Hot Water Extract of Curcuma longa L. Improves Serum Inflammatory Markers and General Health in Subjects with Overweight or Prehypertension/Mild Hypertension: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Enrollment of Participants
2.3. Study Agent
2.4. Intervention
2.5. Measurement of Inflammatory Markers
2.6. Short-Form 36 Health Status Questionnaire
2.7. Profile of Mood States Scale
2.8. Sample Size
2.9. Statistical Analysis
3. Results
3.1. Subjects
3.2. Effect of WEC on Serum Inflammatory Markers
3.3. Effect of WEC on Serum Metabolic Markers
3.4. Effect of WEC on SF-36 Scores
3.5. Effect of WEC on POMS Scores
3.6. Safety
4. Discussion
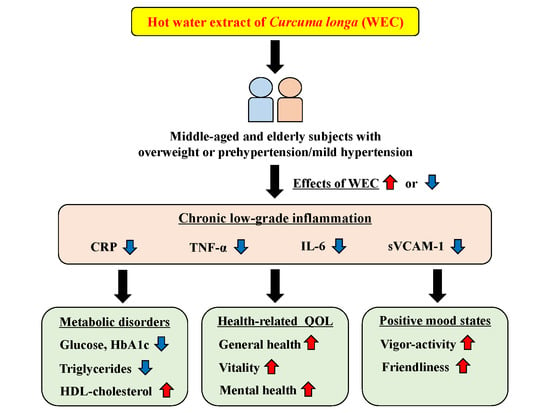
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushner, I.; Samols, D.; Magrey, M. A unifying biologic explanation for “high-sensitivity” C-reactive protein and “low-grade” inflammation. Arthritis Care Res. 2010, 62, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Leon-Pedroza, J.I.; Gonzalez-Tapia, L.A.; del Olmo-Gil, E.; Castellanos-Rodriguez, D.; Escobedo, G.; Gonzalez-Chavez, A. Low-grade systemic inflammation and the development of metabolic diseases: From the molecular evidence to the clinical practice. Cirugía y Cirujanos 2015, 83, 543–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Dore, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Oriowo, M.A. Perivascular adipose tissue, vascular reactivity and hypertension. Med. Princ. Pract. 2015, 24, 29–37. [Google Scholar] [CrossRef]
- Kolb, H.; Mandrup-Poulsen, T. The global diabetes epidemic as a consequence of lifestyle-induced low-grade inflammation. Diabetologia 2010, 53, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Tamakoshi, K.; Yatsuya, H.; Kondo, T.; Hori, Y.; Ishikawa, M.; Zhang, H.; Murata, C.; Otsuka, R.; Zhu, S.; Toyoshima, H. The metabolic syndrome is associated with elevated circulating C-reactive protein in healthy reference range, a systemic low-grade inflammatory state. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M. High-sensitivity C-reactive protein: Potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001, 103, 1813–1818. [Google Scholar] [CrossRef]
- Tao, Q.; Ang, T.F.A.; De Carli, C.; Auerbach, S.H.; Devine, S.; Stein, T.D.; Zhang, X.; Massaro, J.; Au, R.; Qiu, W.Q. Association of Chronic Low-grade Inflammation With Risk of Alzheimer Disease in ApoE4 Carriers. JAMA Netw. Open 2018, 1, e183597. [Google Scholar] [CrossRef]
- Otani, T.; Iwasaki, M.; Sasazuki, S.; Inoue, M.; Tsugane, S. Japan Public Health Center–Based Prospective Study Group. Plasma C-reactive protein and risk of colorectal cancer in a nested case-control study: Japan Public Health Center–based prospective study. Cancer Epidemiol. Prev. Biomark. 2006, 15, 690–695. [Google Scholar] [CrossRef]
- Iqbal, F.; Baker, W.S.; Khan, M.I.; Thukuntla, S.; McKinney, K.H.; Abate, N.; Tuvdendorj, D. Current and future therapies for addressing the effects of inflammation on HDL cholesterol metabolism. Br. J. Pharm. 2017, 174, 3986–4006. [Google Scholar] [CrossRef] [Green Version]
- Garvin, P.; Nilsson, E.; Ernerudh, J.; Kristenson, M. The joint subclinical elevation of CRP and IL-6 is associated with lower health-related quality of life in comparison with no elevation or elevation of only one of the biomarkers. Qual. Life Res. 2016, 25, 213–221. [Google Scholar] [CrossRef]
- DellaGioia, N.; Devine, L.; Pittman, B.; Hannestad, J. Bupropion pre-treatment of endotoxin-induced depressive symptoms. Brain Behav. Immun. 2013, 31, 197–204. [Google Scholar] [CrossRef]
- da Cruz Fernandes, I.M.; Pinto, R.Z.; Ferreira, P.; Lira, F.S. Low back pain, obesity, and inflammatory markers: Exercise as potential treatment. J. Exerc. Rehabil. 2018, 14, 168–174. [Google Scholar] [CrossRef]
- Gumina, S.; Carbone, S.; Perugia, D.; Vestri, A.R.; Postacchini, F. Shoulder adhesive capsulitis in the early freezing phase: Correlations between blood exams and Constant Score. Musculoskelet. Surg. 2011, 95, 37. [Google Scholar] [CrossRef]
- Komulainen, P.; Lakka, T.A.; Kivipelto, M.; Hassinen, M.; Penttila, I.M.; Helkala, E.L.; Gylling, H.; Nissinen, A.; Rauramaa, R. Serum high sensitivity C-reactive protein and cognitive function in elderly women. Age Ageing 2007, 36, 443–448. [Google Scholar] [CrossRef] [Green Version]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef]
- Mehra, K.S.; Mikuni, I.; Gupta, U.; Gode, K.D. Curcuma longa (Linn) drops in corneal wound healing. Tokai J. Exp. Clin. Med. 1984, 9, 27–31. [Google Scholar]
- Yu, Z.F.; Kong, L.D.; Chen, Y. Antidepressant activity of aqueous extracts of Curcuma longa in mice. J. Ethnopharmacol. 2002, 83, 161–165. [Google Scholar] [CrossRef]
- Li, Y.; Shi, X.; Zhang, J.; Zhang, X.; Martin, R.C. Hepatic protection and anticancer activity of curcuma: A potential chemopreventive strategy against hepatocellular carcinoma. Int. J. Oncol. 2014, 44, 505–513. [Google Scholar] [CrossRef]
- Anandakumar, S.; Joseph, J.A.; Bethapudi, B.; Agarwal, A.; Jung, E.-B. Anti-inflammatory effects of turmeric (Curcuma longa L.) extract on acute and chronic inflammation models. J. Korean Soc. Food Sci. Nutr. 2014, 43, 612–617. [Google Scholar] [CrossRef]
- Sengupta, M.; Sharma, G.D.; Chakraborty, B. Hepatoprotective and immunomodulatory properties of aqueous extract of Curcuma longa in carbon tetra chloride intoxicated Swiss albino mice. Asian Pac. J. Trop. Biomed. 2011, 1, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Uchio, R.; Murosaki, S.; Ichikawa, H. Hot water extract of turmeric (Curcuma longa) prevents non-alcoholic steatohepatitis in mice by inhibiting hepatic oxidative stress and inflammation. J. Nutr. Sci. 2018, 7, e36. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, K.; Muroyama, K.; Murosaki, S. Effect of a water extract of Curcuma longa on emotional states in healthy participants. Biosci. Microbiota Food Health 2018, 37, 25–29. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef]
- Shiwaku, K.; Anuurad, E.; Enkhmaa, B.; Nogi, A.; Kitajima, K.; Shimono, K.; Yamane, Y.; Oyunsuren, T. Overweight Japanese with body mass indexes of 23.0-24.9 have higher risks for obesity-associated disorders: A comparison of Japanese and Mongolians. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 152–158. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef]
- Kawasaki, K.; Muroyama, K.; Yamamoto, N.; Murosaki, S. A hot water extract of Curcuma longa inhibits adhesion molecule protein expression and monocyte adhesion to TNF-alpha-stimulated human endothelial cells. Biosci. Biotechnol. Biochem. 2015, 79, 1654–1659. [Google Scholar] [CrossRef]
- Fukuhara, S.; Bito, S.; Green, J.; Hsiao, A.; Kurokawa, K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J. Clin. Epidemiol. 1998, 51, 1037–1044. [Google Scholar] [CrossRef]
- Fukuhara, S.; Ware, J.E., Jr.; Kosinski, M.; Wada, S.; Gandek, B. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J. Clin. Epidemiol. 1998, 51, 1045–1053. [Google Scholar] [CrossRef]
- Heuchert, J.P.; McNair, D.M. Profile of Mood States, POMS-2; Multi-Health Systems Inc.: North Tonawanda, NY, USA, 2012. [Google Scholar]
- Konuma, H.; Hirose, H.; Yokoyama, K. Relationship of the Japanese Translation of the Profile of Mood States Second Edition (POMS 2®) to the First Edition (POMS®). Juntendo Med. J. 2015, 61, 517–519. [Google Scholar] [CrossRef]
- Yokoyama, K.; Watanabe, K. Translation of POMS 2: Profile of Mode States, 2nd ed.; Kaneko Shobo: Tokyo, Japan, 2015. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef]
- Shimura, T.; Kitagawa, M.; Yamada, T.; Ebi, M.; Mizoshita, T.; Tanida, S.; Kataoka, H.; Kamiya, T.; Joh, T. C-reactive protein is a potential prognostic factor for metastatic gastric cancer. Anticancer Res. 2012, 32, 491–496. [Google Scholar]
- Howell, D.C. Statistical Methods for Psychology; Cengage Learning: Belmont, CA, USA, 2009; pp. 461–483. [Google Scholar]
- Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Nikolaidis, M.G. Low vitamin C values are linked with decreased physical performance and increased oxidative stress: Reversal by vitamin C supplementation. Eur. J. Nutr. 2016, 55, 45–53. [Google Scholar] [CrossRef]
- Corpas, R.; Grinan-Ferre, C.; Palomera-Avalos, V.; Porquet, D.; Garcia de Frutos, P.; Franciscato Cozzolino, S.M.; Rodriguez-Farre, E.; Pallas, M.; Sanfeliu, C.; Cardoso, B.R. Melatonin induces mechanisms of brain resilience against neurodegeneration. J. Pineal Res. 2018, 65, e12515. [Google Scholar] [CrossRef]
- Sung, K.C. Seasonal variation of C-reactive protein in apparently healthy Koreans. Int. J. Cardiol. 2006, 107, 338–342. [Google Scholar] [CrossRef]
- Liu, B.; Taioli, E. Seasonal Variations of Complete Blood Count and Inflammatory Biomarkers in the US Population—Analysis of NHANES Data. PLoS ONE 2015, 10, e0142382. [Google Scholar] [CrossRef]
- Arima, H.; Kubo, M.; Yonemoto, K.; Doi, Y.; Ninomiya, T.; Tanizaki, Y.; Hata, J.; Matsumura, K.; Iida, M.; Kiyohara, Y. High-sensitivity C-reactive protein and coronary heart disease in a general population of Japanese: The Hisayama study. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1385–1391. [Google Scholar] [CrossRef]
- Iso, H.; Noda, H.; Ikeda, A.; Yamagishi, K.; Inoue, M.; Iwasaki, M.; Tsugane, S. The impact of C-reactive protein on risk of stroke, stroke subtypes, and ischemic heart disease in middle-aged Japanese: The Japan public health center-based study. J. Atheroscler. Thromb. 2012, 19, 756–766. [Google Scholar] [CrossRef]
- Doi, Y.; Kiyohara, Y.; Kubo, M.; Ninomiya, T.; Wakugawa, Y.; Yonemoto, K.; Iwase, M.; Iida, M. Elevated C-reactive protein is a predictor of the development of diabetes in a general Japanese population: The Hisayama Study. Diabetes Care 2005, 28, 2497–2500. [Google Scholar] [CrossRef]
- Silvestro, A.; Brevetti, G.; Schiano, V.; Scopacasa, F.; Chiariello, M. Adhesion molecules and cardiovascular risk in peripheral arterial disease. Soluble vascular cell adhesion molecule-1 improves risk stratification. Thromb. Haemost. 2005, 93, 559–563. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 1997, 336, 973–979. [Google Scholar] [CrossRef]
- Mizoguchi, M.; Tahara, N.; Tahara, A.; Nitta, Y.; Kodama, N.; Oba, T.; Mawatari, K.; Yasukawa, H.; Kaida, H.; Ishibashi, M.; et al. Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes a prospective, randomized, comparator-controlled study using serial FDG PET/CT imaging study of carotid artery and ascending aorta. JACC Cardiovasc. Imaging 2011, 4, 1110–1118. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Price, J.F.; Fowkes, F.G.; Zanchetti, A.; Roncaglioni, M.C.; Tognoni, G.; Lee, R.; Belch, J.F.; Wilson, M.; Mehta, Z.; et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: Analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet 2012, 379, 1602–1612. [Google Scholar] [CrossRef]
- Devaraj, S.; Jialal, I. C-reactive protein polarizes human macrophages to an M1 phenotype and inhibits transformation to the M2 phenotype. Arter. Thromb. Vasc. Biol. 2011, 31, 1397–1402. [Google Scholar] [CrossRef]
- Devaraj, S.; Davis, B.; Simon, S.I.; Jialal, I. CRP promotes monocyte-endothelial cell adhesion via Fcgamma receptors in human aortic endothelial cells under static and shear flow conditions. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1170–H1176. [Google Scholar] [CrossRef]
- Nada, A.S.; Hawas, A.M.; Amin Nel, D.; Elnashar, M.M.; Abd Elmageed, Z.Y. Radioprotective effect of Curcuma longa extract on gamma-irradiation-induced oxidative stress in rats. Can. J. Physiol. Pharmacol. 2012, 90, 415–423. [Google Scholar] [CrossRef]
- Sun, D.I.; Nizamutdinova, I.T.; Kim, Y.M.; Cai, X.F.; Lee, J.J.; Kang, S.S.; Kim, Y.S.; Kang, K.M.; Chai, G.Y.; Chang, K.C.; et al. Bisacurone inhibits adhesion of inflammatory monocytes or cancer cells to endothelial cells through down-regulation of VCAM-1 expression. Int. Immunopharmacol. 2008, 8, 1272–1281. [Google Scholar] [CrossRef]
- Kawasaki, K.; Okuda-Hanafusa, C.; Aoyagi, M.; Taoka, K.; Yamamoto, N.; Muroyama, K.; Murosaki, S.; Yamamoto, Y. Inhibitory effect of the compounds from the water extract of Curcuma longa on the production of PGE2 and NO in a macrophage cell line stimulated by LPS. Biosci. Biotechnol. Biochem. 2018, 82, 2109–2117. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Liu, L.; Mei, M.; Yang, S.; Li, Q. Roles of chronic low-grade inflammation in the development of ectopic fat deposition. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef]
- Shin, H.S.; Han, J.M.; Kim, H.G.; Choi, M.K.; Son, C.G.; Yoo, H.R.; Jo, H.K.; Seol, I.C. Anti-atherosclerosis and hyperlipidemia effects of herbal mixture, Artemisia iwayomogi Kitamura and Curcuma longa Linne, in apolipoprotein E-deficient mice. J. Ethnopharmacol. 2014, 153, 142–150. [Google Scholar] [CrossRef]
- Lee, S.J.; Han, J.M.; Lee, J.S.; Son, C.G.; Im, H.J.; Jo, H.K.; Yoo, H.R.; Kim, Y.S.; Seol, I.C. ACE Reduces Metabolic Abnormalities in a High-Fat Diet Mouse Model. Evid. Based Complement. Altern. Med. 2015, 2015, 352647. [Google Scholar] [CrossRef]
- Testa, M.A.; Simonson, D.C. Assessment of quality-of-life outcomes. N. Engl. J. Med. 1996, 334, 835–840. [Google Scholar] [CrossRef]
- Lacourt, T.E.; Vichaya, E.G.; Chiu, G.S.; Dantzer, R.; Heijnen, C.J. The High Costs of Low-Grade Inflammation: Persistent Fatigue as a Consequence of Reduced Cellular-Energy Availability and Non-adaptive Energy Expenditure. Front. Behav. Neurosci. 2018, 12, 78. [Google Scholar] [CrossRef] [Green Version]
- Lasselin, J.; Capuron, L. Chronic low-grade inflammation in metabolic disorders: Relevance for behavioral symptoms. Neuroimmunomodulation 2014, 21, 95–101. [Google Scholar] [CrossRef]
- Liukkonen, T. Low-grade Inflammation in Depression, Anxiety and Sleep Disturbances; University of Oulu: Oulu, Finland, 2011. [Google Scholar]
- Seiler, A.; Murdock, K.W.; Fagundes, C.P. Impaired mental health and low-grade inflammation among fatigued bereaved individuals. J. Psychosom. Res. 2018, 112, 40–46. [Google Scholar] [CrossRef]
- Cho, H.J.; Kivimäki, M.; Bower, J.E.; Irwin, M.R. Association of C-reactive protein and interleukin-6 with new-onset fatigue in the Whitehall II prospective cohort study. Psychol. Med. 2013, 43, 1773–1783. [Google Scholar] [CrossRef]
- Matura, L.A.; Ventetuolo, C.E.; Palevsky, H.I.; Lederer, D.J.; Horn, E.M.; Mathai, S.C.; Pinder, D.; Archer-Chicko, C.; Bagiella, E.; Roberts, K.E. Interleukin-6 and Tumor Necrosis Factor-α Are Associated with Quality of Life–Related Symptoms in Pulmonary Arterial Hypertension. Ann. Am. Thorac. Soc. 2015, 12, 370–375. [Google Scholar] [CrossRef]
- Kristjánsdóttir, J.; Olsson, G.I.; Sundelin, C.; Naessen, T. Could SF-36 be used as a screening instrument for depression in a Swedish youth population? Scand. J. Caring Sci. 2011, 25, 262–268. [Google Scholar] [CrossRef]
- Boix-Castejón, M.; Herranz-López, M.; Gago, A.P.; Olivares-Vicente, M.; Caturla, N.; Roche, E.; Micol, V. Hibiscus and lemon verbena polyphenols modulate appetite-related biomarkers in overweight subjects: A randomized controlled trial. Food Funct. 2018, 9, 3173–3184. [Google Scholar] [CrossRef]
- Babaeian, M.; Naseri, M.; Kamalinejad, M.; Ghaffari, F.; Emadi, F.; Feizi, A.; Rafiei, R.; Mazaheri, M.; Hasheminejad, S.A.; Park, J.-W. The efficacy of mentha longifolia in the treatment of patients with postprandial distress syndrome: A double-blind, randomized clinical trial. Iran. Red Crescent Med. J. 2017, 19. [Google Scholar] [CrossRef]
- Vasileva, L.V.; Saracheva Kcapital Ie, C.; Ivanovska, M.V.; Petrova, A.P.; Sucouglu, E.; Murdjeva, M.A.; Getova-Spasova, D.P. Beneficial Effect of Chronic Treatment with Extracts from Rhodiola Rosea L. and Curcuma Longa L. on the Immunoreactivity of Animals Subjected to a Chronic Mild Stress Model. Folia Med. 2017, 59, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Shrestha, A.C.; Kim, H.S.; Ham, H.N.; Kim, J.H.; Kim, Y.J.; Noh, Y.J.; Kim, S.J.; Kim, D.K.; Jo, H.K.; et al. WS-5 Extract of Curcuma longa, Chaenomeles sinensis, and Zingiber officinale Contains Anti-AChE Compounds and Improves beta-Amyloid-Induced Memory Impairment in Mice. Evid. Based Complement. Altern. Med. 2019, 2019, 5160293. [Google Scholar] [CrossRef]
- Ferguson, J.F.; Mulvey, C.K.; Patel, P.N.; Shah, R.Y.; Doveikis, J.; Zhang, W.; Tabita-Martinez, J.; Terembula, K.; Eiden, M.; Koulman, A.; et al. Omega-3 PUFA supplementation and the response to evoked endotoxemia in healthy volunteers. Mol. Nutr. Food Res. 2014, 58, 601–613. [Google Scholar] [CrossRef]
- Cho, H.J.; Eisenberger, N.I.; Olmstead, R.; Breen, E.C.; Irwin, M.R. Preexisting mild sleep disturbance as a vulnerability factor for inflammation-induced depressed mood: A human experimental study. Transl. Psychiatry 2016, 6, e750. [Google Scholar] [CrossRef]
- Miyata, S.; Noda, A.; Iwamoto, K.; Kawano, N.; Banno, M.; Tsuruta, Y.; Noda, Y.; Ozaki, N. Impaired cortical oxygenation is related to mood disturbance resulting from three nights of sleep restriction. Sleep Biol. Rhythm. 2015, 13, 387–394. [Google Scholar] [CrossRef]
- Christian, L.M.; Glaser, R.; Porter, K.; Iams, J.D. Stress-induced inflammatory responses in women: Effects of race and pregnancy. Psychosom. Med. 2013, 75, 658–669. [Google Scholar] [CrossRef]
- Childs, E.; de Wit, H. Regular exercise is associated with emotional resilience to acute stress in healthy adults. Front. Physiol. 2014, 5, 161. [Google Scholar] [CrossRef]
- Kell, G.; Rao, A.; Beccaria, G.; Clayton, P.; Inarejos-García, A.M.; Prodanov, M. affron® a novel saffron extract (Crocus sativus L.) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complement. Ther. Med. 2017, 33, 58–64. [Google Scholar] [CrossRef]
- Hongo, N. Daily Fatigue Reducing Effect of Astaxanthin-A Randomized, Placebo-controlled, Double-blind, Parallel-group Study. Jpn. Phamacol. 2017, 45, 61–72. [Google Scholar]
- Ibironke, G.F.; Alemonu, O.J. Effects of Ethanol Extract of Curcuma Longa Rhizome on Neurobehavioural Activities in Stressed Rats. Afr. J. Biomed. Res. 2013, 16, 193–197. [Google Scholar]
- Kumar, A.; Singh, A. Possible nitric oxide modulation in protective effect of (Curcuma longa, Zingiberaceae) against sleep deprivation-induced behavioral alterations and oxidative damage in mice. Phytomedicine 2008, 15, 577–586. [Google Scholar] [CrossRef]

| Placebo (0.9 g/3 Tablets) | WEC (0.9 g/3 Tablets) | |
|---|---|---|
| Energy (kcal) | 3.4 | 3.4 |
| Carbohydrate (g) | 0.820 | 0.760 |
| Protein (g) | 0 | 0.019 |
| Lipid (g) | 0.013 | 0.031 |
| Sodium chloride (mg) | 0.74 | 0.45 |
| Bisacurone (μg) | 0 | 400 |
| Turmeronol A (μg) | 0 | 80 |
| Turmeronol B (μg) | 0 | 20 |
| Placebo Group (n = 44) | WEC Group (n = 43) | p Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Sex (n, Male/Female) | 22/22 | 23/20 | 0.746 | ||
| Age (years) | 58.5 | 5.5 | 58.8 | 5.3 | 0.803 |
| Physical measurements and tests | |||||
| BMI (kg/m2) | 25.1 | 2.5 | 25.0 | 1.8 | 0.561 |
| SBP (mmHg) | 131.0 | 16.8 | 129.2 | 15.3 | 0.626 |
| DBP (mmHg) | 82.4 | 11.6 | 81.6 | 10.5 | 0.642 |
| Serum inflammatory markers | |||||
| CRP (mg/dL) | 0.105 | 0.081 | 0.090 | 0.064 | 0.354 |
| TNF-α (pg/mL) | 1.557 | 0.358 | 1.936 | 1.945 | 0.215 |
| IL-1β (pg/mL) | 0.057 | 0.091 | 0.051 | 0.102 | 0.771 |
| IL-6 (pg/mL) | 1.125 | 0.412 | 4.381 | 11.566 | 0.072 |
| sVCAM-1 (ng/mL) | 571.0 | 121.7 | 599.3 | 129.7 | 0.297 |
| Metabolic markers | |||||
| Glucose (mg/dL) | 84.2 | 6.0 | 86.0 | 6.9 | 0.178 |
| HbA1c (%) | 5.48 | 0.24 | 5.51 | 0.28 | 0.535 |
| Triglyceride (mg/dL) | 112.5 | 56.2 | 127.5 | 74.1 | 0.272 |
| Total cholesterol (mg/dL) | 220.1 | 38.6 | 218.3 | 33.5 | 0.778 |
| LDL-cholesterol (mg/dL) | 138.1 | 34.2 | 132.8 | 29.9 | 0.384 |
| HDL-cholesterol (mg/dL) | 54.3 | 14.9 | 56.0 | 12.3 | 0.579 |
| Placebo Group (n = 44) | WEC Group (n = 43) | p Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| SF-36 scores (points) | |||||
| Physical functioning (PF) | 91.7 | 8.8 | 94.3 | 6.5 | 0.121 |
| Role physical (RP) | 91.3 | 14.3 | 95.6 | 11.0 | 0.120 |
| Bodily pain (BP) | 78.7 | 16.3 | 78.5 | 17.6 | 0.958 |
| General health (GH) | 74.5 | 15.9 | 74.8 | 13.6 | 0.910 |
| Vitality (VT) | 66.3 | 20.2 | 67.2 | 14.2 | 0.828 |
| Social functioning (SF) | 90.3 | 18.7 | 94.8 | 11.6 | 0.189 |
| Role emotional (RE) | 90.2 | 15.5 | 93.2 | 11.4 | 0.295 |
| Mental health (MH) | 77.2 | 13.4 | 79.0 | 13.5 | 0.536 |
| Physical component summary (PCS) | 51.0 | 7.9 | 53.3 | 5.2 | 0.117 |
| Mental component summary (MCS) | 53.5 | 8.3 | 53.6 | 6.9 | 0.951 |
| POMS scores (points) | |||||
| Anger–hostility (AH) | 46.9 | 7.1 | 47.1 | 5.9 | 0.896 |
| Confusion–bewilderment (CB) | 47.9 | 8.2 | 47.2 | 9.1 | 0.716 |
| Depression–dejection (DD) | 47.4 | 5.5 | 46.7 | 6.7 | 0.602 |
| Fatigue–inertia (FI) | 47.6 | 8.3 | 44.8 | 6.1 | 0.077 |
| Tension–anxiety (TA) | 48.0 | 9.5 | 46.8 | 9.2 | 0.563 |
| Vigor–activity (VA) | 56.3 | 10.3 | 56.1 | 9.3 | 0.932 |
| Friendliness (F) | 56.8 | 9.3 | 56.1 | 10.1 | 0.770 |
| Total mood disturbance (TMD) | 45.8 | 8.2 | 44.6 | 7.8 | 0.496 |
| Change from Baseline | Repeated Measures Two-Way ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 4 | Week 8 | Week 12 | Group | Time | Interaction | ||||
| Mean | SD | Mean | SD | Mean | SD | ||||
| CRP (mg/dL) | |||||||||
| Placebo | 0.016 | 0.072 | 0.036 | 0.092 | 0.057 | 0.138 | 0.077 | 0.098 | 0.243 |
| WEC | 0.006 | 0.086 | −0.001* | 0.071 | 0.015 ** | 0.068 | |||
| TNF-α (pg/mL) | |||||||||
| Placebo | 0.718 | 0.335 | 0.824 | 0.448 | 0.215 | 0.342 | 0.165 | < 0.001 | 0.696 |
| WEC | 0.381 ** | 1.806 | 0.410 ** | 1.754 | −0.153 ** | 1.718 | |||
| IL-1β (pg/mL) | |||||||||
| Placebo | 0.013 | 0.105 | 0.029 | 0.110 | 0.019 | 0.120 | 0.474 | 0.270 | 0.020 |
| WEC | 0.067 | 0.144 | 0.010 | 0.120 | 0.033 | 0.159 | |||
| IL-6 (pg/mL) | |||||||||
| Placebo | 0.21 | 1.41 | 0.49 | 1.12 | 0.33 | 0.70 | 0.150 | 0.042 | 0.021 |
| WEC | −2.26 ** | 10.32 | −2.41 ** | 10.91 | −1.33 ** | 11.09 | |||
| sVCAM-1 (mg/dL) | |||||||||
| Placebo | 12.7 | 85.9 | 6.1 | 59.6 | 33.5 | 59.9 | 0.112 | 0.024 | 0.780 |
| WEC | −14.1 * | 92.7 | −10.3 | 98.9 | 5.9 * | 95.7 | |||
| Change from Baseline | Repeated Measures Two-Way ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 4 | Week 8 | Week 12 | Group | Time | Interaction | ||||
| Mean | SD | Mean | SD | Mean | SD | ||||
| Glucose (mg/dL) | |||||||||
| Placebo | 2.2 | 4.6 | 2.7 | 4.7 | 2.0 | 5.0 | 0.070 | 0.153 | 0.900 |
| WEC | 0.1 ** | 6.5 | 1.0 * | 5.5 | −0.1 ** | 6.6 | |||
| HbA1c (%) | |||||||||
| Placebo | 0.132 | 0.096 | 0.018 | 0.108 | 0.016 | 0.103 | 0.174 | < 0.001 | 0.174 |
| WEC | 0.128 | 0.093 | −0.005 | 0.097 | −0.033 ** | 0.125 | |||
| Triglycerides (mg/dL) | |||||||||
| Placebo | 15.8 | 72.4 | 14.0 | 55.5 | −4.0 | 47 | 0.135 | 0.005 | 0.784 |
| WEC | 3.1 | 36.8 | 2.0 * | 50.1 | −12.6 | 54.8 | |||
| Total cholesterol (mg/dL) | |||||||||
| Placebo | 5.5 | 15.1 | 2.3 | 18.2 | 3.5 | 15.1 | 0.751 | 0.506 | 0.635 |
| WEC | 4.0 | 16.6 | 3.8 | 15.8 | 2.0 | 17.8 | |||
| LDL−cholesterol (mg/dL) | |||||||||
| Placebo | 1.5 | 18.2 | 0.6 | 18.0 | 2.5 | 13.3 | 0.911 | 1.000 | 0.619 |
| WEC | 2.1 | 14.6 | 2.0 | 16.3 | 1.4 | 14.3 | |||
| HDL−cholesterol (mg/dL) | |||||||||
| Placebo | 1.4 | 5.1 | 1.3 | 5.8 | 2.8 | 4.5 | 0.099 | 0.022 | 0.155 |
| WEC | 2.1 | 5.0 | 4.3 ** | 5.5 | 4.2 | 8.6 | |||
| Change from Baseline | Repeated Measures Two-Way ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 4 | Week 8 | Week 12 | Group | Time | Interaction | ||||
| Mean | SD | Mean | SD | Mean | SD | ||||
| Physical functioning (PF) | |||||||||
| Placebo | 0.11 | 5.23 | −0.80 | 5.49 | −0.80 | 7.85 | 0.674 | 0.182 | 0.571 |
| WEC | −0.23 | 5.87 | −2.21 | 10.02 | −0.58 | 6.83 | |||
| Role physical (RP) | |||||||||
| Placebo | −0.43 | 10.56 | 1.56 | 9.82 | −0.71 | 16.68 | 0.696 | 0.345 | 0.267 |
| WEC | −2.33 | 11.41 | −0.58 | 9.53 | 1.16 | 8.10 | |||
| Bodily pain (BP) | |||||||||
| Placebo | 1.43 | 17.91 | −2.09 | 17.61 | −0.95 | 19.36 | 0.475 | 0.177 | 0.829 |
| WEC | 3.00 | 17.28 | −0.14 | 19.96 | 2.67 | 17.13 | |||
| General health (GH) | |||||||||
| Placebo | −2.5 | 8.55 | −4.05 | 8.83 | −3.61 | 9.90 | 0.047 | 0.858 | 0.503 |
| WEC | −0.05 | 11.60 | 0.65 ** | 10.45 | 0.09 ** | 9.36 | |||
| Vitality (VT) | |||||||||
| Placebo | −0.14 | 14.36 | −1.28 | 11.95 | −2.27 | 15.61 | 0.153 | 0.820 | 0.176 |
| WEC | 1.16 | 12.29 | 1.16 | 9.38 | 3.78 ** | 11.74 | |||
| Social functioning (SF) | |||||||||
| Placebo | 2.84 | 15.69 | 2.84 | 14.23 | −1.42 | 25.46 | 0.511 | 0.950 | 0.008 |
| WEC | −1.74 | 16.27 | −2.62 * | 13.79 | 2.62 | 11.43 | |||
| Role emotional (RE) | |||||||||
| Placebo | 1.89 | 13.31 | 2.65 | 11.33 | −0.19 | 12.90 | 0.415 | 0.939 | 0.127 |
| WEC | −0.19 | 11.57 | −1.55* | 11.39 | 0.97 | 11.67 | |||
| Mental health (MH) | |||||||||
| Placebo | 2.16 | 8.79 | −1.02 | 9.74 | −0.91 | 13.17 | 0.300 | 0.085 | 0.062 |
| WEC | 1.98 | 9.83 | 0.81 | 10.06 | 3.60 ** | 11.56 | |||
| Physical component summary (PCS) | |||||||||
| Placebo | 0.17 | 4.64 | 0.86 | 4.10 | −0.21 | 8.69 | 0.234 | 0.963 | 0.247 |
| WEC | −0.97 | 4.65 | −1.42* | 6.42 | −0.31 | 4.59 | |||
| Mental component summary (MCS) | |||||||||
| Placebo | 0.47 | 5.45 | −1.06 | 4.70 | −1.16 | 7.32 | 0.079 | 0.228 | 0.064 |
| WEC | 1.03 | 5.43 | 0.71* | 5.27 | 1.99 ** | 5.08 | |||
| Change from Baseline | Repeated Measures Two-Way ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Week 4 | Week 8 | Week 12 | Group | Time | Interaction | ||||
| Mean | SD | Mean | SD | Mean | SD | ||||
| Anger−hostility (AH) | |||||||||
| Placebo | −1.86 | 4.64 | −1.91 | 6.45 | −2.05 | 9.20 | 0.412 | 0.378 | 0.482 |
| WEC | −1.09 | 5.27 | −0.16 | 4.63 | −2.07 | 5.32 | |||
| Confusion−bewilderment (CB) | |||||||||
| Placebo | 0.18 | 5.62 | −0.16 | 5.85 | 0.16 | 8.95 | 0.667 | 0.928 | 0.751 |
| WEC | −0.35 | 5.40 | −0.05 | 5.98 | −0.84 | 6.18 | |||
| Depression−dejection (DD) | |||||||||
| Placebo | −1.32 | 3.84 | −0.77 | 4.98 | −0.34 | 7.76 | 0.616 | 0.602 | 0.591 |
| WEC | −0.58 | 4.72 | 0.09 | 5.10 | −0.65 | 3.96 | |||
| Fatigue−inertia (FI) | |||||||||
| Placebo | −1.25 | 5.36 | −2.48 | 6.10 | −2.61 | 7.86 | 0.486 | 0.247 | 0.548 |
| WEC | −1.19 | 4.61 | −0.91 | 5.84 | −2.23 | 5.23 | |||
| Tension−anxiety (TA) | |||||||||
| Placebo | −0.80 | 5.97 | −0.61 | 6.91 | −0.91 | 7.73 | 0.976 | 0.641 | 0.874 |
| WEC | −0.6 | 5.90 | −0.35 | 5.96 | −1.26 | 6.88 | |||
| Vigor−activity (VA) | |||||||||
| Placebo | −1.64 | 6.85 | −1.52 | 7.05 | −2.14 | 7.88 | 0.225 | 0.044 | 0.007 |
| WEC | −2.21 | 7.25 | −0.07 | 7.89 | 1.91* | 7.28 | |||
| Friendliness (F) | |||||||||
| Placebo | −0.86 | 5.99 | −1.36 | 6.92 | −1.23 | 7.74 | 0.284 | 0.238 | 0.089 |
| WEC | −1.21 | 6.92 | 0.14 | 6.82 | 1.51* | 6.06 | |||
| Total mood disturbance (TMD) | |||||||||
| Placebo | −0.75 | 4.02 | −1.02 | 4.89 | −0.77 | 8.85 | 0.990 | 0.289 | 0.206 |
| WEC | −0.26 | 4.13 | −0.30 | 4.53 | −2.02 | 4.76 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchio, R.; Muroyama, K.; Okuda-Hanafusa, C.; Kawasaki, K.; Yamamoto, Y.; Murosaki, S. Hot Water Extract of Curcuma longa L. Improves Serum Inflammatory Markers and General Health in Subjects with Overweight or Prehypertension/Mild Hypertension: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 1822. https://doi.org/10.3390/nu11081822
Uchio R, Muroyama K, Okuda-Hanafusa C, Kawasaki K, Yamamoto Y, Murosaki S. Hot Water Extract of Curcuma longa L. Improves Serum Inflammatory Markers and General Health in Subjects with Overweight or Prehypertension/Mild Hypertension: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2019; 11(8):1822. https://doi.org/10.3390/nu11081822
Chicago/Turabian StyleUchio, Ryusei, Koutarou Muroyama, Chinatsu Okuda-Hanafusa, Kengo Kawasaki, Yoshihiro Yamamoto, and Shinji Murosaki. 2019. "Hot Water Extract of Curcuma longa L. Improves Serum Inflammatory Markers and General Health in Subjects with Overweight or Prehypertension/Mild Hypertension: A Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 11, no. 8: 1822. https://doi.org/10.3390/nu11081822
APA StyleUchio, R., Muroyama, K., Okuda-Hanafusa, C., Kawasaki, K., Yamamoto, Y., & Murosaki, S. (2019). Hot Water Extract of Curcuma longa L. Improves Serum Inflammatory Markers and General Health in Subjects with Overweight or Prehypertension/Mild Hypertension: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 11(8), 1822. https://doi.org/10.3390/nu11081822