14C-Cobalamin Absorption from Endogenously Labeled Chicken Eggs Assessed in Humans Using Accelerator Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
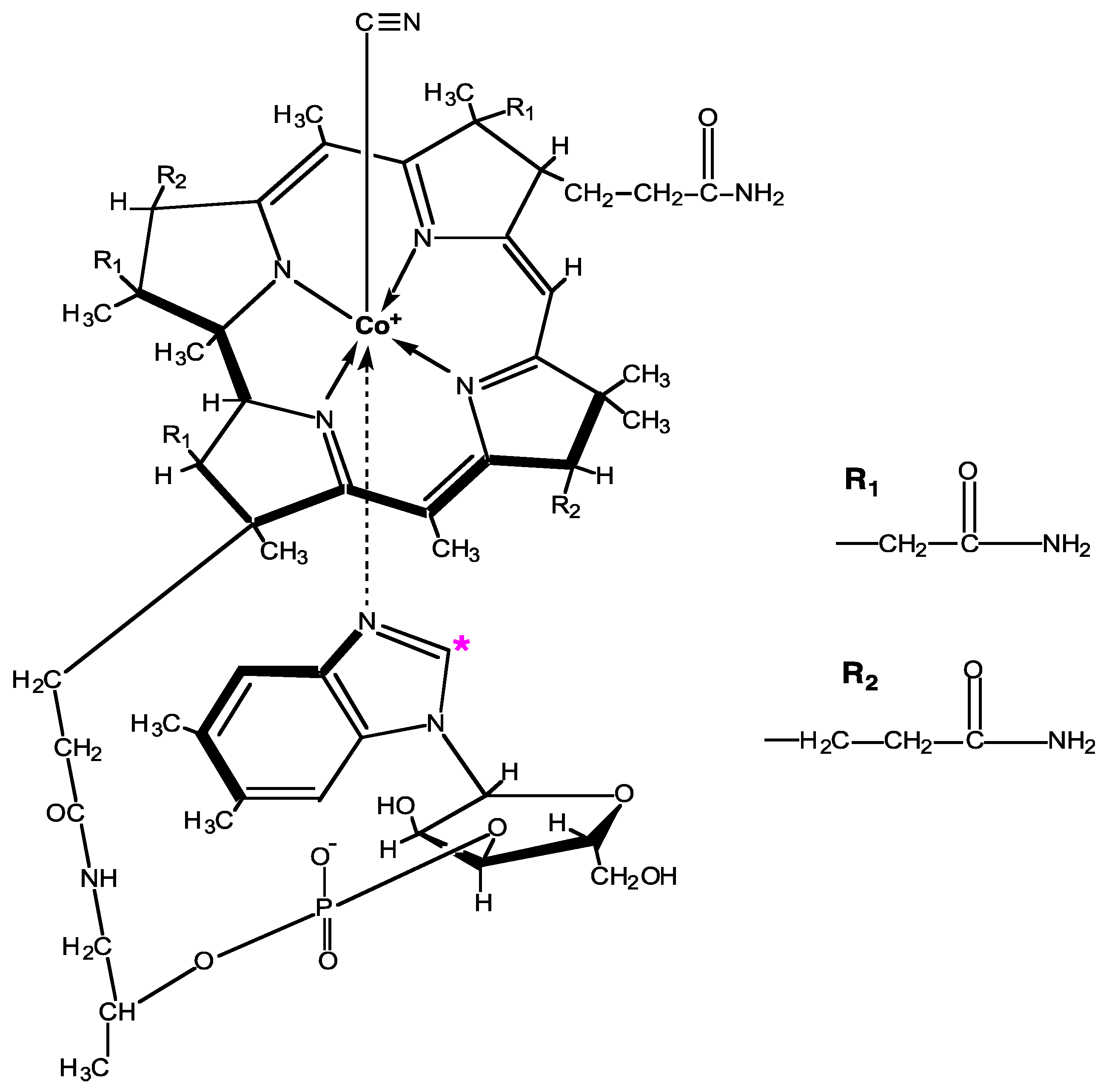
2.1. 14C-B12 Synthesis
2.2. Production of Labeled Eggs
2.3. Human Subjects, Dosing and Sample Collection
2.4. Analysis of 14C
2.5. Calculation of % Bioavailability
2.6. Statistics
3. Results
3.1. Appearance of 14C Label in Eggs
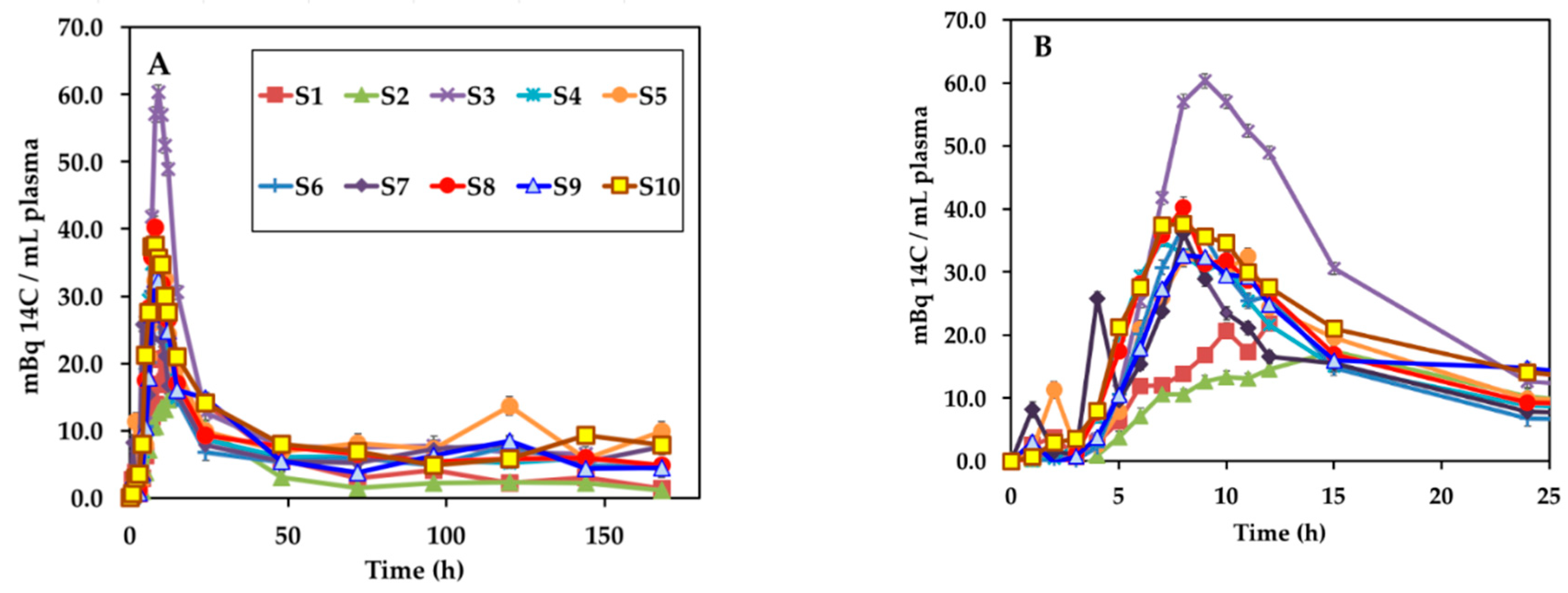
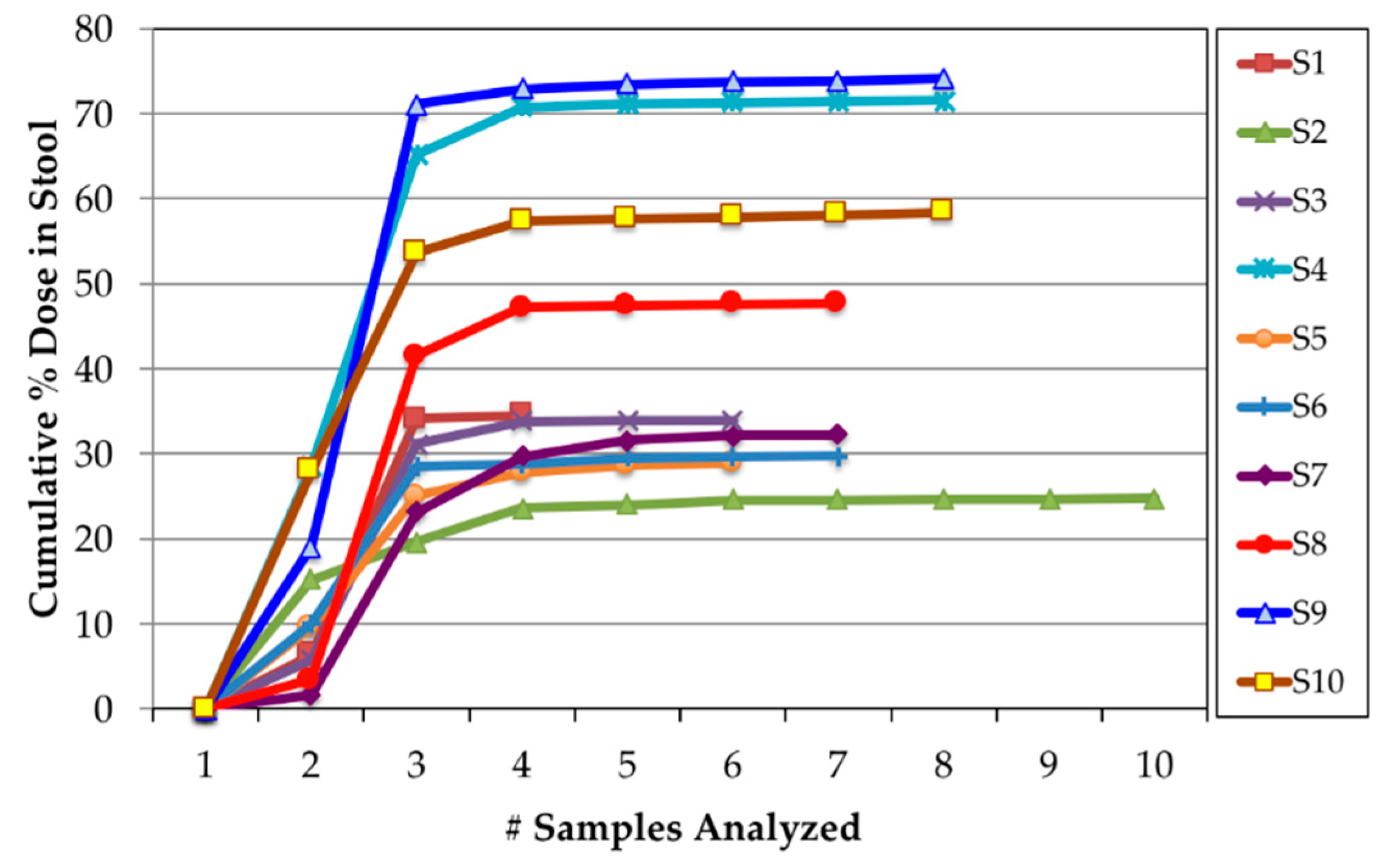
3.2. Appearance of 14C Label in Plasma, Uurine, and Feces
3.3. Calculation of Bioavailability
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Recruitment, Screening and Participation of Subjects

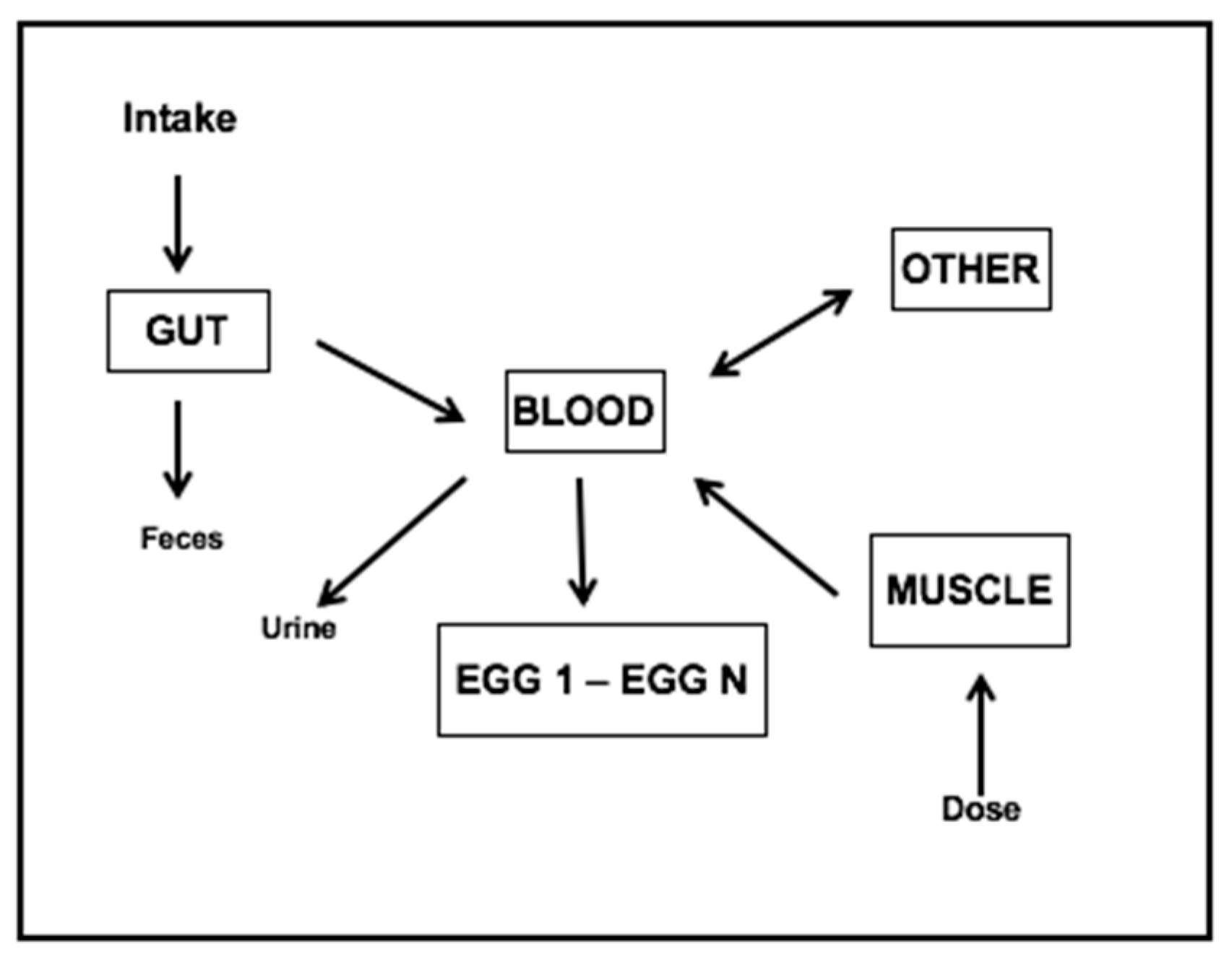
Appendix B. Egg Labeling Model
| Reference | Protocol | Dose 1 | ODE | PME | OPE | PPE | ODB | PMB |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | |||
| Edwards [23] | 1 | 0.5 µCi 57Co-B12 (IM) | 52 | 52 | 8 | 11 | 35 | 37 |
| Edwards [23] | 2 | 0.5 µCi 57Co-B12 (FED) | 33 | 32 | 5 | 4 | 18 | 19 |
| Edwards [23] | 3 | 0.072 µCi 60Co-B12 (IM) | 63 | 59 | 11 | 12 | 45 | 41 |
| Edwards [23] | 4 | 0.072 µCi 60Co-B12 (FED) | 36 | 37 | 7 | 5 | 30 | 27 |
| Denton [24] | 5 | 3 µg/d crystal B12 (IM) | 33 | 28 | 4 | 4 | NA | NA |
| Denton [24] | 6 | 3 µg/d crystal B12 (FED) | 20 | 15 | 1.5 | 1.3 | NA | NA |


References
- Brito, A.; Habeych, E.; Silva-Zolezzi, I.; Galaffu, N.; Allen, L.H. Methods to assess vitamin B12 bioavailability and technologies to enhance its absorption. Nutr. Rev. 2018, 76, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Hvas, A.M.; Morkbak, A.L.; Hardlei, T.F.; Nexo, E. The vitamin B12 absorption test, CobaSorb, identifies patients not requiring vitamin B12 injection therapy. Scand. J. Clin. Lab. Investig. 2011, 71, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Carmel, R. Malabsorption of food cobalamin. Baillieres Clin. Haematol. 1995, 8, 639–655. [Google Scholar] [CrossRef]
- Allen, L.H.; Miller, J.W.; de Groot, L.; Rosenberg, I.H.; Smith, A.D.; Refsum, H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J. Nutr. 2018, 148, 1995S–2027S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, K.L.; Rich, S.; Rosenberg, I.; Jacques, P.; Dallal, G.; Wilson, P.W.; Selhub, J. Plasma vitamin B-12 concentrations relate to intake source in the Framingham Offspring study. Am. J. Clin. Nutr. 2000, 71, 514–522. [Google Scholar] [CrossRef]
- Doscherholmen, A.; McMahon, J.; Ripley, D. Vitamin B12 absorption from eggs. Proc. Soc. Exp. Biol. Med. 1975, 149, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Doscherholmen, A.; Swaim, W.R. Impaired assimilation of egg Co 57 vitamin B 12 in patients with hypochlorhydria and achlorhydria and after gastric resection. Gastroenterology 1973, 64, 913–919. [Google Scholar] [CrossRef]
- Doets, E.L.; Szczecinska, A.; Dhonukshe-Rutten, R.A.; Cavelaars, A.E.; van‘t Veer, P.; Brzozowska, A.; de Groot, L.C. Systematic review on daily vitamin B12 losses and bioavailability for deriving recommendations on vitamin B12 intake with the factorial approach. Ann. Nutr. Metab. 2013, 62, 311–322. [Google Scholar] [CrossRef]
- Heyssel, R.M.; Bozian, R.C.; Darby, W.J.; Bell, M.C. Vitamin B12 turnover in man. The assimilation of vitamin B12 from natural foodstuff by man and estimates of minimal daily dietary requirements. Am. J. Clin. Nutr. 1966, 18, 176–184. [Google Scholar] [CrossRef]
- Doscherholmen, A. Inhibition by raw eggs of vitamin B12 absorption. JAMA 1978, 240, 2045. [Google Scholar] [CrossRef]
- Reizenstein, P.G.; Nyberg, W. Intestinal absorption of liver-bound radiovitamin B12 in patients with pernicious anaemia and in controls. Lancet 1959, 2, 248–252. [Google Scholar] [CrossRef]
- Kittang, E.; Hamborg, B.; Schjonsby, H. Absorption of food cobalamins assessed by the double-isotope method in healthy volunteers and in patients with chronic diarrhoea. Scand. J. Gastroenterol. 1985, 20, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Aimone-Gastin, I.; Pierson, H.; Jeandel, C.; Bronowicki, J.P.; Plenat, F.; Lambert, D.; Nabet-Belleville, F.; Gueant, J.L. Prospective evaluation of protein bound vitamin B12 (cobalamin) malabsorption in the elderly using trout flesh labelled in vivo with 57Co-cobalamin. Gut 1997, 41, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Doscherholmen, A.; McMahon, J.; Economon, P. Vitamin B12 absorption from fish. Proc. Soc. Exp. Biol. Med. 1981, 167, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Schade, S.G.; Schilling, R.F. Effect of pepsin on the absorption of food vitamin B12 and iron. Am. J. Clin. Nutr. 1967, 20, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Chanarin, I. The Megaloblastic Anemias, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1979. [Google Scholar]
- Doscherholmen, A.; McMahon, J.; Ripley, D. Vitamin B12 assimilation from chicken meat. Am. J. Clin. Nutr. 1978, 31, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.S.; Turteltaub, K.W.; Finkel, R.; Nelson, D.E. Accelerator mass spectrometry. Anal. Chem. 1995, 67, 353A–359A. [Google Scholar] [CrossRef]
- Carkeet, C.; Dueker, S.R.; Lango, J.; Buchholz, B.A.; Miller, J.W.; Green, R.; Hammock, B.D.; Roth, J.R.; Anderson, P.J. Human vitamin B12 absorption measurement by accelerator mass spectrometry using specifically labeled (14)C-cobalamin. Proc. Natl. Acad. Sci. USA 2006, 103, 5694–5699. [Google Scholar] [CrossRef]
- Vogel, J.S.; Love, A.H. Quantitating isotopic molecular labels with accelerator mass spectrometry. Methods Enzymol. 2005, 402, 402–422. [Google Scholar]
- Lin, Y.; Dueker, S.R.; Follett, J.R.; Fadel, J.G.; Arjomand, A.; Schneider, P.D.; Miller, J.W.; Green, R.; Buchholz, B.A.; Vogel, J.S.; et al. Quantitation of in vivo human folate metabolism. Am. J. Clin. Nutr. 2004, 80, 680–691. [Google Scholar] [CrossRef]
- The Incredible Egg. Available online: http://www.incredibleegg.org/health-and-nutrition/egg-nutrients (accessed on 29 August 2019).
- Edwards, H.M., Jr. Vitamin B12. The absorption of 57Co or 60Co-B12 by the laying hen and mature rooster. Poult. Sci. 1969, 48, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.A.; Kellog, W.L.; Sizemore, J.R.; Lillie, R.J. Effect of injecting and feeding vitamin B12 to hens on content of the vitamin in the egg and blood. J. Nutr. 1954, 54, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Universities Federation for Animal Welfare Handbook on the Care and Management of Laboratory Animals, 8th ed.; Hubrecht, R.C.; Kirkwood, J. (Eds.) John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Latimer, G.; Horowitz, W. Official Methods of Analysis of AOAC International, 18th ed.; AOAC Intl: Gaithersburg, MD, USA, 2001. [Google Scholar]
- Green, R.; Miller, J.W. Vitamin B12. In Handbook of Vitamins, 5th ed.; Zempleni, J., Suttie, J.W., Gregory, J.F., Stover, P.J., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 447–489. [Google Scholar]
- Garrod, M.G.; Buchholz, B.A.; Miller, J.W.; Haack, K.W.; Green, R.; Allen, L.H. Vitamin B12 added as a fortificant to flour retains high bioavailability when baked in bread. Nucl. Inst. Methods Phys. Res. B 2019, 438, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Ognibene, T.J.; Bench, G.; Vogel, J.S.; Peaslee, G.F.; Murov, S. A high-throughput method for the conversion of CO2 obtained from biochemical samples to graphite in septa-sealed vials for quantification of 14C via accelerator mass spectrometry. Anal. Chem. 2003, 75, 2192–2196. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, B.A.; Freeman, S.P.H.T.; Haack, K.W.; Vogel, J.S. Tips and traps in the 14C bio-AMS preparation laboratory. Nucl. Instrum. Methods Phys. Res. B 2000, 172, 404–408. [Google Scholar] [CrossRef]
- Zhao, C.; Hwang, S.H.; Buchholz, B.A.; Carpenter, T.S.; Lightstone, F.C.; Yang, J.; Hammock, B.D.; Casida, J.E. GABAA receptor target of tetramethylenedisulfotetramine. Proc. Natl. Acad. Sci. USA 2014, 111, 8607–8612. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health, Office of Dietary Supplements. Vitamin B12 Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/#en18 (accessed on 29 August 2019).
- McCartt, A.D.; Ognibene, T.J.; Bench, G.; Turteltaub, K.W. Quantifying carbon-14 for biology using cavity ring-down spectroscopy. Anal. Chem. 2016, 88, 8714–8719. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, N.A.; Dueker, S.R.; Muri, D.; Senn, C.; Yoon, H.; Yu, B.Y.; Lee, G.H.; Dong, F.; Otteneder, M.B. Nanotracing and cavity-ring down spectroscopy: A new ultrasensitive approach in large molecule drug disposition studies. PLoS ONE 2018, 13, e0205435. [Google Scholar] [CrossRef] [PubMed]


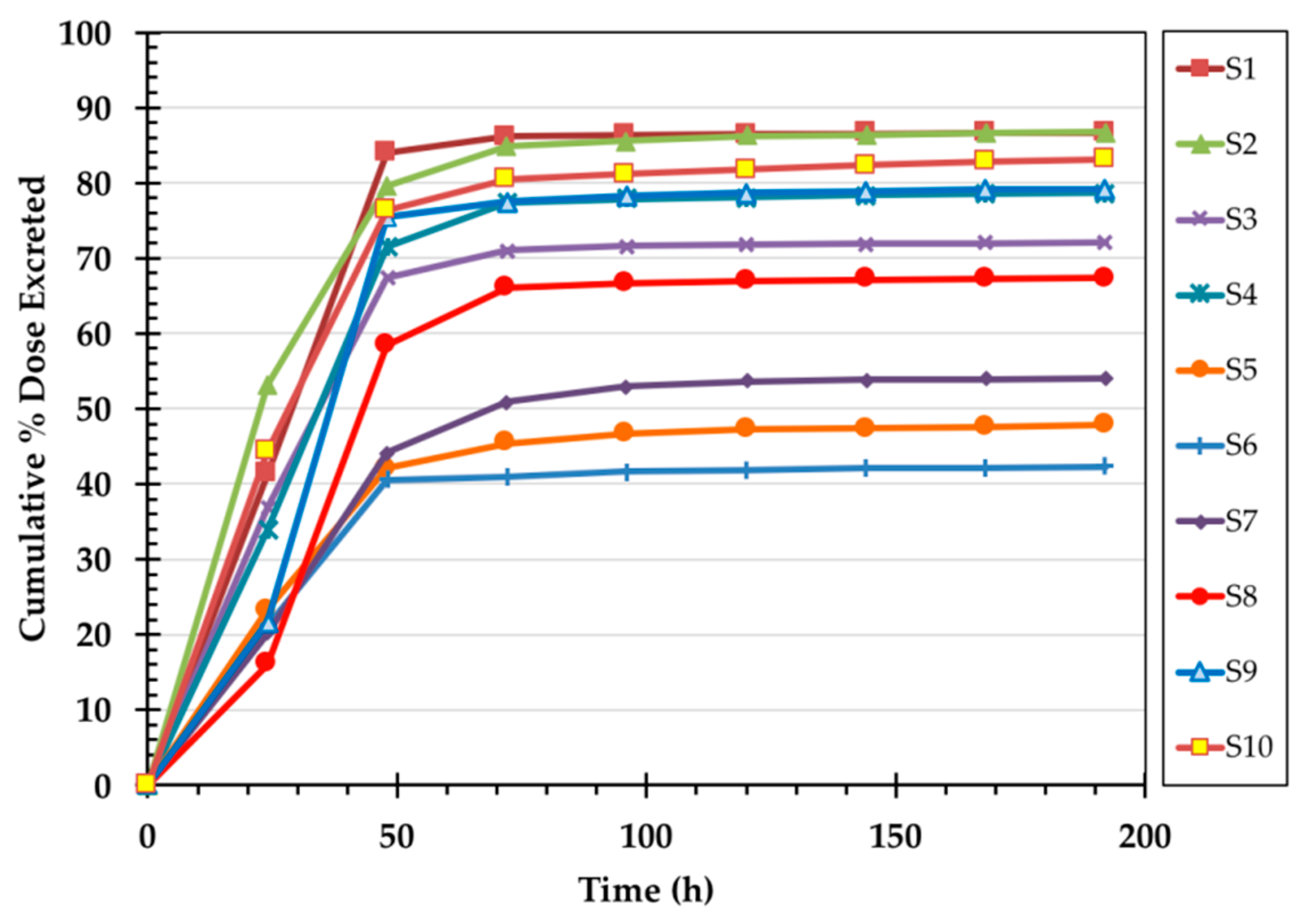

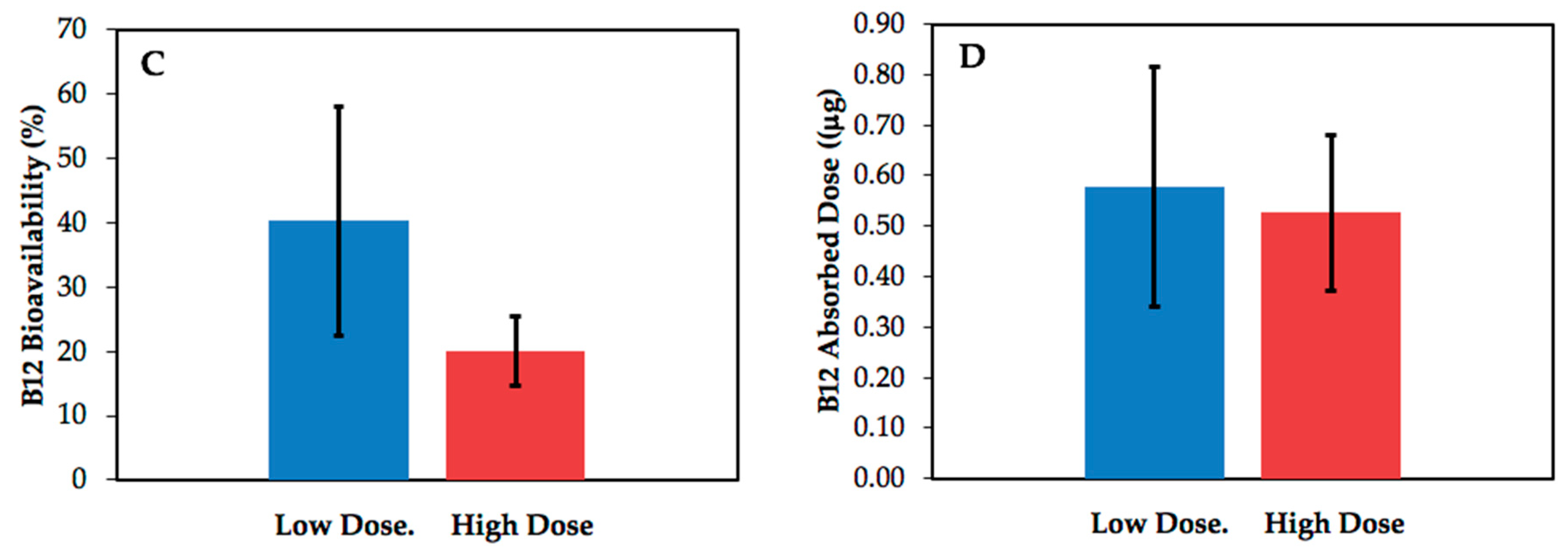
| Subject | Age (y) | Sex 1 | BMI 2 (kg/m2) | Serum | B12 | 14C-B12 | Specific |
|---|---|---|---|---|---|---|---|
| B12 | Dose | Dose | Activity | ||||
| (pmol/L) | (μg) | (kBq) | (Bq/μg) | ||||
| S1 | 29 | F | 24.4 | 331 | 2.38 | 1.114 | 468 |
| S2 | 43 | M | 28.2 | 441 | 1.66 | 0.777 | 468 |
| S3 | 29 | M | 24.5 | 358 | 2.65 | 1.159 | 437 |
| S4 | 24 | F | 21.2 | 323 | 2.65 | 1.158 | 437 |
| S5 | 53 | F | 20.8 | 365 | 1.43 | 1.089 | 761 |
| S6 | 25 | M | 23.9 | 371 | 1.40 | 1.083 | 774 |
| S7 | 24 | M | 24.5 | 306 | 1.40 | 1.093 | 781 |
| S8 | 26 | M | 23.8 | 325 | 1.44 | 1.089 | 756 |
| S9 | 22 | F | 35.1 | 227 | 2.64 | 1.131 | 428 |
| S10 | 24 | M | 23.1 | 386 | 2.64 | 1.131 | 428 |
| Subject | B12 | 14C-B12 | Cmax | Tmax | Plasma | Plasma |
|---|---|---|---|---|---|---|
| Dose | Dose | Plasma | Plasma | AUC 1 0–24 h | AUC 1 0–168 h | |
| (μg) | (kBq) | (Bq/L) | (h) | (Bq·h/L) | (Bq·h/L) | |
| S1 | 2.38 | 1.114 | 21.9 | 12 | 323 | 940 |
| S2 | 1.66 | 0.777 | 17.4 | 15 | 250 | 657 |
| S3 | 2.65 | 1.159 | 60.3 | 9 | 649 | 1745 |
| S4 | 2.65 | 1.158 | 34.9 | 7 | 386 | 1241 |
| S5 | 1.43 | 1.089 | 32.5 | 11 | 410 | 1649 |
| S6 | 1.40 | 1.083 | 37.2 | 8 | 363 | 1185 |
| S7 | 1.40 | 1.093 | 36.2 | 8 | 357 | 1291 |
| S8 | 1.44 | 1.089 | 40.2 | 8 | 417 | 1336 |
| S9 | 2.64 | 1.131 | 32.6 | 8 | 399 | 1308 |
| S10 | 2.64 | 1.131 | 37.6 | 8 | 483 | 1585 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrod, M.G.; Rossow, H.A.; Calvert, C.C.; Miller, J.W.; Green, R.; Buchholz, B.A.; Allen, L.H. 14C-Cobalamin Absorption from Endogenously Labeled Chicken Eggs Assessed in Humans Using Accelerator Mass Spectrometry. Nutrients 2019, 11, 2148. https://doi.org/10.3390/nu11092148
Garrod MG, Rossow HA, Calvert CC, Miller JW, Green R, Buchholz BA, Allen LH. 14C-Cobalamin Absorption from Endogenously Labeled Chicken Eggs Assessed in Humans Using Accelerator Mass Spectrometry. Nutrients. 2019; 11(9):2148. https://doi.org/10.3390/nu11092148
Chicago/Turabian StyleGarrod, Marjorie G., Heidi A. Rossow, Christopher C. Calvert, Joshua W. Miller, Ralph Green, Bruce A. Buchholz, and Lindsay H. Allen. 2019. "14C-Cobalamin Absorption from Endogenously Labeled Chicken Eggs Assessed in Humans Using Accelerator Mass Spectrometry" Nutrients 11, no. 9: 2148. https://doi.org/10.3390/nu11092148





