Effect of Rice Processing towards Lower Rapidly Available Glucose (RAG) Favors Idli, a South Indian Fermented Food Suitable for Diabetic Patients
Abstract
:1. Introduction
2. Dietary Factors and Type 2 Diabetes
3. Contemporary Perceptions of the Deterrent Levels of Starch/Carbohydrate/Fiber/Fat in Diet
4. Rice and the Glycemic Index Mechanism
5. Glycemic Index and Glycemic Load
6. The Roles of Rapidly Available Glucose (RAG) and Slowly Available Glucose (SAG) in Diabetic Patients
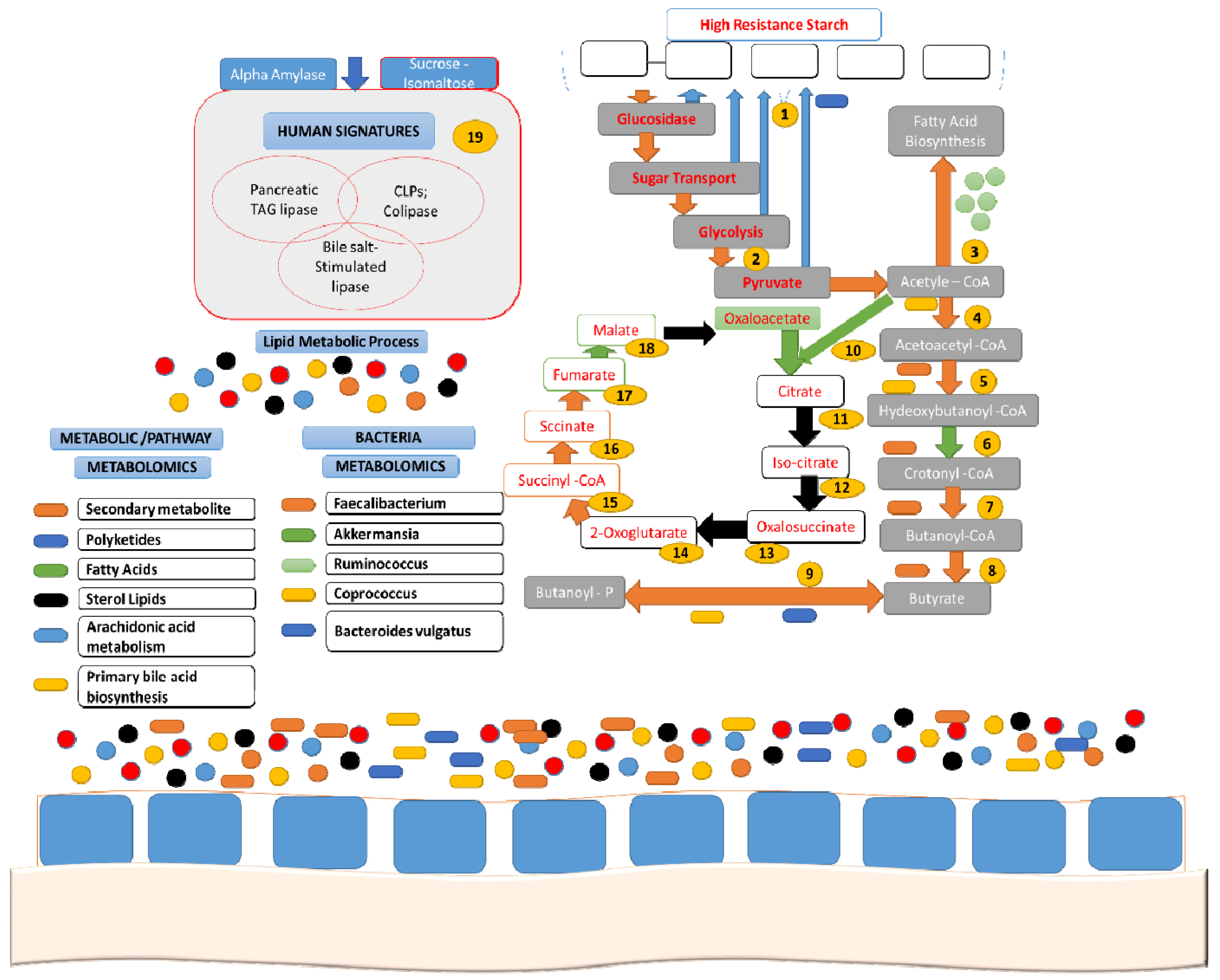
7. Resistant Starch and Gut Microbiota Modulation
8. Resistant Starch (RS), Insulin, Glucose Metabolism, Glycemic Load (GL), and Glycemic Index (GI)
9. Fermented Foods—Idli
10. Limitations of the Glycemic Index (over 77 ± 2, Considered High) and the Glycemic Load (over 40.04, Considered Hiked) for a Serving Size of 250 g
11. Processing Method
12. Physical Treatment
13. Microstructure of Rice Grains
14. Glycemic Index
15. Rapidly Available Glucose (RAG) and Slowly Available Glucose (SAG)
16. Processing Method and Resistant Starch
17. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geiss, L.S.; Wang, J.; Cheng, Y.J.; Thompson, T.J.; Barker, L.; Li, Y.; Gregg, E.W. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA 2014, 312, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Narayan, K.V.; Boyle, J.P.; Thompson, T.J.; Sorensen, S.W.; Williamson, D.F. Lifetime risk for diabetes mellitus in the United States. JAMA 2003, 290, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Yan, Y.; Colditz, G.A.; Herrick, C.J. Prenatal counseling on type 2 diabetes risk, exercise, and nutrition affects the likelihood of postpartum diabetes screening after gestational diabetes. J. Perinatol. 2018, 38, 315. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Kumar, A.; Purohit, S.R.; Rao, P.S. Impact of fermentation and extrusion processing on physicochemical, sensory and bioactive properties of rice-black gram mixed flour. LWT 2018, 89, 155–163. [Google Scholar] [CrossRef]
- Rani, M.; Amane, D.; Ananthanarayan, L. Impact of partial replacement of rice with other selected cereals on idli batter fermentation and idli characteristics. J. Food Sci. Technol. 2019, 56, 1192–1201. [Google Scholar] [CrossRef]
- Lehmann, U.; Robin, F. Slowly digestible starch–its structure and health implications: A review. Trends Food Sci. Technol. 2007, 18, 346–355. [Google Scholar] [CrossRef]
- Arns, B.; Bartz, J.; Radunz, M.; do Evangelho, J.A.; Pinto, V.Z.; da Rosa Zavareze, E.; Dias, A.R.G. Impact of heat-moisture treatment on rice starch, applied directly in grain paddy rice or in isolated starch. Lwt-Food Sci. Technol. 2015, 60, 708–713. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.J.; Liu, Q.; Hoover, R. Impact of annealing and heat-moisture treatment on rapidly digestible, slowly digestible and resistant starch levels in native and gelatinized corn, pea and lentil starches. Carbohydr. Polym. 2009, 75, 436–447. [Google Scholar] [CrossRef]
- Hu, F.B.; Van Dam, R.M.; Liu, S. Diet and risk of type II diabetes: The role of types of fat and carbohydrate. Diabetologia 2001, 44, 805–817. [Google Scholar] [CrossRef]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Brennan, C.S. Dietary fibre, glycaemic response, and diabetes. Mol. Nutr. Food Res. 2005, 49, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Hoover, R.; Zhou, Y. In vitro and in vivo hydrolysis of legume starches by α-amylase and resistant starch formation in legumes—A review. Carbohydr. Polym. 2003, 54, 401–417. [Google Scholar] [CrossRef]
- Gangoiti, J.; Pijning, T.; Dijkhuizen, L. Biotechnological potential of novel glycoside hydrolase family 70 enzymes synthesizing α-glucans from starch and sucrose. Biotechnol. Adv. 2018, 36, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Donlao, N.; Matsushita, Y.; Ogawa, Y. Influence of postharvest drying conditions on resistant starch content and quality of non-waxy long-grain rice (Oryza sativa L.). Drying Technol. 2018, 36, 952–964. [Google Scholar] [CrossRef]
- Lin, A.H.M.; Lee, B.H.; Chang, W.J. Small intestine mucosal α-glucosidase: A missing feature of in vitro starch digestibility. Food Hydrocoll. 2016, 53, 163–171. [Google Scholar] [CrossRef]
- Ashwar, B.A.; Gani, A.; Shah, A.; Wani, I.A.; Masoodi, F.A. Preparation, of resistant starch—A review. Starch-Stärke 2016, 68, 287–301. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, B.; Jin, Z.; BeMiller, J.N. Microbial Starch-Converting Enzymes: Recent Insights and Perspectives. Comp. Rev. Food Sci. Food Saf. 2018, 17, 1238–1260. [Google Scholar] [CrossRef] [Green Version]
- Palacios, O.M.; Kramer, M.; Maki, K.C. Diet and prevention of type 2 diabetes mellitus: Beyond weight loss and exercise. Expert Rev. Endocrinol. Metab. 2019, 14, 1–12. [Google Scholar]
- Willett, W.; Manson, J.; Liu, S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am. J. Clin. Nutr. 2002, 76, 274S–280S. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Willett, W.C.; Stampfer, M.J.; Hu, F.B.; Franz, M.; Sampson, L.; Hennekens, C.H.; Manson, J.E. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am. J. Clin. Nutr. 2000, 71, 1455–1461. [Google Scholar] [CrossRef] [Green Version]
- Chiu, S.; Mulligan, K.; Schwarz, J.M. Dietary carbohydrates and fatty liver disease: De novo lipogenesis. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Chandler-Laney, P.C.; Schneider, C.R.; Gower, B.A.; Granger, W.M.; Mancuso, M.S.; Biggio, J.R. Association of late-night carbohydrate intake with glucose tolerance among pregnant A frican A merican women. Matern. Child Nutr. 2016, 12, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Quddus, A.; Stinson, L.; Shikany, J.M.; Howard, B.V.; Kutob, R.M.; Lu, B.; Manson, J.E.; Eaton, C.B. Artificially sweetened beverages, sugar-sweetened beverages, plain water, and incident diabetes mellitus in postmenopausal women: The prospective Women’s Health Initiative observational study. Am. J. Clin. Nutr. 2017, 106, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Sami, W.; Ansari, T.; Butt, N.S.; Ab Hamid, M.R. Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. 2017, 11, 65. [Google Scholar]
- Yu, Z.; Ley, S.H.; Sun, Q.; Hu, F.B.; Malik, V.S. Cross-sectional association between sugar-sweetened beverage intake and cardiometabolic biomarkers in US women. Br. J. Nutr. 2018, 119, 570–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamothe, L.M.; Lê, K.A.; Samra, R.A.; Roger, O.; Green, H.; Macé, K. The scientific basis for healthful carbohydrate profile. Crit. Rev. Food Sci. Nutr. 2019, 59, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef]
- Sardá, F.A.H.; Giuntini, E.B.; Nazare, J.A.; Koenig, D.; Bahia, L.R.; Lajolo, F.M.; Menezes, E.W.D. Effectiveness of carbohydrates as a functional ingredient in glycemic control. Food Sci. Technol. 2018, 38, 561–576. [Google Scholar] [CrossRef] [Green Version]
- Young-Hyman, D.; De Groot, M.; Hill-Briggs, F.; Gonzalez, J.S.; Hood, K.; Peyrot, M. Psychosocial care for people with diabetes: A position statement of the American Diabetes Association. Diabetes Care 2016, 39, 2126–2140. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef]
- McMacken, M.; Shah, S. A plant-based diet for the prevention and treatment of type 2 diabetes. J. Geriatr. Cardiol. 2017, 14, 342. [Google Scholar] [PubMed]
- Dyson, P.; McArdle, P.; Mellor, D.; Guess, N. James Lind Alliance research priorities: What role do carbohydrates, fats and proteins have in the management of Type 2 diabetes, and are there risks and benefits associated with particular approaches? Diabet. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Haase, N.U. The In Vitro Digestibility of Carbohydrates in Boiled and Processed Potatoes. Potato Res. 2015, 58, 91–102. [Google Scholar] [CrossRef]
- Sloth, B.; Krog-Mikkelsen, I.; Flint, A.; Tetens, I.; Björck, I.; Vinoy, S.; Elmståhl, H.; Astrup, A.; Lang, V.; Raben, A. No difference in body weight decrease between a low glycemic index and high glycemic index diet but reduced LDL cholesterol after 10 wk adlibutum intake of the low glycemic index. Am. J. Clin. Nutr. 2004, 80, 337–347. [Google Scholar] [CrossRef] [PubMed]
- EURESTA. European Flair-concerted Action on Resistant Starch; Newsletter IV, September; Human Nutrition Department, Wageningen Agriculture University: Wageningen, The Netherlands, 1993; p. 2. [Google Scholar]
- Tobaruela, E.D.C.; Santos, A.D.O.; de Almeida-Muradian, L.B.; Araujo, E.D.S.; Lajolo, F.M.; Menezes, E.W. Application of dietary fiber method AOAC 2011.25 in fruit and comparison with AOAC 991.43 method. Food Chem. 2018, 238, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Perera, A.; Meda, V.; Tyler, R.T. Resistant starch: A review of analytical protocols for determining resistant starch and of factors affecting the resistant starch content of foods. Food Res. Int. 2010, 43, 1959–1974. [Google Scholar] [CrossRef]
- Tovar, J. Bioavailability of Starch in Processed Legumes. Importance of Physical Inaccessibility and Retrogradation. SC. Ph.D. Thesis, Department of Applied Nutrition and Food Chemistry, University of Lund, Scania, Sweden, 1992. [Google Scholar]
- Tamura, M.; Singh, J.; Kaur, L.; Ogawa, Y. Impact of the degree of cooking on starch digestibility of rice—An in vitro study. Food Chem. 2016, 191, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Kong, L. Starch-guest inclusion complexes: Formation, structure, and enzymatic digestion. Crit. Rev. Food Sci. Nutr. 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jyothsna, E.; Hymavathi, T.V. Resistant starch: Importance, categories, food sources and physiological effects. J. Pharmacogn. Phytochem. 2017, 6, 67–69. [Google Scholar]
- Saura-Calixto, F.; Abia, R. Resistant starch: An indigestible fraction of foods. Grasas Y Aceites 1991, 3, 239–242. [Google Scholar] [CrossRef]
- Englyst, H.N.; Macfarlane, G.T. Breakdown of resistant starch and readily digestible starch by human gut bacteria. J. Sci. Food Agric. 1986, 37, 699–706. [Google Scholar] [CrossRef]
- Björck, I.; Granfeldt, Y.; Liljeberg, H.; Tovar, J.; Asp, N.-G. Food properties affecting the digestion and absorption of carbohydrates. Am. J. Clin. Nutr. 1994, 59, 699S–705S. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Iozzo, P.; Virtanen, K.A.; Honka, M.J.; Bucci, M.; Nuutila, P. Adipose tissue and skeletal muscle insulin-mediated glucose uptake in insulin resistance: Role of blood flow and diabetes. Am. J. Clin. Nutr. 2018, 108, 749–758. [Google Scholar] [CrossRef]
- Wagenmakers, A.J.; Strauss, J.A.; Shepherd, S.O.; Keske, M.A.; Cocks, M. Increased muscle blood supply and transendothelial nutrient and insulin transport induced by food intake and exercise: Effect of obesity and ageing. J. Physiol. 2016, 594, 2207–2222. [Google Scholar] [CrossRef] [PubMed]
- Mandhania, M.H.; Paul, D.; Suryavanshi, M.V.; Sharma, L.; Chowdhury, S.; Diwanay, S.S.; Diwanay, S.S.; Shouche, Y.S.; Patole, M.S. Diversity and Succession of Microbiota during Fermentation of the Traditional Indian Food Idli. Appl. Environ. Microbial. 2019, 85, 00368-19. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important resistant starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 2), S33–S50. [Google Scholar]
- Chelliah, R.; Ramakrishnan, S.R.; Premkumar, D.; Antony, U. Accelerated fermentation of Idli batter using Eleusinecoracana and Pennisetumglaucum. J. Food Sci. Technol. 2017, 54, 2626–2637. [Google Scholar] [CrossRef]
- Sharma, D. Studies on Utilization of Gudmar (Gymnemasylvestre) Leaf Extract as Antidiabetic in Fennel Rts Beverage. Ph.D. Thesis, Vasantrao Naik Marathwada Krishi Vidyapeeth, Parbhani, India, 2017. [Google Scholar]
- De Pilli, T.; Alessandrino, O. Effects of different cooking technologies on biopolymers modifications of cereal-based foods: Impact on nutritional and quality characteristics review. Crit. Rev. Food Sci. Nutr. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Heiniö, R.L.; Noort, M.W.J.; Katina, K.; Alam, S.A.; Sozer, N.; De Kock, H.L.; Hersleth, M.; Poutanen, K. Sensory characteristics of wholegrain and bran-rich cereal foods—A review. Trends Food Sci. Technol. 2016, 47, 25–38. [Google Scholar] [CrossRef]
- Goelema, J.O.; Spreeuwenberg, M.A.M.; Hof, G.; van der Poel, A.F.B.; Tamminga, S. Effect of pressure toasting on the rumen degradability and intestinal digestibility of whole and broken peas, lupins and faba beans and a mixture of these feedstuffs. Anim. Feed Sci. Technol. 1998, 76, 35–50. [Google Scholar] [CrossRef]
- Mukherjee, S.K.; Albury, M.N.; Pederson, C.S.; Van Veen, A.G.; Steinkraus, K.H. Role of Leuconostocmesenteroides in leavening the Batter of Idli, a Fermented Food of India. Appl. Microbiol. 1965, 13, 227–231. [Google Scholar] [PubMed]
- Lorenz, K.; Kulp, K. Physico-chemical properties of defatted heat-moisture treated starches. Starch/Stärke 1983, 35, 123–129. [Google Scholar] [CrossRef]
- Takahashi, T.; Miura, M.; Ohisa, N.; Mori, K.; Kobayashi, S. Heat Treatments of Milled Rice and Properties of the Flours. Cereal Chem. 2005, 82, 228–232. [Google Scholar] [CrossRef]
- Eliasson, A.C. Starch: Physicochemical and functional aspects. In Carbohydrates in Food, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 501–600. [Google Scholar]
- Chelliah, R. Anna University: Chennai, India, Unpublished work. 2017.
- Caplice, E.; Fitzgerald, G.F. Food fermentations: Role of microorganisms in food production and preservation. Int. J. Food Microbial. 1999, 50, 131–149. [Google Scholar] [CrossRef]
- Goddard, M.S.; Young, G.; Marcus, R. The effect of amylose content on insulin and glucose response to ingested rice. Am. J. Clin. Nutr. 1984, 39, 388–392. [Google Scholar] [CrossRef]
- Collar, C.; Armero, E. Functional and Thermal Behaviours of Heat-Moisture-Treated Starch-Rich Wheat-Based Blended Matrices: Impact of Treatment of Non-wheat Flours. Food Biopro. Technol. 2019, 1–14. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant Starch—A Review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef]
- Lawal, O.S.; Adebowale, K.O. An Assessment of Changes in Thermal and Physico-Chemical Parameters of Jack Bean (Canavaliaensiformis) Starch Following Hydrothermal Modifications; European Food Research and Technology Zeitschriftfür Lebensmittel- Untersuchung und -Forschung A; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Theurer, C.B. Grain processing effects on starch utilization by ruminants. J. Anim. Sci. 1986, 63, 1649–1662. [Google Scholar] [CrossRef]
- Gunaratne, A. Heat-Moisture Treatment of Starch. In Physical Modifications of Starch; Springer: Singapore, 2018; pp. 15–36. [Google Scholar] [CrossRef]
- Berry, C.S. Resistant starch: Formation and measurement of starch that survives exhaustive digestion with amylolytic enzymes during the determination of dietary fibre. J. Cereal Sci. 1986, 4, 301–314. [Google Scholar] [CrossRef]
- Fredriksson, H.; Bjo¨rck, I.; Andersson, R.; Liljeberg, H.; Silverio, J.; Eliasson, A.C.; Åmana, P. Studies on alpha-amylase degradation of retrograded starch gels from waxy maize and high-amylopectin potato. Carbohydr. Polym. 2000, 43, 81–87. [Google Scholar] [CrossRef]
- Silverio, J.; Fredriksson, H.; Andersson, R.; Eliasson, A.C.; Aman, P. The effect of temperature cycling on the amylopectin retrogradation of starches with different amylopectin unit-chain length distribution. Carbohydr. Polym. 2000, 42, 175–184. [Google Scholar] [CrossRef]
- Eliasson, A.C.; Gudmundsson, M. Starch: Physicochemical and functional aspects. In Carbohydrates in Food; Eliasson, A.C., Ed.; Dekker: New York, NY, USA, 1996; pp. 431–503. [Google Scholar]
- Franco, C.M.L.; Ciacco, C.F.; Tavares, D.Q. Effect of heat-moist treatment on enzymatic susceptibility. Starke/Starch 1995, 47, 223–228. [Google Scholar]
- Han, J.-A.; BeMiller, J.N. Preparation and physical characteristics of slowly digesting modiWed food starches. J. Carbpol. 2007, 67, 366–374. [Google Scholar]
- Jenkins, D.J.A.; Jerkins, A.L.; Kendall, C.W.C.; Augustine, L.; Vuksan, V. Dietary fiber, carbohydrate metabolism, and chronic disease. In Advanced Dietary Fiber Technology; McCleary, B.V., Prosky, L., Eds.; Iowa State University Press: Ames, IA, USA, 2001; pp. 162–167. [Google Scholar]
- Guraya, H.S.; James, C.; Champagne, E.T. Effect of enzyme concentration and storage temperature on the formation of slowly digestible starch from cooked debranched rice starch. Starch/Stärke 2001, 53, 131–139. [Google Scholar] [CrossRef]
- Englyst, K.N.; Englyst, H.N.; Hudson, G.J.; Cole, T.J.; Cumming, J.H. Rapidly available glucose in foods: An in vitro measurement that reflects the glycemic responses. Am. J. Clin. Nutr. 1999, 69, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Han, J.A.; Lim, S.T. Effect of presoaking on textural, thermal, and digestive properties of cooked brown rice. Cereal Chem. 2009, 86, 100–105. [Google Scholar] [CrossRef]
- Kim, J.H.; Tanhehco, E.J.; Ng, P.K.W. Effect of extrusion conditions on resistant starch formation from pastry wheat flour. Food Chem. 2006, 99, 718–723. [Google Scholar] [CrossRef]
- Koksel, H.; Masatcioglu, T.; Kahraman, K.; Ozturk, S.; Basman, A. Improving effect of lyophilization on functional properties of resistant starch preparations formed by acid hydrolysis and heat treatment. J. Cereal Sci. 2008, 47, 275–282. [Google Scholar] [CrossRef]
- Zhao, X.H.; Lin, Y. The impact of coupled acid or pullulanasedebranching on the formation of resistant starch from maize starch with autoclaving–cooling cycles. Eur. Food Res. Technol. 2009, 230, 179. [Google Scholar] [CrossRef]
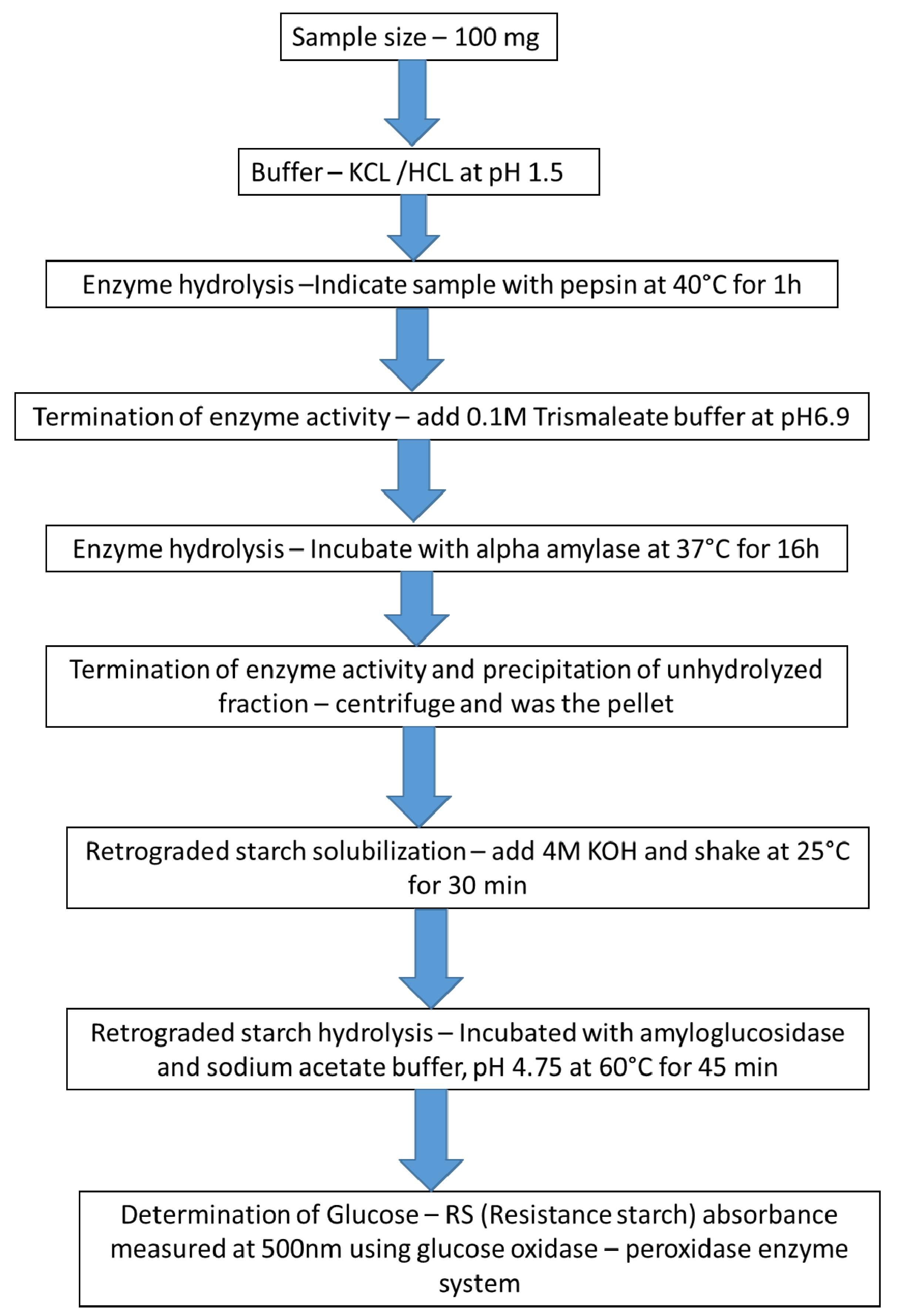
- Goñi, I.; Garcia-Diz, L.; Mañas, E.; Saura-Calixto, F. Analysis of resistant starch: A method for foods and food products. Food Chem. 1996, 56, 445–449. [Google Scholar] [CrossRef]




| Source | Total Starch | Total Dietary Fiber | Resistant Starch |
|---|---|---|---|
| Legumes | |||
| Red beans | 42.4 | 36.2 | 24.4 |
| Pulses | 53.2 | 33.1 | 25.3 |
| Vigna unguiculata (Peas) | 53.6 | 32.6 | 17.5 |
| Cereal grains | |||
| Barley (Hordeum vulgare) | 55.2 | 17 | 18.3 |
| Corn Starch | 77.5 | 19.3 | 25.7 |
| Arborio rice | 95.2 | 1.2 | 14.2 |
| Wheat | 50.4 | 17.2 | 13.2 |
| Oats | 43.2 | 37.2 | 7.2 |
| Flours | |||
| Corn | 84.2 | 2.3 | 11.3 |
| Wheat | 68.5 | 12.6 | 1.4 |
| Rice | 86.3 | 5.4 | 1.5 |
| Potato | 81 | 2.7 | 1.6 |
| Grain-based food products | |||
| Spaghetti | 73.9 | 5.7 | 3.1 |
| Rolled oats | 56.4 | 10.3 | 8.2 |
| Cereal products | |||
| Crisp bread | 67.2 | N/A | 1.4 |
| White bread | 46.3 | N/A | 1.6 |
| Granary bread | 44.6 | N/A | 6.2 |
| Extruded oat cereal | 57.1 | N/A | 0.5 |
| Puffed wheat cereal | 67.7 | N/A | 1.5 |
| Oat porridge | 9.2 | N/A | 0.4 |
| Cooked spaghetti | N/A | N/A | 2.7 |
| Cooked rice | N/A | N/A | 3.8 |
| Potato products | |||
| Boiled potatoes | N/A | N/A | 2.1 |
| Chips | 29.1 | N/A | 4.5 |
| Mashed potatoes | N/A | N/A | 2.1 |
| Starch Source | Treatment | Resistant Starch (%) | Reference |
|---|---|---|---|
| Rice starches | Native | 6.1–10.4 | |
| Heat–moisture treatment (HMT) | 18.3–23.1 | ||
| Acid and HMT | 30.1–38 | ||
| Corn | Native | 4.3 | [48] |
| Autoclaving | 24–31 | ||
| a-amylase and pullulanase | 58.83 | [8] | |
| Partial acid hydrolysis followed by HMT | 63.2 | [8] | |
| Potato starch | Raw | 75 | |
| Wheat starch | Cross-linking with sodium trimetaphosphate sodium tripolyphosphate, epichlorohydrinandphosphoryl chloride | 75.2–85.3 | |
| [8] | |||
| phosphorylation | |||
| Bean starch | Acetylation |
| C∞ (%) | k × 10−2 (min−1) | HI | eGI | ||
|---|---|---|---|---|---|
| Homogenized | R | 41.0 ± 3.7 c | 4.51 ± 0.54 b | 39.3 ± 2.5 d | 61.3 ± 1.4 d |
| MH | 69.0 ± 4.6 ab | 19.11 ± 1.26 a | 78.0 ± 5.1 b | 82.5 ± 2.8 b | |
| CC | 78.1 ± 1.0 a | 22.25 ± 6.94 a | 88.5 ± 2.1 a | 88.3 ± 1.1 a | |
| Non-Homogenized | R | 8.6 ± 1.4 d | 3.07 ± 0.52 b | 8.1 ± 1.0 e | 43.8 ± 0.4 e |
| MH | 63.2 ± 9.1 b | 3.03 ± 0.49 b | 54.1 ± 4.8 c | 69.4 ± 2.6 c | |
| CC | 66.3 ± 2.2 b | 3.28 ± 0.67 b | 58.4 ± 3.1 c | 71.8 ± 1.7 c |
| Starch Source | Treatment | RS | Method of RS Analysis | Reference |
|---|---|---|---|---|
| Japonica brown rice | Pre-soaking in water at 30 or 40 °C to reach 25% humidity + heated | 30.2–30.4% | [48] | [8] |
| Pre-soaked in water at 20 or 45 °C to reach 30% humidity + heated | 21.7–27.9% | |||
| Waxy rice | Natural waxy rice starch | 10.30% | [48] | [76] |
| Gelatinized starch | 3.00% | |||
| Ascetically gelatinized starch at 60 °C for 5 min | 8.60% | |||
| Partially gelatinized starch at 70 °C for 5 min | 7.70% | |||
| Pastry wheat flour | Extracted at 20% humidity; 150/200/250 rpm; 40–120 °C; stored at 4 °C/0 days | 0.48–0.52% | Megazyme® assay | [77] |
| Extruded at 20% humidity; 150/200/250 rpm; 40–120 °C; stored at 4 °C/7–14 days | 1.21–1.35% | |||
| Extracted at 40% humidity; 150/200/250 rpm; 40–120 °C; stored at 4 °C/0 days | 0.63–0.67% | |||
| Extracted at 40% humidity; 150/200/250 rpm; 40–120 °C; stored at 4 °C/7–14 days | 1.52–1.86% | |||
| Extracted at 60% humidity; 150/200/250 rpm; 40–120 °C; stored at 4 °C/0 days | 2.54–2.65% | |||
| Extracted at 60% humidity; 150/200/250 rpm; 40–120 °C; stored at 4 °C/7–14 days | 3.55–4.25% | |||
| Corn | Acid modified with 1.64 M HCl at 40 °C for 4 h + gelatinized + sterilized at 121 °C for15 min + freeze dried | 5% | AOAC 991.43 [36] | [78] |
| Acid reformed with 1.64 M Hydrochloric acid at 40 °C for 4 h + gelatinized + sterilized at 121°C for15 min + Stored at 95 °C for 48 h + freeze dried | 12% | |||
| Corn | Normal corn starch | 19.70% | modified as per [79] | [76] |
| Galvanized with high moisture for 24 h at 50 °C | 18.30% | |||
| Moist–heat management (30% moisture + 24 h at ambient temperature + 120 °C for 24 h) | 16.90% | |||
| Galvanized with high humidity for 24 h at 50 °C + Moist–heat treatment (30% humidity + 24 h ambient temperature + 120 °C for 24 h) | 17.30% | |||
| Moist–heat treatment (30% moisture + 24 h ambient temperature + 120 °C for 24 h) + annealed with excess water for 24 h at 50 °C | 19.70% | |||
| High-amylose corn | Sterilized at 121 °C + Stored at 4 °C for 24 h (repeated twice) | 30% | [36] | [36] |
| Sterilized at 121 °C + Stored at 4 °C for 24 h (repeated twice) + hydrolyzed with 0.1 M organic acid | 39% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelliah, R.; Chandrashekar, S.; Saravanakumar, K.; Ramakrishnan, S.R.; Rubab, M.; Daliri, E.B.-M.; Barathikannan, K.; Tyagi, A.; Kwame Ofosu, F.; Chen, X.; et al. Effect of Rice Processing towards Lower Rapidly Available Glucose (RAG) Favors Idli, a South Indian Fermented Food Suitable for Diabetic Patients. Nutrients 2019, 11, 1497. https://doi.org/10.3390/nu11071497
Chelliah R, Chandrashekar S, Saravanakumar K, Ramakrishnan SR, Rubab M, Daliri EB-M, Barathikannan K, Tyagi A, Kwame Ofosu F, Chen X, et al. Effect of Rice Processing towards Lower Rapidly Available Glucose (RAG) Favors Idli, a South Indian Fermented Food Suitable for Diabetic Patients. Nutrients. 2019; 11(7):1497. https://doi.org/10.3390/nu11071497
Chicago/Turabian StyleChelliah, Ramachandran, Sangeeta Chandrashekar, Kandasamy Saravanakumar, Sudha Rani Ramakrishnan, Momna Rubab, Eric Banan-Mwine Daliri, Kaliyan Barathikannan, Akanksha Tyagi, Fred Kwame Ofosu, Xiuqin Chen, and et al. 2019. "Effect of Rice Processing towards Lower Rapidly Available Glucose (RAG) Favors Idli, a South Indian Fermented Food Suitable for Diabetic Patients" Nutrients 11, no. 7: 1497. https://doi.org/10.3390/nu11071497
APA StyleChelliah, R., Chandrashekar, S., Saravanakumar, K., Ramakrishnan, S. R., Rubab, M., Daliri, E. B.-M., Barathikannan, K., Tyagi, A., Kwame Ofosu, F., Chen, X., Kim, S.-H., Elahi, F., NaKyeong, H., Wang, M.-H., Raman, V., Antony, U., & Oh, D.-H. (2019). Effect of Rice Processing towards Lower Rapidly Available Glucose (RAG) Favors Idli, a South Indian Fermented Food Suitable for Diabetic Patients. Nutrients, 11(7), 1497. https://doi.org/10.3390/nu11071497








