Increase of the Adiponectin/Leptin Ratio in Patients with Obesity and Type 2 Diabetes after Roux-en-Y Gastric Bypass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Analytical Procedures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- The GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Kiortsis, D.N.; Catalán, V. Precision medicine: Diagnosis and management of obesity. Lancet Diabetes Endocrinol. 2018, 6, 164–166. [Google Scholar] [CrossRef]
- Bray, G.A.; Frühbeck, G.; Ryan, D.H.; Wilding, J.P. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [CrossRef] [Green Version]
- Frühbeck, G. Bariatric and metabolic surgery: A shift in eligibility and success criteria. Nat. Rev. Endocrinol. 2015, 11, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The science of obesity management: An Endocrine Society scientific statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef]
- Gloy, V.L.; Briel, M.; Bhatt, D.L.; Kashyap, S.R.; Schauer, P.R.; Mingrone, G.; Bucher, H.C.; Nordmann, A.J. Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 347, f5934. [Google Scholar] [CrossRef]
- Ikramuddin, S.; Korner, J.; Lee, W.J.; Connett, J.E.; Inabnet, W.B.; Billington, C.J.; Thomas, A.J.; Leslie, D.B.; Chong, K.; Jeffery, R.W.; et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: The Diabetes Surgery Study randomized clinical trial. JAMA 2013, 309, 2240–2249. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cummings, D.E. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care 2016, 39, 893–901. [Google Scholar] [CrossRef]
- Davies, S.W.; Efird, J.T.; Guidry, C.A.; Penn, R.I.; Sawyer, R.G.; Schirmer, B.D.; Hallowell, P.T. Long-term diabetic response to gastric bypass. J. Surg. Res. 2014, 190, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Still, C.D.; Wood, G.C.; Benotti, P.; Petrick, A.T.; Gabrielsen, J.; Strodel, W.E.; Ibele, A.; Seiler, J.; Irving, B.A.; Celaya, M.P.; et al. Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: A retrospective cohort study. Lancet Diabetes Endocrinol. 2014, 2, 38–45. [Google Scholar] [CrossRef]
- Zhang, R.; Borisenko, O.; Telegina, I.; Hargreaves, J.; Ahmed, A.R.; Sanchez Santos, R.; Pring, C.; Funch-Jensen, P.; Dillemans, B.; Hedenbro, J.L. Systematic review of risk prediction models for diabetes after bariatric surgery. Br. J. Surg. 2016, 103, 1420–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.J.; Almulaifi, A.; Tsou, J.J.; Ser, K.H.; Lee, Y.C.; Chen, S.C. Laparoscopic sleeve gastrectomy for type 2 diabetes mellitus: Predicting the success by ABCD score. Surg. Obes. Relat. Dis. 2015, 11, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.C.; Mirshahi, T.; Still, C.D.; Hirsch, A.G. Association of DiaRem Score with cure of type 2 diabetes following bariatric surgery. JAMA Surg. 2016, 151, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.F.; Scopinaro, N.; Cordera, R. Adipokine pattern after bariatric surgery: Beyond the weight loss. Obes. Surg. 2016, 26, 2793–2801. [Google Scholar] [CrossRef] [PubMed]
- Catalán, V.; Gómez-Ambrosi, J.; Ramírez, B.; Rotellar, F.; Pastor, C.; Silva, C.; Rodríguez, A.; Gil, M.J.; Cienfuegos, J.A.; Frühbeck, G. Proinflammatory cytokines in obesity: Impact of type 2 diabetes mellitus and gastric bypass. Obes. Surg. 2007, 17, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Salvador, J.; Frühbeck, G. Adipokines in the treatment of diabetes mellitus and obesity. Expert Opin. Pharmacother. 2009, 10, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Linscheid, P.; Christ-Crain, M.; Stoeckli, R.; Reusch, C.E.; Lutz, T.A.; Muller, B.; Keller, U. Increase in high molecular weight adiponectin by bariatric surgery-induced weight loss. Diabetes Obes. Metab. 2008, 10, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Gómez-Ambrosi, J. Control of body weight: A physiologic and transgenic perspective. Diabetologia 2003, 46, 143–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.F.; Gradaschi, R.; Andraghetti, G.; Scopinaro, N.; Cordera, R. Serum leptin and adiponectin concentration in type 2 diabetes patients in the short and long term following biliopancreatic diversion. Obes. Surg. 2016, 26, 2442–2448. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Gómez-Ambrosi, J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018, 7, 57–62. [Google Scholar]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Colina, I.; Gómez-Ambrosi, J. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutritents 2019, 11, 454. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Portincasa, P.; Colina, I.; Gómez-Ambrosi, J. Involvement of the leptin-adiponectin axis in inflammation and oxidative stress in the metabolic syndrome. Sci. Rep. 2017, 7, 6619. [Google Scholar] [CrossRef]
- Vega, G.L.; Grundy, S.M. Metabolic risk susceptibility in men is partially related to adiponectin/leptin ratio. J. Obes. 2013, 2013, 409679. [Google Scholar] [CrossRef]
- Jung, C.H.; Rhee, E.J.; Choi, J.H.; Bae, J.C.; Yoo, S.H.; Kim, W.J.; Park, C.Y.; Mok, J.O.; Kim, C.H.; Lee, W.Y.; et al. The relationship of adiponectin/leptin ratio with homeostasis model assessment insulin resistance index and metabolic syndrome in apparently healthy korean male adults. Korean Diabetes J. 2010, 34, 237–243. [Google Scholar] [CrossRef]
- Musil, F.; Blaha, V.; Ticha, A.; Hyspler, R.; Haluzik, M.; Lesna, J.; Smahelova, A.; Sobotka, L. Effects of body weight reduction on plasma leptin and adiponectin/leptin ratio in obese patients with type 1 diabetes mellitus. Physiol. Res. 2015, 64, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Maehata, E.; Yano, M.; Taniyama, M.; Suzuki, S. Correlation between the adiponectin-leptin ratio and parameters of insulin resistance in patients with type 2 diabetes. Metabolism 2005, 54, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Yano, M.; Yamakado, M.; Maehata, E.; Suzuki, S. Relationship between the adiponectin-leptin ratio and parameters of insulin resistance in subjects without hyperglycemia. Metabolism 2006, 55, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Vigouroux, C.; Maachi, M.; Nguyen, T.H.; Coussieu, C.; Gharakhanian, S.; Funahashi, T.; Matsuzawa, Y.; Shimomura, I.; Rozenbaum, W.; Capeau, J.; et al. Serum adipocytokines are related to lipodystrophy and metabolic disorders in HIV-infected men under antiretroviral therapy. AIDS 2003, 17, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Tiliscan, C.; Arama, V.; Mihailescu, R.; Munteanu, D.; Iacob, D.G.; Popescu, C.; Catana, R.; Negru, A.; Lobodan, A.; Arama, S.S. Association of adiponectin/leptin ratio with carbohydrate and lipid metabolism parameters in HIV-infected patients during antiretroviral therapy. Endocr. Res. 2018, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Catalán, V.; Rodríguez, A.; Andrada, P.; Ramírez, B.; Ibañez, P.; Vila, N.; Romero, S.; Margall, M.A.; Gil, M.J.; et al. Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care 2014, 37, 2813–2821. [Google Scholar] [CrossRef]
- Muruzábal, F.J.; Frühbeck, G.; Gómez-Ambrosi, J.; Archanco, M.; Burrell, M.A. Immunocytochemical detection of leptin in non-mammalian vertebrate stomach. Gen. Comp. Endocrinol. 2002, 128, 149–152. [Google Scholar] [CrossRef]
- Lancha, A.; Frühbeck, G.; Gómez-Ambrosi, J. Peripheral signalling involved in energy homeostasis control. Nutr. Res. Rev. 2012, 25, 223–248. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, A.; Ezquerro, S.; Mendez-Gimenez, L.; Becerril, S.; Frühbeck, G. Revisiting the adipocyte: A model for integration of cytokine signaling in the regulation of energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E691–E714. [Google Scholar] [CrossRef]
- Lasa, A.; Miranda, J.; Bullo, M.; Casas, R.; Salas-Salvado, J.; Larretxi, I.; Estruch, R.; Ruiz-Gutierrez, V.; Portillo, M.P. Comparative effect of two Mediterranean diets versus a low-fat diet on glycaemic control in individuals with type 2 diabetes. Eur. J. Clin. Nutr. 2014, 68, 767–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oda, N.; Iniamura, S.; Fujita, T.; Uchida, Y.; Inagaki, K.; Kakizawa, H.; Hayakawa, N.; Suzuki, A.; Takeda, J.; Horikawa, Y.; et al. The ratio of leptin to adiponectin can be used as an index of insulin resistance. Metab. Clin. Exp. 2008, 57, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Masquio, D.C.; de Piano, A.; Sanches, P.L.; Corgosinho, F.C.; Campos, R.M.; Carnier, J.; da Silva, P.L.; Caranti, D.A.; Tock, L.; Oyama, L.M.; et al. The effect of weight loss magnitude on pro-/anti-inflammatory adipokines and carotid intima-media thickness in obese adolescents engaged in interdisciplinary weight loss therapy. Clin. Endocrinol. 2013, 79, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Raselli, S.; Grigore, L.; Garlaschelli, K.; Dozio, E.; Magni, P.; Catapano, A.L. Leptin:adiponectin ratio is an independent predictor of intima media thickness of the common carotid artery. Stroke 2007, 38, 2844–2846. [Google Scholar] [CrossRef] [PubMed]
- Satoh, N.; Naruse, M.; Usui, T.; Tagami, T.; Suganami, T.; Yamada, K.; Kuzuya, H.; Shimatsu, A.; Ogawa, Y. Leptin-to-adiponectin ratio as a potential atherogenic index in obese type 2 diabetic patients. Diabetes Care 2004, 27, 2488–2490. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ambrosi, J.; Salvador, J.; Rotellar, F.; Silva, C.; Catalán, V.; Rodríguez, A.; Gil, M.J.; Frühbeck, G. Increased serum amyloid A concentrations in morbid obesity decrease after gastric bypass. Obes. Surg. 2006, 16, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, Y.J. Prediction of Diabetes Remission in Morbidly Obese Patients After Roux-en-Y Gastric Bypass. Obes. Surg. 2016, 26, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, M.; Mari, A.; Anselmino, M.; Baldi, S.; Barsotti, E.; Guarino, D.; Camastra, S.; Bellini, R.; Berta, R.D.; Ferrannini, E. The role of β-cell function and insulin sensitivity in the remission of type 2 diabetes after gastric bypass surgery. J. Clin. Endocrinol. Metab. 2011, 96, E1372–E1379. [Google Scholar] [CrossRef]
- Mallipedhi, A.; Min, T.; Prior, S.L.; MacIver, C.; Luzio, S.D.; Dunseath, G.; Bracken, R.M.; Islam, S.; Barry, J.D.; Caplin, S.; et al. Association between the preoperative fasting and postprandial C-peptide AUC with resolution of type 2 diabetes 6 months following bariatric surgery. Metabolism 2015, 64, 1556–1563. [Google Scholar] [CrossRef]
- Lee, W.J.; Chong, K.; Ser, K.H.; Chen, J.C.; Lee, Y.C.; Chen, S.C.; Su, Y.H.; Tsai, M.H. C-peptide predicts the remission of type 2 diabetes after bariatric surgery. Obes. Surg. 2012, 22, 293–298. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Silva, C.; Rotellar, F.; Gil, M.J.; Cienfuegos, J.A.; Salvador, J.; Frühbeck, G. Expression of caveolin-1 in human adipose tissue is upregulated in obesity and obesity-associated type 2 diabetes mellitus and related to inflammation. Clin. Endocrinol. 2008, 68, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G. Obesity: Aquaporin enters the picture. Nature 2005, 438, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Escuredo, J.M.; Gómez-Ambrosi, J.; Catalán, V.; Domingo, P.; Giralt, M.; Frühbeck, G.; Villarroya, F. Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. Int. J. Obes. 2015, 39, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Gómez-Ambrosi, J. Modulation of the leptin-induced white adipose tissue lipolysis by nitric oxide. Cell. Signal. 2001, 13, 827–833. [Google Scholar] [CrossRef]
- Frühbeck, G.; Gómez-Ambrosi, J.; Salvador, J. Leptin-induced lipolysis opposes the tonic inhibition of endogenous adenosine in white adipocytes. FASEB J. 2001, 15, 333–340. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Before Surgery | After Surgery |
|---|---|---|
| n (males, females) | 25 (11, 14) | 25 (11, 14) |
| Age (years) | 50 ± 2 | 51 ± 2 |
| BMI (kg/m2) | 44.2 ± 1.3 | 33.6 ± 1.6 *** |
| Body fat (%) | 49.9 ± 1.5 | 39.6 ± 2.0 *** |
| Waist (cm) | 128 ± 3 | 107 ± 3 *** |
| Waist-to-hip ratio | 1.00 ± 0.01 | 0.97 ± 0.02 ** |
| SBP (mmHg) | 128 ± 3 | 120 ± 3 ** |
| DBP (mmHg) | 80 ± 2 | 73 ± 1 *** |
| Fasting glucose (mg/dL) | 133 ± 7 | 115 ± 9 |
| Fasting insulin (μU/mL) | 21.3 ± 2.5 | 8.0 ± 1.2 *** |
| HOMA | 6.6 ± 0.8 | 2.1 ± 0.3 *** |
| QUICKI | 0.302 ± 0.006 | 0.363 ± 0.010 *** |
| HbA1c (%) | 7.2 ± 0.2 | 6.4 ± 0.2 ** |
| Triglycerides (mg/dL) | 140 ± 10 | 97 ± 11 ** |
| Total cholesterol (mg/dL) | 185 ± 7 | 159 ± 7 * |
| LDL-cholesterol (mg/dL) | 111 ± 6 | 90 ± 5 * |
| HDL-cholesterol (mg/dL) | 46 ± 2 | 51 ± 2 * |
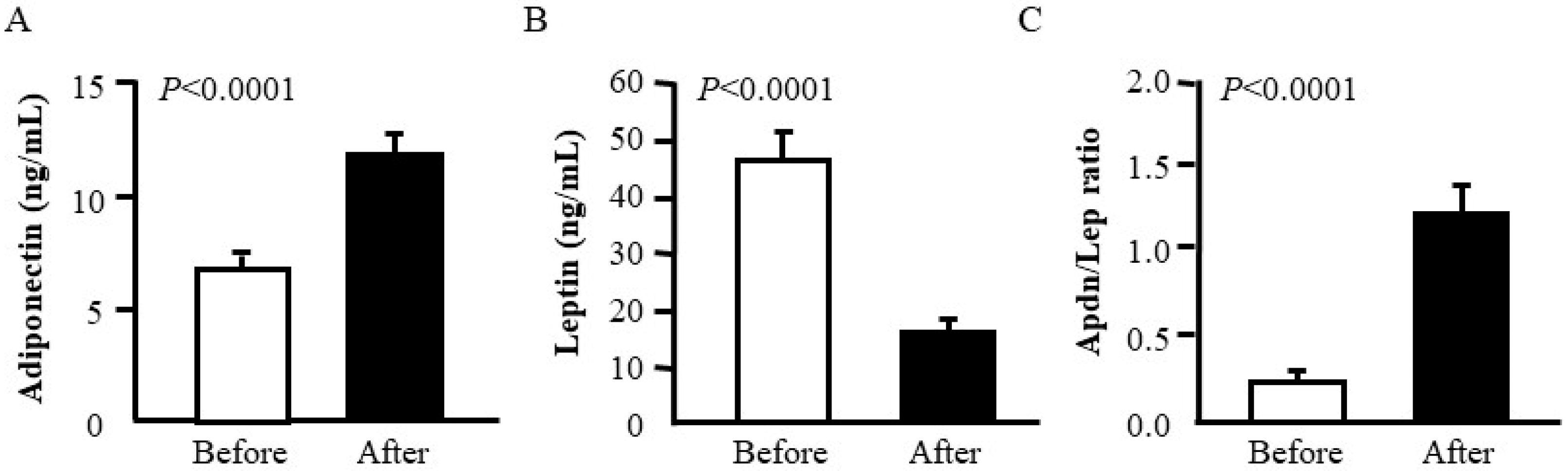
| Leptin (ng/mL) | 45.3 ± 5.6 | 15.1 ± 2.4 *** |
| Adiponectin (µg/mL) | 6.73 ± 0.67 | 11.68 ± 0.81 *** |
| Adpn/Lep ratio | 0.21 ± 0.03 | 1.20 ± 0.19 *** |
| Uric acid (mg/dL) | 5.8 ± 0.3 | 4.5 ± 0.2 *** |
| Creatinine (mg/dL) | 0.80 ± 0.04 | 0.77 ± 0.03 * |
| CRP (mg/L) | 8.5 ± 1.6 | 2.1 ± 1.0 * |
| Fibrinogen (mg/dL) | 385 ± 21 | 348 ± 23 |
| von Willebrand factor (%) | 152 ± 11 | 138 ± 14 |
| Homocysteine (μmol/L) | 10.2 ± 1.2 | 9.3 ± 1.1 |
| AST (IU/L) | 15 ± 1 | 18 ± 2 |
| ALT (IU/L) | 21 ± 2 | 27 ± 5 |
| AST/ALT | 0.78 ± 0.07 | 0.87± 0.07 |
| γ-GT (IU/L) | 35 ± 6 | 16 ± 2 ** |
| Adpn/Lep Ratio Before Surgery | Adpn/Lep Ratio After Surgery | |||
|---|---|---|---|---|
| r | p | r | p | |
| Δ BMI | −0.36 | 0.077 | 0.58 | 0.002 |
| Δ BF | −0.02 | 0.912 | 0.79 | <0.001 |
| Δ Waist | −0.14 | 0.526 | 0.72 | <0.001 |
| Δ WHR | 0.37 | 0.087 | 0.58 | 0.004 |
| Δ Adiponectin | Δ Leptin | Δ Adpn/Lep Ratio | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Δ BMI | −0.49 | 0.013 | 0.73 | <0.001 | −0.64 | <0.001 |
| Δ BF | −0.48 | 0.014 | 0.25 | 0.234 | −0.80 | <0.001 |
| Δ Waist | −0.45 | 0.030 | 0.58 | 0.004 | −0.76 | <0.001 |
| Δ WHR | −0.06 | 0.788 | 0.06 | 0.783 | −0.52 | 0.011 |
| Responders | Non-Responders | p (R vs. NR) | ||||
|---|---|---|---|---|---|---|
| Before BS | After BS | Before BS | After BS | Before BS | After BS | |
| n | 18 | 18 | 7 | 7 | - | - |
| Age | 49 ± 3 | 50 ± 3 | 58 ± 2 | 59 ± 2 | 0.014 | 0.014 |
| BMI (kg/m2) | 45.2 ± 1.7 | 33.8 ± 1.6 *** | 41.4 ± 1.9 | 32.3 ± 2.6 ** | 0.226 | 0.601 |
| Body fat (%) | 50.7 ± 1.9 | 40.2 ± 2.5 *** | 49.2 ± 2.9 | 38.4 ± 4.1 * | 0.273 | 0.701 |
| Waist (cm) | 127 ± 4 | 106 ± 4 *** | 105 ± 4 | 110 ± 6 ** | 0.731 | 0.730 |
| Waist-to-hip ratio | 0.99 ± 0.01 | 0.96 ± 0.02 | 1.02 ± 0.02 | 0.95 ± 0.02 * | 0.246 | 0.836 |
| SBP (mmHg) | 127 ± 4 | 117 ± 3 * | 130 ± 4 | 126 ± 7 ** | 0.679 | 0.220 |
| DBP (mmHg) | 81 ± 3 | 72 ± 2 *** | 77 ± 4 | 74 ± 3 * | 0.439 | 0.628 |
| Fasting glucose (mg/dL) | 125 ± 6 | 97 ± 4 *** | 166 ± 16 | 172 ± 25 | 0.004 | 0.002 |
| Fasting insulin (μU/mL) | 21.9 ± 3.2 | 9.5 ± 1.5 ** | 19.1 ± 4.9 | 4.7 ± 1.0 * | 0.649 | 0.008 |
| HOMA | 6.3 ± 1.0 | 2.3 ± 1.4 ** | 7.8 ± 2.2 | 1.8 ± 0.5 ** | 0.487 | 0.516 |
| QUICKI | 0.304 ± 0.007 | 0.358 ± 0.012 *** | 0.298 ± 0.017 | 0.362 ± 0.020 * | 0.728 | 0.869 |
| HbA1c (%) | 7.3 ± 0.6 | 6.1 ± 0.4 ** | 8.1 ± 0.5 | 6.7 ± 0.2 | 0.005 | 0.172 |
| Triglycerides (mg/dL) | 141 ± 12 | 84 ± 10 *** | 129 ± 28 | 135 ± 30 | 0.422 | 0.041 |
| Total cholesterol (mg/dL) | 194 ± 10 | 150 ± 7 *** | 162 ± 8 | 184 ± 9 | 0.222 | 0.025 |
| LDL-cholesterol (mg/dL) | 122 ± 8 | 86 ± 7 ** | 90 ± 9 | 100 ± 6 | 0.190 | 0.263 |
| HDL-cholesterol (mg/dL) | 45 ± 3 | 48 ± 2 | 45 ± 2 | 57 ± 4 * | 0.513 | 0.058 |
| Leptin (ng/mL) | 48.8 ± 6.9 | 16.9 ± 3.1 *** | 38.1 ± 10.8 | 11.8 ± 3.1 * | 0.415 | 0.830 |
| Adiponectin (µg/mL) | 6.49 ± 0.90 | 11.80 ± 1.06 *** | 7.34 ± 0.64 | 11.39 ± 1.14 | 0.582 | 0.356 |
| Adpn/Lep ratio | 0.17 ± 0.03 | 1.11 ± 0.21 *** | 0.32 ± 0.09 | 1.43 ± 0.44 * | 0.150 | 0.478 |
| Uric acid (mg/dL) | 5.9 ± 0.4 | 4.4 ± 0.3 ** | 5.3 ± 0.3 | 4.6 ± 0.3 | 0.325 | 0.759 |
| Creatinine (mg/dL) | 0.78 ± 0.04 | 1.29 ± 0.54 | 0.80 ± 0.09 | 0.74 ± 0.07 * | 0.551 | 0.811 |
| CRP (mg/L) | 9.7 ± 4.2 | 3.0 ± 1.6 | 7.3 ± 3.1 | 0.9 ± 0.2 * | 0.447 | 0.352 |
| Fibrinogen (mg/dL) | 378 ± 24 | 373 ± 24 | 357 ± 72 | 271 ± 32 | 0.828 | 0.154 |
| von Willebrand factor (%) | 159 ± 23 | 140 ± 18 | 161 ± 28 | 136 ± 27 * | 0.940 | 0.971 |
| Homocysteine (μmol/L) | 7.92 ± 1.67 | 9.47 ± 1.44 | 11.30 ± 1.70 | 9.13 ± 1.11 | 0.722 | 0.838 |
| AST (IU/L) | 16 ± 2 | 17 ± 2 | 18 ± 1 | 31 ± 3 | 0.913 | 0.262 |
| ALT (IU/L) | 23 ± 2 | 26 ± 5 | 23 ± 3 | 26 ± 12 | 0.273 | 0.656 |
| AST/ALT | 0.70 ± 0.04 | 0.79 ± 0.06 | 1.00 ± 0.18 | 1.07 ± 0.21 | 0.135 | 0.240 |
| γ-GT (IU/L) | 32 ± 5 | 15 ± 2 ** | 43 ± 18 | 24 ± 7 | 0.439 | 0.233 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unamuno, X.; Izaguirre, M.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Valentí, V.; Moncada, R.; Silva, C.; Salvador, J.; et al. Increase of the Adiponectin/Leptin Ratio in Patients with Obesity and Type 2 Diabetes after Roux-en-Y Gastric Bypass. Nutrients 2019, 11, 2069. https://doi.org/10.3390/nu11092069
Unamuno X, Izaguirre M, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Becerril S, Valentí V, Moncada R, Silva C, Salvador J, et al. Increase of the Adiponectin/Leptin Ratio in Patients with Obesity and Type 2 Diabetes after Roux-en-Y Gastric Bypass. Nutrients. 2019; 11(9):2069. https://doi.org/10.3390/nu11092069
Chicago/Turabian StyleUnamuno, Xabier, Maitane Izaguirre, Javier Gómez-Ambrosi, Amaia Rodríguez, Beatriz Ramírez, Sara Becerril, Víctor Valentí, Rafael Moncada, Camilo Silva, Javier Salvador, and et al. 2019. "Increase of the Adiponectin/Leptin Ratio in Patients with Obesity and Type 2 Diabetes after Roux-en-Y Gastric Bypass" Nutrients 11, no. 9: 2069. https://doi.org/10.3390/nu11092069
APA StyleUnamuno, X., Izaguirre, M., Gómez-Ambrosi, J., Rodríguez, A., Ramírez, B., Becerril, S., Valentí, V., Moncada, R., Silva, C., Salvador, J., Portincasa, P., Frühbeck, G., & Catalán, V. (2019). Increase of the Adiponectin/Leptin Ratio in Patients with Obesity and Type 2 Diabetes after Roux-en-Y Gastric Bypass. Nutrients, 11(9), 2069. https://doi.org/10.3390/nu11092069