL) | 209.60 | 5.71 | 213.77 | 8.84 | 212.10 | 5.56 |
| High-density lipoprotein (mg/dL) | 44.70 | 4.63 | 44.30 | 2.32 | 44.46 | 2.16 |
| Low-density lipoprotein (mg/dL) | 130.95 | 4.37 | 133.28 | 1.54 | 132.35 | 1.87 |
| Triglycerides (mg/dL) | 161.70 | 2.56 | 158.06 | 4.45 | 159.52 | 2.80 |
| Glycated haemoglobin HbA1c (%) | 6.95 | 0.30 | 6.98 | 0.30 | 6.97 | 0.20 |
| Supplement | Time (min) | Serum Glucose (mmol/L) | Insulin (mU/L) | GLP-1 (pmol/L) | GIP (pg/mL) |
|---|---|---|---|---|---|
| 0 | 6.52 ± 0.07 | 6.44 ± 0.32 | 6.26 ± 0.28 | 29.64 ± 0.50 | |
| 30 | 10.14 ± 0.07 bc | 22.84 ± 1.00 bc | 12.93 ± 0.21 bc | 55.44 ± 0.58 bc | |
| 60 | 10.80 ± 0.12 bd | 33.91 ± 0.97 bc | 8.35 ± 0.22 bc | 62.27 ± 0.89 bc | |
| ET | 90 | 9.39 ± 0.16 b | 36.20 ± 0.64 bc | 7.78 ± 0.15 b | 74.68 ± 0.72 bc |
| 120 | 8.76 ± 0.17 b | 25.90 ± 0.70 bc | 7.16 ± 0.27 b | 71.23 ± 0.36 bc | |
| 150 | 8.14 ± 0.21 bc | 16.80 ± 0.56 bc | 6.55 ± 0.12 b | 67.77 ± 0.50 bc | |
| 180 | 7.13 ± 0.21 b | 9.91 ± 0.81 | 5.99 ± 0.21 b | 62.86 ± 1.26 bc | |
| 0 | 6.64 ± 0.08 | 6.49 ± 0.11 | 6.95 ± 0.36 c | 29.97 ± 0.40 | |
| 30 | 6.68 ± 0.13 ac | 17.24 ± 0.31 a | 18.16 ± 0.26 ac | 45.57 ± 0.42 a | |
| 60 | 9.10 ± 0.06 ac | 18.16 ± 0.24 ac | 14.75 ± 0.24 ac | 50.85 ± 0.15 ac | |
| GS | 90 | 8.53 ± 0.07 ac | 19.09 ± 0.20 a | 12.73 ± 0.24 ac | 54.23 ± 0.21 ac |
| 120 | 7.92 ± 0.05 ac | 14.38 ± 0.16 ac | 11.63 ± 0.14 ac | 55.99 ± 1.09 ac | |
| 150 | 6.98 ± 0.12 a | 12.93 ± 0.19 a | 10.86 ± 0.19 ac | 57.87 ± 0.31 a | |
| 180 | 6.16±0.10 a | 10.87 ± 0.18 | 8.91 ± 0.21 ac | 52.85 ± 1.69 ac | |
| 0 | 6.66 ± 0.10 | 6.50 ± 0.40 | 6.20 ± 0.53 | 28.70 ± 1.07 | |
| 30 | 7.47 ± 0.12 ab | 19.04 ± 0.27 a | 14.51 ± 0.22 ab | 46.00 ± 0.71 a | |
| 60 | 10.17 ± 0.05 ab | 24.86 ± 0.35 ab | 10.26 ± 0.11 ab | 56.51 ± 1.12 ab | |
| DI | 90 | 9.10 ± 0.05 b | 21.14 ± 0.36 a | 8.09 ± 0.17 b | 63.78 ± 0.63 ab |
| 120 | 8.56 ± 0.07 b | 17.98 ± 0.30 ab | 7.35 ± 0.14 b | 61.10 ± 0.51 ab | |
| 150 | 7.33 ± 0.07 a | 13.24 ± 0.25 a | 6.95 ± 0.09 b | 57.52 ± 0.50 a | |
| 180 | 6.61 ± 0.12 | 11.13 ± 0.21 | 6.56 ± 0.20 b | 45.19 ± 0.96 ab |
| Supplement | Time (min) | Hunger (mm) | Fullness (mm) | Desire to Eat (mm) | Prospective Food Consumption (mm) | SA (mm) |
|---|---|---|---|---|---|---|
| 0 | 63.80 ± 1.70 | 34.30 ± 0.88 | 61.80 ± 1.14 | 21.40 ± 1.88 | 53.18 ± 0.40 | |
| 30 | 33.60 ± 2.45 | 62.50 ± 1.60 b | 32.10 ± 1.19 | 28.90 ± 2.04 b | 33.03 ± 0.91 bc | |
| 60 | 37.10 ± 1.10 bc | 55.70 ± 2.32 b | 42.60 ± 1.11 bc | 35.50 ± 0.91 bc | 40.08 ± 0.56 bc | |
| ET | 90 | 46.00 ± 1.00 bc | 38.40 ± 3.14 bc | 43.40 ± 0.82 bc | 38.00 ± 1.97 b | 47.05 ± 1.08 bc |
| 120 | 47.20 ± 1.58 bc | 32.60 ± 2.02 bc | 46.00 ± 1.00 bc | 41.80 ± 0.95 bc | 50.60 ± 0.53 bc | |
| 150 | 56.60 ± 1.41 bc | 30.40 ± 2.10 bc | 54.30 ± 1.39 bc | 46.80 ± 1.44 b | 56.83 ± 0.74 bc | |
| 180 | 70.60 ± 1.92 bc | 20.50 ± 1.71 bc | 73.60 ± 1.06 bc | 55.90 ± 1.27 b | 69.90 ± 0.91 bc | |
| 0 | 66.40 ± 1.42 | 32.50 ± 1.76 | 63.50 ± 0.93 | 19.20 ± 0.80 | 54.15 ± 0.62 | |
| 30 | 30.80 ± 1.78 | 70.10 ± 2.12 a | 30.80 ± 1.65 | 20.60 ± 0.69 a | 28.03 ± 0.85 a | |
| 60 | 28.50 ± 1.66 a | 65.90 ± 1.57 a | 27.80 ± 1.80 ac | 22.00 ± 1.09 ac | 28.10 ± 0.58 ac | |
| GS | 90 | 27.30 ± 1.95 ac | 63.70 ± 1.92 ac | 31.00 ± 1.69 a | 24.40 ± 1.75 ac | 29.75 ± 0.93 ac |
| 120 | 29.80 ± 1.70 ac | 58.60 ± 1.99 ac | 35.60 ± 0.83 a | 33.30 ± 1.12 a | 35.03 ± 0.77 ac | |
| 150 | 34.30 ± 1.16 ac | 46.00 ± 1.74 ac | 43.90 ± 1.16 a | 36.60 ± 0.90 ac | 40.20 ± 0.42 ac | |
| 180 | 62.30 ± 1.51 a | 34.90 ± 1.47 ac | 58.80 ± 1.90 a | 45.00 ± 1.32 ac | 57.80 ± 0.64 ac | |
| 0 | 63.20 ± 1.30 | 32.80 ± 1.18 | 65.20 ± 1.24 | 21.40 ± 1.27 | 54.25 ± 0.59 | |
| 30 | 32.30 ± 1.50 a | 68.40 ± 1.38 | 29.60 ± 1.97 | 23.90 ± 1.30 | 29.35 ± 0.85 a | |
| 60 | 28.60 ± 2.03 ab | 60.60 ± 1.35 | 34.60 ± 1.48 ab | 25.50 ± 0.91 ab | 32.03 ± 0.68 ab | |
| DI | 90 | 33.90 ± 1.38 ab | 54.80 ± 1.17 ab | 35.00 ± 1.22 a | 35.00 ± 1.02 b | 37.28 ± 0.64 ab |
| 120 | 39.00 ± 1.13 ab | 45.90 ± 1.49 ab | 36.90 ± 1.17 a | 36.70 ± 1.27 a | 40.93 ± 0.58 ab | |
| 150 | 44.90 ± 0.99 ab | 38.30 ± 2.39 ab | 47.40 ± 1.97 a | 44.90 ± 1.50 b | 49.73 ± 0.63 ab | |
| 180 | 60.60 ± 2.02 a | 27.00 ± 1.71 ab | 61.90 ± 2.36 a | 52.00 ± 1.21 b | 61.88 ± 076 ab |
| Hunger | Fullness | Desire to Eat | Prospective Food Consumption | Subjective Appetite | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ET | r | p | r | p | r | p | r | p | r | p |
| Glycaemia | 0.060 | 0.868 | −0.687 | 0.028 | 0.217 | 0.547 | −0.025 | 0.945 | 0.659 | 0.038 |
| Insulin | −0.215 | 0.552 | 0.713 | 0.021 | −0.046 | 0.900 | 0.362 | 0.304 | −0.437 | 0.321 |
| GLP-1 | −0.133 | 0.714 | −0.756 | 0.011 | 0.392 | 0.262 | −0.543 | 0.105 | 0.321 | 0.540 |
| GIP | 0.219 | 0.544 | −0.082 | 0.821 | 0.486 | 0.155 | 0.399 | 0.253 | 0.540 | 0.107 |
| DI | r | p | r | p | r | p | r | p | r | p |
| Glycaemia | 0.004 | 0.992 | −0.226 | 0.530 | −0.069 | 0.849 | 0.173 | 0.633 | 0.357 | 0.311 |
| Insulin | 0.190 | 0.599 | −0.163 | 0.652 | −0.455 | 0.187 | −0.196 | 0.587 | −0.254 | 0.479 |
| GLP-1 | 0.483 | 0.158 | 0.140 | 0.700 | 0.158 | 0.662 | −0.407 | 0.243 | 0.098 | 0.787 |
| GIP | 0.294 | 0.410 | 0.099 | 0.785 | −0.615 | 0.058 | 0.069 | 0.850 | −0.540 | 0.107 |
| GS | r | p | r | p | r | p | r | p | r | p |
| Glycaemia | −0.192 | 0.595 | 0.019 | 0.958 | −0.152 | 0.674 | 0.128 | 0.726 | −0.154 | 0.672 |
| Insulin | 0.466 | 0.175 | −0.020 | 0.957 | 0.217 | 0.548 | −0.175 | 0.628 | 0.344 | 0.330 |
| GLP-1 | 0.308 | 0.386 | 0.072 | 0.843 | 0.436 | 0.208 | −0.421 | 0.226 | 0.191 | 0.596 |
| GIP | 0.231 | 0.521 | −0.046 | 0.900 | −0.135 | 0.710 | −0.076 | 0.834 | 0.086 | 0.813 |
| Treatment Groups | Mean ± SEM |
|---|---|
| Glycaemic Index (GI) | |
| ET | 56.40 ± 0.43 bc |
| DI | 51.44 ± 0.60 ab |
| GS | 47.59 ± 0.49 ac |
| Glycaemic Load (GL) | |
| ET | 23.69 ± 0.18 bc |
| DI | 12.04 ± 0.14 ac |
| GS | 11.42 ± 0.12 ab |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angarita Dávila, L.; Bermúdez, V.; Aparicio, D.; Céspedes, V.; Escobar, M.C.; Durán-Agüero, S.; Cisternas, S.; de Assis Costa, J.; Rojas-Gómez, D.; Reyna, N.; et al. Effect of Oral Nutritional Supplements with Sucromalt and Isomaltulose versus Standard Formula on Glycaemic Index, Entero-Insular Axis Peptides and Subjective Appetite in Patients with Type 2 Diabetes: A Randomised Cross-Over Study. Nutrients 2019, 11, 1477. https://doi.org/10.3390/nu11071477
Angarita Dávila L, Bermúdez V, Aparicio D, Céspedes V, Escobar MC, Durán-Agüero S, Cisternas S, de Assis Costa J, Rojas-Gómez D, Reyna N, et al. Effect of Oral Nutritional Supplements with Sucromalt and Isomaltulose versus Standard Formula on Glycaemic Index, Entero-Insular Axis Peptides and Subjective Appetite in Patients with Type 2 Diabetes: A Randomised Cross-Over Study. Nutrients. 2019; 11(7):1477. https://doi.org/10.3390/nu11071477
Chicago/Turabian StyleAngarita Dávila, Lisse, Valmore Bermúdez, Daniel Aparicio, Virginia Céspedes, Ma. Cristina Escobar, Samuel Durán-Agüero, Silvana Cisternas, Jorge de Assis Costa, Diana Rojas-Gómez, Nadia Reyna, and et al. 2019. "Effect of Oral Nutritional Supplements with Sucromalt and Isomaltulose versus Standard Formula on Glycaemic Index, Entero-Insular Axis Peptides and Subjective Appetite in Patients with Type 2 Diabetes: A Randomised Cross-Over Study" Nutrients 11, no. 7: 1477. https://doi.org/10.3390/nu11071477





